Abstract
Autologous T cells modified to recognize novel antigen targets are a novel form of therapy for cancer. We review the various potential forms of observed and hypothetical toxicities associated with genetically modified T cells. Despite the focus on toxicities in this review, re-directed T cells represent a powerful and highly effective form of anti-cancer therapy; we remain optimistic that the common toxicities will become routinely manageable and that some theoretical toxicity will be exceedingly rare, if ever observed.
Keywords: CAR T cells, adoptive therapy, toxicity
Bedoya and colleagues review the observed and theoretical clinical toxicities of genetically modified T cells, focusing on T cells redirected with chimeric antigen receptors (CARs). The observed toxicities reflect the power of re-directed T cells, which still hold great potential as immunotherapies for cancer and other serious diseases.
Main Text
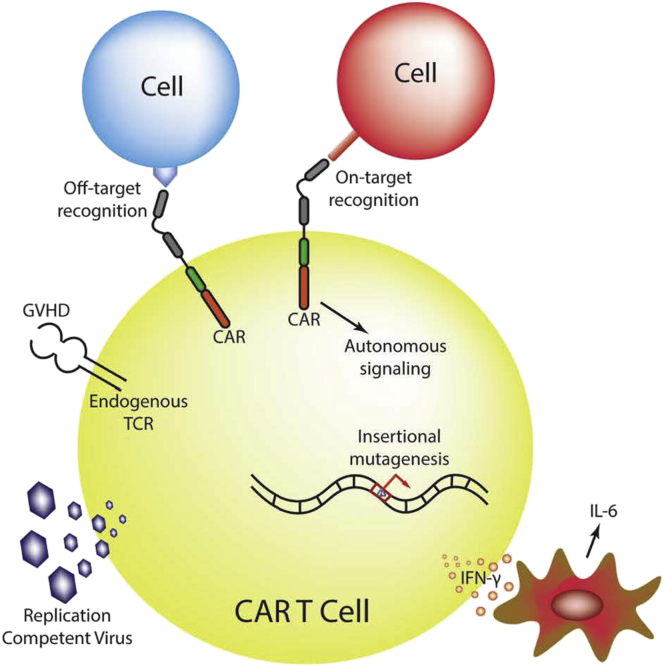
Adoptive cellular therapy using chimeric antigen receptor (CAR) T cells has been successful in the treatment of refractory or relapsed (r/r) B cell malignancies. Despite this early success, broader applications of CAR T cell therapy may be limited by the availability of targetable antigens and the mitigation of their various toxicity profiles. Toxicities discussed here included bystander effects leading to systemic inflammatory reactions such as the previously described cytokine release syndrome (CRS), neurologic toxicities, on- and off-target effects of CARs, hypersensitivity reactions to CAR constructs, and theoretical toxicities, including insertional mutagenesis mediated by transgene delivery, autonomous CAR signaling, autoimmunity or graft-versus-host disease (GVHD) caused by T cell products, and generation of a replication-competent virus (Figure 1).1, 2 Despite the focus on toxicities in this review, we remain optimistic that toxicity mitigation can be overcome through experience in clinical management and optimized T cell engineering strategies, while some of the theoretical toxicities may never materialize in the clinical setting.
Figure 1.
Potential Mechanisms of Toxicity of CAR T Cells
Some mechanisms of CAR-T cell-associated toxicities, both real and hypothetical, are depicted in the diagram. Cytokine release syndrome (CRS), the most relevant toxicity described so far, has been associated with the activation of CAR T cells, leading to IFN-γ production and concomitant high levels of systemic IL-6 secreted by bystander macrophage cells. Additional toxicities, which have not been observed in the clinical setting, could result from autonomous signaling of the CAR; off-target recognition of the scFv; insertional mutagenesis of the CAR transgene; activation of endogenous TCR, leading to GVHD; and formation of a replication-competent virus.
Bystander Innate Cells
One well-described CAR toxicity is CRS, which results from activation of bystander innate immune cells, including macrophages. CRS has been observed in trials using CAR constructs targeting various antigens but has become associated with anti-CD19 CARs and the anti-CD19 bispecific T cell engaging antibody, blinatumomab.3 CRS can range from mild flu-like symptoms such as fever, myalgias, fatigue, and mild hypotension to a more serious presentation of severe inflammatory response syndrome (SIRS) involving significant hypotension requiring pressors, vascular leak with associated respiratory failure, coagulopathy, and multi-organ system failure. Cytokine profiling of patients with CRS has repeatedly demonstrated significantly elevated levels of interleukin (IL)-6, IL-10, granulocyte colony-stimulating factor (G-CSF), and interferon (IFN)-γ, with levels often correlating with rapid CAR T cell activation and expansion. The severity of CRS does not appear to correlate with overall disease response, but most responding patients demonstrate some degree of CRS.3, 4, 5, 6, 7 In patients with acute lymphoblastic leukemia (ALL), CRS has been correlated with disease burden at the time of infusion, and experience has demonstrated that more potent conditioning regimens may increase the likelihood of severe CRS and/or CAR-related toxicity.8
Patients with severe CRS can also develop a macrophage activation syndrome (MAS) reminiscent of hemophagocytic lymphohistiocytosis, as demonstrated by overlapping cytokine profiles, hyperferritinemia, and evidence of hemophagocytosis on bone marrow biopsy.3, 7 In all witnessed cases to date, MAS appears to resolve with the resolution of CRS. Current theories on the mechanism of action include high levels of IFN-γ production in the setting of rapid T cell activation and cytotoxicity, resulting in robust macrophage activation.9 Given the importance of IFN-γ in T cell cytotoxicity, and the clinically available anti-cytokine therapies, management of CRS involves the blockade of the pro-inflammatory cytokine IL-6 via blockade of the IL-6 receptor with tocilizumab. Neutralization of IL-6 with siltuximab has also been attempted, but it is not as well established as tocilizumab.7 High-dose corticosteroids have been used in patients, although there is some evidence that they may have a detrimental effect on T cell proliferation; therefore, their use is reserved for management of continuing severe CRS and/or severe neurologic toxicity.10 Predictive biomarkers of which patients are likely to experience CRS are being explored.11 More extensive reviews of the grading and management of CRS have been published,7, 12 and the algorithms for clinical management are still in development by the various sponsors of CAR T cell therapies.
Neurotoxicity
One type of unexpected toxicity is the range of transient neurologic complications that have been observed in almost all trials targeting T cells to CD19 with either CAR T cells or bispecific T cell engagers (BiTEs). Manifestations vary and include confusion, obtundation, seizures, hallucinations, aphasia, ataxia, and more recently and rarely, profound cerebral edema. In some instances, these symptoms can correlate with the onset of CRS, but neurologic symptoms can also occur before or following resolution of CRS. These symptoms are usually self-limiting and are managed with high-dose steroids and anti-epileptic drugs as needed; tocilizumab does not appear to have a beneficial effect in ameliorating neurologic toxicity, though it has not been formally tested in this context.
Given the toxicity overlap with blinatumomab and the absence of neurotoxicity seen with other CAR constructs, there may be a correlation with single-chain fragment variable (scFv) cross-reactivity with a yet unidentified antigen expressed in the nervous system.13, 14 CAR T cells have been shown to infiltrate the cerebrospinal fluid and central nervous system (CNS); however, their presence does not appear to be directly correlated with neurologic toxicity and may be beneficial in diseases such as ALL, in which the CNS is a sanctuary site for the leukemia cells. Alternatively, CAR T cell neurotoxicity may be associated with cytokines and inflammatory factors released with immune activation. Recent work identified an association of severe CRS with the peak serum levels of 24 cytokines, including IFN-γ, IL-6, sgp130, and soluble IL-6 receptor (sIL-6R).11 It is therefore possible that specific cytokine profiling may help predict which patients are at high risk for neurologic toxicities. For example, it is well established that high levels of IFN-γ in the blood trigger monocyte/macrophage activation and migration to inflamed tissues.15 Cells of the myeloid lineage present in the CNS may become activated by these same inflammatory factors, causing a localized cytokine release in the CNS. This may also partially explain the neurologic toxicity observed with blinatumomab.3
Juno Therapeutics reported a series of three cases of lethal neurotoxicity in adult patients with r/r ALL treated with JCAR015, which includes the CD28 and CD3z signaling domains. Although the study went through a temporary clinical hold by the U.S. Food and Drug Administration (FDA), it was rapidly re-instated after the conditioning regimen was modified to eliminate fludarabine; this trial now uses only cyclophosphamide in the conditioning regimen. Because fludarabine has not been independently associated with lethal or severe neurotoxicity as a single agent or with other forms of CAR T cells, it is likely to remain an important drug in the conditioning regimens for other CAR T cells. Since the initial writing of this manuscript, this trial has been placed on clinical hold after more cases of lethal cerebral edema have occurred.
On-Target Toxicities and Target Antigen Selection
One of the most important factors in CAR design is choosing the right antigen target. Ideally, the antigen should be expressed only on the surface of the tumor cells, not on healthy tissue. With few exceptions, such as EGFRvIII in glioblastoma, most antigens that have been selected thus far are also present at some level in normal tissues. Because the main driver initiating CAR T cell activation is the direct engagement of the scFv with its cognate antigen, the coexpression of this antigen on any non-tumor cell leads to simultaneous elimination of both tumor and non-tumor targets; the degree of on-target toxicity is likely related to the affinity of the CAR, the level of antigen expression on the healthy tissue, the potency of the CAR, and the relative functional importance of the healthy tissue target. One notable example is that of a CAR recognizing Her2/neu, an antigen that is frequently overexpressed in epithelial malignancies, including gastric, colon, and breast cancer. In 2010, a single patient with Her2+ metastatic colon cancer was treated with a high dose of Her2-targeted CAR therapy and within 15 min of infusion, developed new respiratory failure requiring intubation and intensive care unit (ICU) management; the patient died from CRS and CAR T cell targeting of Her2 on lung epithelium.16 However, other studies targeting Her2 with lower doses and different CAR constructs, based on either a lower-affinity scFv or less potent signaling domains, show that Her2-targeted CAR T cells may be safe.17, 18, 19 Some antigens that are expressed on normal tissues may still be targetable by CARs without causing dose-limiting toxicity, such as mesothelin and prostate-specific membrane antigen (PSMA) (S.F. Slovin et al., 2013, J Clin Oncol., abstract);20 however, neither of these targets has brought about dramatic clinical efficacy, and it is possible that these two outcomes are inexorably linked. Other examples of on-target, off-tumor toxicities associated with the most commonly targeted antigens used in CAR pre-clinical studies and clinical trials are shown in Table 1.
Table 1.
Examples of On-Target, Off-Tumor Toxicities of CAR T Cell Therapies
| Target Antigen | Disease | On-Target, Off-Tumor Toxicity | References |
|---|---|---|---|
| CD19 | B cell malignancies | B cell aplasia | Maude et al.50 |
| Porter et al.51 | |||
| HER2/ERBB2 | colon cancer | lethal pulmonary failure | Morgan et al.16 |
| Carbonic anhydrase-IX (CA-IX) | renal cancer | bile duct epithelium infiltration (cholestasis) | Lamers et al.52 |
| Lamers et al.53 | |||
| CD30 | Hodgkin’s lymphoma | lymph node, tonsils, thymus infiltration | Wang et al.54 |
| CD123 | acute myeloid leukemia | elimination of hematopoietic stem cell and myeloid compartments | Mardiros et al.55 |
| Gill et al.56 | |||
| Kappa light chain | non-Hodgkin’s lymphoma/multiple myeloma | elimination of kappa-expressing B and plasma cells | Ramos et al.57 |
In the case of CD19 antigen (expressed on the B cell lineage), some on-target, off-tumor toxicities, such as B cell aplasia, are clinically manageable and may not be unacceptably detrimental following CAR T cell therapy.21, 22, 23 Patients with B cell aplasia often receive intravenous immunoglobulin (IVIG), but this may not be necessary in all patients (S.J. Schuster et al., 2016, J Clin Oncol., abstract). The prolonged absence of CD19-expressing cells reflects the exceptional capacity of CAR T cells to persist and function in vivo.
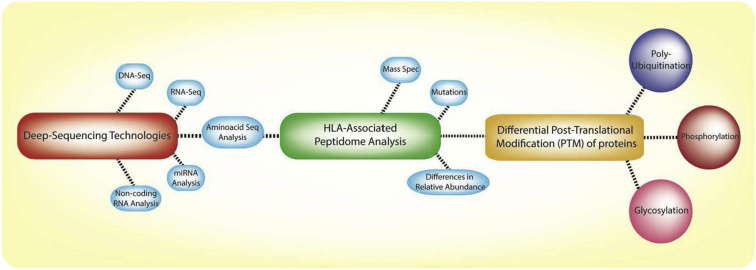
Given the marked toxicities described thus far and the apparent power of T cell-based therapy, the importance of pre-clinical assays to determine the potential for on-target, off-tumor expression of antigens is apparent. Various approaches are under way to screen for antigens expressed exclusively in tumors (Figure 2). Bulk- and single-cell deep sequencing technologies such as DNA sequencing (DNA-seq), RNA sequencing (RNA-seq), and tumor exome analysis have yielded a better understanding of unique tumor antigens.24, 25 An established database of proteins expressed by healthy tissues, on a single-cell level, would complement this approach.
Figure 2.
Novel Analytical Tools to Identify Antigens Expressed by Tumor Cells
Deep-sequencing technologies such as RNA-seq and DNA-seq are being used in the identification of tumor neoantigens and tumor-specific elements such as microRNAs (miRNAs) and non-coding RNAs. In addition, advances in analytical methods such as mass spectrometry have led to the discovery of a vast number of tumor antigens (normally associated with HLA complexes) bearing post-translational modifications (e.g., phosphorylation, glycosylation, and poly-ubiquitination), broadening the range of the T cell-targetable peptidome for multiple cancers.
Although most CAR targets have been limited to natural cell-surface antigens in the form of proteins, it is possible to target post-translational modifications of proteins26, 27 and glycosphingolipids with CARs.28 T cells can also be genetically modified with natural or engineered T cell receptors (TCRs), which recognize peptides presented in the context of major histocompatibility complex (MHC). By combining genetic analysis of mutations in cancer cells with immune-peptidome analysis by mass spectrometry, a wave of new peptide antigen targets that can be recognized by endogenous TCRs29, 30 has potential for therapeutic development. Furthermore, new molecular methods that allow for the analysis of post-translationally modified (PTM) proteins has further increased the repertoire of new candidates amenable for T cell recognition. Cobbold et al.31 emphasized the increasing importance of these post-translational modifications in human malignancies. They demonstrated that phosphopeptide antigens derived from cancer-related phosphoproteins could serve as immunologic signatures of the “transformed self” and were immunogenic in patients with and without cancer. These neo-antigen phosphopeptides were then identified on primary human malignant tissue and were often derived from oncogenes linked to leukemogenesis, making them exciting targets for future T cell-targeted therapy independent of overall mutational load.31
Most of the neoantigens studied are derived from modified intracellular proteins and have been proposed and tested as candidates for TCR-engineered T cells. Nevertheless, the discovery of neoantigens on the surface of cancer cells will likely provide a valuable source of targets for CAR T cells. Recent work has demonstrated the possibility of scFv fragments capable of identifying intracellular neoantigens loaded on endogenous MHC.32 The possibility of CAR T cells being able to target both extra-cellular and intra-cellular proteins is promising, though screening for on-target toxicities may be more challenging to carry out with available, practical assays in the pre-clinical setting.
Off-Target Toxicities
One benefit of CAR-based approaches is that their specificity is dictated by antibody-like binding, independent of MHC expression. Whereas CAR T cell therapy has yet to demonstrate clear off-target effects mediate by inappropriate scFv recognition of a non-target antigen, TCR-modified T cell therapies have revealed the possibility of TCR promiscuity and the risk of off-target effects.
One example of such off-target toxicities was observed in patients treated with human leukocyte antigen (HLA)-A1-restricted, MAGE-A3-specific, enhanced-affinity TCR-modified T cells. Despite extensive pre-clinical safety studies, 5 days following T cell infusion, both infused patients died of acute cardiac failure and robust T cell infiltration. No MAGE-A3 expression could be identified on cardiac myocytes. Further investigation revealed that the affinity enhancement of the TCR resulted in recognition of a similar peptide expressed in cardiac muscle, leading to acute cardiac rejection.33
Following these unexpected deaths, an extensive investigation was undertaken, with initial experiments demonstrating no cross-reactivity with standard myocyte cell culture models. Only the use of highly purified human cardiomyocytes derived from induced pluripotent stem cells revealed the reactivity with the titin peptide expressed in beating cardiac myocytes.34 Despite advances in modern technology, the ability for pre-clinical models to detect the cross-reactivity of various TCR constructs remains challenging, and no standardized tests or assays can reliably predict all potential off-target toxicities.
Allergic Reactions to CARs
Most CAR constructs in use today derive their scFv from available murine monoclonal antibodies. These murine components are often immunogenic and able to elicit both cellular and humoral host immune responses.35 Cellular immune responses to either the murine components or the junction components of CARs may play a role in limiting the long-term persistence of CAR T cells in some patients and may decrease overall CAR T cell efficacy.36 Although many drugs and immunotherapies can be administered recursively, immune responses to foreign proteins can also result in allergic sensitization. One case of host-mediated CAR T cell allergy has been reported, and it occurred in a patient treated with mesothelin-directed CAR T cells based on a murine antibody. The patient received three doses of CAR T cells and immediately developed anaphylactic shock and cardiac arrest following the third infusion; fortunately, the patient was successfully resuscitated. High levels of anti-mouse antibodies and elevated serum tryptase levels implied an immunoglobulin E (IgE)-mediated anaphylactic reaction.37 To facilitate long-term engraftment and allow for repetitive dosing as necessary, several investigators are making efforts to generate fully humanized CAR constructs.
Autonomous Signaling or GVHD
When endogenous T cells encounter antigen, they require both antigen specificity and costimulation to achieve activation. Antigen engagement without costimulation leads to anergy, or refractory non-responsiveness. Because CAR-based therapies provide both activation (through the CD3z domain) and costimulation (CD28 and/or 41BB domains) in a single protein, without transcriptional or temporal regulation, it is possible that CAR T cells will have autonomous signaling through the CAR. Antigen-independent signaling has been observed in CARs specific for c-Met and GD2, with constitutive T cell proliferation, function, and eventual exhaustion, along with spontaneous CAR clustering on the T cells in vitro.38, 39 Studies in animal models demonstrated a deleterious effect on T cell function and persistence, likely mediated by activation-induced cell death (AICD) and/or T cell exhaustion. This phenotype was abrogated with the addition of 41BB costimulation. The relative effects of antigen-independent versus antigen-dependent activation are not yet well understood, especially with regard to their role in maintaining T cell function and memory after infusion.
Constitutively activated T cells have the potential to induce autoimmunity or GVHD, even if the T cell activation is mediated due to CAR signaling over endogenous TCR signaling. To date, no examples of induced autoimmunity have been observed with autologous CAR T cells, but patients with underlying autoimmune disease are usually excluded from these trials. Even in cases in which CAR T cells are generated from the same donor in patients who have received a prior allogeneic stem cell transplant, CAR T cells have not mediated autoimmune disease or GVHD,40 though the patient populations have been highly selected to exclude those at greatest risk for developing GVHD. Compared to donor-lymphocyte infusions, which are infusions of resting T cells that can carry a significant risk of developing acute and/or chronic GVHD,41 there have been no documented cases of acute or chronic GVHD following allogeneic CAR T cell therapy, including among those patients who received pre-infusion lymphodepletion.4, 10, 21, 42 CAR-T cell-mediated GVHD may be encountered more frequently with off-the-shelf allogeneic T cell products, whether they use viral-specific T cell populations and/or endogenous TCR knockdown43 to dampen the risk of GVHD. Ideally, success with allogeneic CAR T cells would allow a broader, more cost-effective, and faster utilization of T cell-based therapies.
Replication Competent Virus
Strategies to deliver the CAR transgene include non-viral transposon-based “sleeping beauty” systems, transiently expressed mRNA, and chromosome-integrating vectors derived from gammaretroviruses or lentiviruses.44 More recently, clustered regularly-interspaced short palindromic repeats (CRISPR)-based gene delivery approaches have demonstrated promising results with the possibility of non-viral targeting transgene insertion.45 Various transposase-based systems have now entered clinical trials, but their overall safety profile has yet to be fully evaluated. Transient mRNA expression seems appealing from a safety perspective but lacks the long-term expression needed for maximal CAR T cell function and persistence. Most studies continue to use viral delivery systems such as gammaretroviral and lentivirus approaches.
One theoretical risk of gammaretroviral and lentivirus approaches is the possibility of generating replication-competent virus generation. Current retroviral and lentiviral delivery systems are designed to only transduce cells, but early-generation retroviruses were susceptible to helper virus contamination, allowing the production of infectious virions and leading to T cell lymphomas in animal models.46 In more than 500 patient-years of follow-up, no replication competent retrovirus has been identified.47 Early replication-competent lentivirus could be generated in vitro by recombination of vector plasmids or in vivo by mobilization of vector DNA in the presence of other infectious lentivirus such as HIV.44 Currently used self-inactivating lentiviral vectors generated from three- or four-plasmid transfections are substantially less susceptible to generation of recombinant virus; furthermore, transgene expression can be driven from a heterologous internal promoter, preventing in vivo recombination48 and enhancing long-term stable expression. To date, there have been no cases of replication-competent virus identified in cell products or patients.
Insertional Site Oncogenesis
One of biggest concerns in early CAR T cell trials was the perceived risk of insertional oncogenesis mediated by transgene integration. Driving these concerns were notable examples such as the 2008 study in which four of ten patients treated with retrovirus-mediated gene transfer for X-linked severe combined immunodeficiency (SCID-X1) resulted in the development of T cell leukemia.49 In contrast to current CAR T cell trials, which use fully differentiated peripheral T cell populations, the SCID-X1 study used CD34+ hematopoietic stem cells, which are more susceptible to a transformational event given their undifferentiated nature. The causative event found in three of the four patients involved the LIM domain-only 2 (LMO2) proto-oncogene, a gene otherwise silenced in peripheral T cells.
Reassuringly, there have been no reported cases of cellular transformation by insertional mutagenesis by viral vectors used to modify T cells. Work by Scholler et al. examined patients treated with retrovirus transduced CAR-T cells between 1998 and 2005, a period encompassing >500 patient years. They found CAR T cell persistence in 98% of these patients for upward of 11 years without evidence of transformation.47 Emphasizing their findings, of the >1,000 individuals who have been infused with CAR T cells to date, no documented transformational event has been observed.
Conclusions
Despite the potential for observed and theoretical toxicities described here, the field of adoptive T cell therapy is rapidly growing and offers the potential for curative treatment of otherwise refractory disease. There will surely be additional toxicities revealed as new T cell drugs are brought into the clinic. The addition of ancillary lymphodepleting drugs, “suicide gene” systems, and cytokine-modulating therapies will likely carry their own toxicity profiles. Nevertheless, re-directed CAR T cells are powerful “living drugs,” and they represent a new form of therapy that offers the possibility of dramatically extending the lives of patients with cancer.
Author Contributions
F.B., M.J.F., and M.V.M. wrote the manuscript.
Conflicts of Interest
M.V.M., M.J.F., and F.B. are listed as inventors on patents related to the use of CAR T cells.
Acknowledgments
M.V.M. was supported by NIH K08CA166039, Gabrielle's Angel Foundation, and the V Foundation.
References
- 1.Bonifant C.L., Jackson H.J., Brentjens R.J., Curran K.J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brudno J.N., Kochenderfer J.N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teachey D.T., Rheingold S.R., Maude S.L., Zugmaier G., Barrett D.M., Seif A.E., Nichols K.E., Suppa E.K., Kalos M., Berg R.A. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochenderfer J.N., Dudley M.E., Feldman S.A., Wilson W.H., Spaner D.E., Maric I., Stetler-Stevenson M., Phan G.Q., Hughes M.S., Sherry R.M. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maude S.L., Barrett D., Teachey D.T., Grupp S.A. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herper M. Juno therapeutics stops trial of cancer-killing cells after 3 patient deaths. Forbes. 2016 http://www.forbes.com/sites/matthewherper/2016/07/07/juno-therapeutics-stops-trial-of-cancer-killing-cells-after-3-patient-deaths/#20e164b15d89 Published online July 7, 2016. [Google Scholar]
- 9.Tang Y.M., Xu X.J. Advances in hemophagocytic lymphohistiocytosis: pathogenesis, early diagnosis/differential diagnosis, and treatment. Sci. World J. 2011;11:697–708. doi: 10.1100/tsw.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teachey D.T., Lacey S.F., Shaw P.A., Melenhorst J.J., Maude S.L., Frey N., Pequignot E., Gonzalez V.E., Chen F., Finklestein J. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–679. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M., Grupp S.A., Mackall C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goebeler M.E., Knop S., Viardot A., Kufer P., Topp M.S., Einsele H., Noppeney R., Hess G., Kallert S., Mackensen A. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J. Clin. Oncol. 2016;34:1104–1111. doi: 10.1200/JCO.2014.59.1586. [DOI] [PubMed] [Google Scholar]
- 14.Topp M.S., Gökbuget N., Stein A.S., Zugmaier G., O’Brien S., Bargou R.C., Dombret H., Fielding A.K., Heffner L., Larson R.A. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg M., Belkowski L.S., Bloom B.R. Regulation of macrophage function by interferon-gamma. Somatic cell genetic approaches in murine macrophage cell lines to mechanisms of growth inhibition, the oxidative burst, and expression of the chronic granulomatous disease gene. J. Clin. Invest. 1990;85:563–569. doi: 10.1172/JCI114473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed N., Salsman V.S., Kew Y., Shaffer D., Powell S., Zhang Y.J., Grossman R.G., Heslop H.E., Gottschalk S. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin. Cancer Res. 2010;16:474–485. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed N., Brawley V.S., Hegde M., Robertson C., Ghazi A., Gerken C., Liu E., Dakhova O., Ashoori A., Corder A. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J. Clin. Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Moon E., Carpenito C., Paulos C.M., Liu X., Brennan A.L., Chew A., Carroll R.G., Scholler J., Levine B.L. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beatty G.L., Haas A.R., Maus M.V., Torigian D.A., Soulen M.C., Plesa G., Chew A., Zhao Y., Levine B.L., Albelda S.M. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett D.M., Singh N., Porter D.L., Grupp S.A., June C.H. Chimeric antigen receptor therapy for cancer. Annu. Rev. Med. 2014;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava P.K. Neoepitopes of cancers: looking back, looking ahead. Cancer Immunol. Res. 2015;3:969–977. doi: 10.1158/2326-6066.CIR-15-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen C.J., Gartner J.J., Horovitz-Fried M., Shamalov K., Trebska-McGowan K., Bliskovsky V.V., Parkhurst M.R., Ankri C., Prickett T.D., Crystal J.S. Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. J. Clin. Invest. 2015;125:3981–3991. doi: 10.1172/JCI82416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posey A.D., Jr., Schwab R.D., Boesteanu A.C., Steentoft C., Mandel U., Engels B., Stone J.D., Madsen T.D., Schreiber K., Haines K.M. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity. 2016;44:1444–1454. doi: 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumaresan P.R., Manuri P.R., Albert N.D., Maiti S., Singh H., Mi T., Roszik J., Rabinovich B., Olivares S., Krishnamurthy J. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc. Natl. Acad. Sci. USA. 2014;111:10660–10665. doi: 10.1073/pnas.1312789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prapa M., Caldrer S., Spano C., Bestagno M., Golinelli G., Grisendi G., Petrachi T., Conte P., Horwitz E.M., Campana D. A novel anti-GD2/4-1BB chimeric antigen receptor triggers neuroblastoma cell killing. Oncotarget. 2015;6:24884–24894. doi: 10.18632/oncotarget.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gfeller D., Bassani-Sternberg M., Schmidt J., Luescher I.F. Current tools for predicting cancer-specific T cell immunity. OncoImmunology. 2016;5:e1177691. doi: 10.1080/2162402X.2016.1177691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leone P., Shin E.C., Perosa F., Vacca A., Dammacco F., Racanelli V. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J. Natl. Cancer Inst. 2013;105:1172–1187. doi: 10.1093/jnci/djt184. [DOI] [PubMed] [Google Scholar]
- 31.Cobbold M., De La Peña H., Norris A., Polefrone J.M., Qian J., English A.M., Cummings K.L., Penny S., Turner J.E., Cottine J. MHC class I-associated phosphopeptides are the targets of memory-like immunity in leukemia. Sci. Transl. Med. 2013;5:203ra125. doi: 10.1126/scitranslmed.3006061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeda H., Akahori Y., Yoneyama, Orito Y., Miyahara Y., Amaishi Y., Okamoto S., Mineno J., Takesako K., Fujiwara H. Immunotherapy with chimeric antigen receptor targeting intracellular WT1 gene product complexed with HLA-a*24:02 molecule. Blood. 2015;126:4292. [Google Scholar]
- 33.Cameron B.J., Gerry A.B., Dukes J., Harper J.V., Kannan V., Bianchi F.C., Grand F., Brewer J.E., Gupta M., Plesa G. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linette G.P., Stadtmauer E.A., Maus M.V., Rapoport A.P., Levine B.L., Emery L., Litzky L., Bagg A., Carreno B.M., Cimino P.J. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamers C.H., Willemsen R., van Elzakker P., van Steenbergen-Langeveld S., Broertjes M., Oosterwijk-Wakka J., Oosterwijk E., Sleijfer S., Debets R., Gratama J.W. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 36.Jensen M.C., Popplewell L., Cooper L.J., DiGiusto D., Kalos M., Ostberg J.R., Forman S.J. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transplant. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maus M.V., Haas A.R., Beatty G.L., Albelda S.M., Levine B.L., Liu X., Zhao Y., Kalos M., June C.H. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frigault M.J., Lee J., Basil M.C., Carpenito C., Motohashi S., Scholler J., Kawalekar O.U., Guedan S., McGettigan S.E., Posey A.D., Jr. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol. Res. 2015;3:356–367. doi: 10.1158/2326-6066.CIR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long A.H., Haso W.M., Shern J.F., Wanhainen K.M., Murgai M., Ingaramo M., Smith J.P., Walker A.J., Kohler M.E., Venkateshwara V.R. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brudno J.N., Somerville R.P., Shi V., Rose J.J., Halverson D.C., Fowler D.H., Gea-Banacloche J.C., Pavletic S.Z., Hickstein D.D., Lu T.L. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J. Clin. Oncol. 2016;34:1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frey N.V., Porter D.L. Graft-versus-host disease after donor leukocyte infusions: presentation and management. Best Pract. Res. Clin. Haematol. 2008;21:205–222. doi: 10.1016/j.beha.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kochenderfer J.N., Dudley M.E., Carpenter R.O., Kassim S.H., Rose J.J., Telford W.G., Hakim F.T., Halverson D.C., Fowler D.H., Hardy N.M. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y., Jacoby E., Fry T.J. Challenges and opportunities of allogeneic donor-derived CAR T cells. Curr. Opin. Hematol. 2015;22:509–515. doi: 10.1097/MOH.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.June C.H., Blazar B.R., Riley J.L. Engineering lymphocyte subsets: tools, trials and tribulations. Nat. Rev. Immunol. 2009;9:704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sander J.D., Joung J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donahue R.E., Kessler S.W., Bodine D., McDonagh K., Dunbar C., Goodman S., Agricola B., Byrne E., Raffeld M., Moen R. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J. Exp. Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scholler J., Brady T.L., Binder-Scholl G., Hwang W.T., Plesa G., Hege K.M., Vogel A.N., Kalos M., Riley J.L., Deeks S.G. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine B.L., Humeau L.M., Boyer J., MacGregor R.R., Rebello T., Lu X., Binder G.K., Slepushkin V., Lemiale F., Mascola J.R. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc. Natl. Acad. Sci. USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hacein-Bey-Abina S., Garrigue A., Wang G.P., Soulier J., Lim A., Morillon E., Clappier E., Caccavelli L., Delabesse E., Beldjord K. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maude S.L., Fitzgerald J.C., Fisher B.T., Li Y., Huang Y.S., Torp K., Seif A.E., Kavcic M., Walker D.M., Leckerman K.H. Outcome of pediatric acute myeloid leukemia patients receiving intensive care in the United States. Pediatr. Crit. Care Med. 2014;15:112–120. doi: 10.1097/PCC.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porter D.L., Hwang W.T., Frey N.V., Lacey S.F., Shaw P.A., Loren A.W., Bagg A., Marcucci K.T., Shen A., Gonzalez V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamers C.H.J., Sleijfer S., Vulto A.G., Kruit W.H., Kliffen M., Debets R., Gratama J.W., Stoter G., Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 53.Lamers C.H., Sleijfer S., van Steenbergen S., van Elzakker P., van Krimpen B., Groot C., Vulto A., den Bakker M., Oosterwijk E., Debets R., Gratama J.W. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol. Ther. 2013;21:904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C., Wu Z., Wang Y., Guo Y., Dai H., Wang X.H., Li X., Zhang Y.J., Zhang W.Y., Chen M.X. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory Hodgkin’s lymphoma: an open-label phase I trial. Clin. Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1365. Published online August 31, 2016. [DOI] [PubMed] [Google Scholar]
- 55.Mardiros A., Dos Santos C., McDonald T., Brown C.E., Wang X., Budde L.E., Hoffman L., Aguilar B., Chang W.C., Bretzlaff W. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122:3138–3148. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gill S., Tasian S.K., Ruella M., Shestova O., Li Y., Porter D.L., Carroll M., Danet-Desnoyers G., Scholler J., Grupp S.A. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123:2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramos C.A., Savoldo B., Torrano V., Ballard B., Zhang H., Dakhova O., Liu E., Carrum G., Kamble R.T., Gee A.P. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J. Clin. Invest. 2016;126:2588–2596. doi: 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]