Abstract
Objective:
The clinical use of doxorubicin, which is a strong antineoplastic agent, is limited due to its cardiotoxic side effects. Metformin is a drug with antihyperglycemic effects, and it has been shown to have a cardioprotective effect on left ventricular function in experimental animal models of myocardial ischemia. The present study investigated the cardioprotective effect of metformin in rats with doxorubicin cardiotoxicity.
Methods:
Wistar albino rats were used in the study. Forty male, 10-week-old Wistar albino rats were randomly divided four groups. The control group rats were intraperitoneally administered saline solution twice a week, four doses in total. The doxorubicin group rats received doxorubicin (4 mg/kg, twice a week, cumulative dose: 16 mg/kg) intraperitoneally. The metformin group rats received metformin (250 mg/kg/day, every day for 14 days) via gavage. The doxorubicin + metformin group rats received doxorubicin and metformin at the same dose. Left ventricular functions were evaluated by using M-mode echocardiography one day after the last dose of doxorubicin. Heart tissue samples were histopathologically examined. Cardiomyocyte apoptosis was detected using in situ terminal deoxynucleotide transferase assay (TUNEL). Serum brain natriuretic peptide and C-type natriuretic peptide levels were measured. Catalase, superoxide dismutase, glutathione peroxidase, and tumor necrosis factor alpha levels were analyzed in the heart tissue. The assumptions of equality of variances and normal distribution were checked for all variables (Shapiro-Wilk test and Q-Q graphics). To identify intergroup differences, one-way variant analysis or the Kruskal-Wallis test was used. A p<0.05 value was accepted as statistically significant.
Results:
Our results showed that doxorubicin treatment caused significant deterioration in left ventricular functions by echocardiography, histological heart tissue damage, and increase in cardiomyocyte apoptosis. Doxorubicin + metformin group showed protection in left ventricular function, elimination of histopathologic change, and reduced of cardiomyocyte apoptosis.
Conclusion:
The present study provided evidence that metformin has cardioprotective effects against doxorubicin cardiotoxicity.
Keywords: doxorubicin, cardiotoxicity, metformin, antioxidant, echocardiography
Introduction
Since the mid-20th century, great progress has been achieved in the survival rates of children with cancer, owing to the development of chemotherapeutic agents. Doxorubicin is one of the strong antineoplastic agents that are often used in clinics. However, its use is limited due to its significant cardiotoxic side effects. Cardiotoxicity leads to additional morbidity and mortality risk for patients (1). Most patients are likely to have more cardiovascular mortality risk compared with tumor recurrence when treated with doxorubicin. The odds of heart disease-related mortality are eight-fold higher in patients who survived pediatric cancer (2, 3). More than 7.5% of patients who receive 250 mg/m2 anthracycline develop congestive heart failure 30 years after chemotherapy (4). A detailed cardiac evaluation of children who received anthracycline treatment shows anomalies including decreased left ventricle mass and wall thickness, increased left ventricle afterload, and decreased left ventricle contractility in more than half of the children (5).
Among the protective prevention strategies against doxorubicin cardiotoxicity, the development of cardioprotective agents is still popular. For this purpose, experimental studies have provided evidence that various pharmacological agents have protective effects against doxorubicin cardiotoxicity (1).
Metformin is an oral antihyperglycemic drug that is used in type 2 diabetic patients. It has been reported to be cardioprotective in addition to decreasing basal and postprandial glucose levels, helping weight loss, and decreasing plasma lipid levels. Experimental animal models of isolated myocardial infarction and heart failure have shown that metformin increases the tolerance of the myocardium to ischemia-reperfusion injury, and decreases the development of heart failure after infarction (6, 7). The aim of the present work was to investigate whether the metformin is able to reduce the cardiotoxic doxorubicin effects.
Methods
Animals
Forty male, 10-week-old Wistar albino rats (weight: 150-210 g) were used in the study. The rats were stored at 25°C room temperature under a 12/12 light/dark cycle, with ad libitum access to water and a standard diet. This study was approved by Erciyes University animal experiments local ethics committee. The experimental model was established in the Experimental Studies Applied Research Center.
Experimental study design
In this study, doxorubicin and metformin treatment regimen were adopted on the basis of the study by Ashour et al. (8). The control group (n=10) received saline solution (twice a week, 2.5 mL/kg, four doses) intraperitoneally. The doxorubicin group received doxorubicin (4 mg/kg, twice a week, cumulative dose: 16 mg/kg) intraperitoneally. The metformin group received metformin (250 mg/kg/day, every day for 14 days) with gavage. The doxorubicin + metformin group received doxorubicin (4 mg/kg, twice a week, cumulative dose: 16 mg/kg) intraperitoneally and metformin (250 mg/kg/day, for 14 days, starting three days prior to doxorubicin treatment) orally with gavage.
Evaluation of left ventricular systolic function by M-mode echocardiography
The rats were anesthetized (xylazine and ketamine) 24 hours after the last dose of doxorubicin. The chest was shaved, and the animals were positioned on their left side. Diastolic interventricular septum thickness (IVSTd), left ventricular end-diastolic diameter (LVEDD), diastolic left ventricular posterior wall thickness (LVPWTd), and systolic interventricular septum thickness (IVSTs), left ventricular end-systolic diameter (LVESD), systolic left ventricular posterior wall thickness (LVPWTs) were measured with the M-mode echocardiographic method using a GE Vivid 7 with 10 S transducer (GE Medical Systems, Horten, Norway) by a sample-blinded investigator. Ejection fraction (EF) and fractional shortening (FS) parameters were obtained using the automatic calculation of the echocardiography device.
Serum and tissue samples collection
The animals’ abdominal skin was cut under anesthesia. Five mL blood samples were collected from the inferior vena cava. The rats were sacrificed after taking the blood samples. A portion of the heart tissue was fixed in 4% of formaldehyde for histological examinations. The remaining portion was stored at -80°C for enzyme-linked immunosorbent assay (ELISA) analyses.
Serum brain natriuretic peptide (BNP) measurement
Serum BNP levels were measured by using a Rat BNP ELISA Kit (sensitivity: 1.445 ng/L and 2-400 ng/L; Shanghai Sunred Biological Technology Co., Ltd, Shanghai, China). Standards were prepared according to the manufacturer’s instructions. First, standards were added to a well plate. Next, samples and BNP antibody were added. Afterwards, streptavidin-HRP was added to the wells, and the plate was incubated at 37°C for 60 minutes. Chromogen solution A and B were added, and the plate was incubated at 37°C for 10 minutes. Stop solution was added, and optical density was measured at 450 nm within 15 minutes.
Serum C-type natriuretic peptide (CNP) measurement
Serum CNP levels were measured using a Rat CNP ELISA Kit (sensitivity: 2.572 ng/L and 3-900 ng/L; Shanghai Sunred Biological Technology Co., Ltd, Shanghai, China). Standards were prepared according to the manufacturer’s instructions. First, standards were added to a well plate. Next, samples and CNP antibody were added. Streptavidin-HRP was subsequently added to the wells, and the plate was incubated at 37°C for 60 minutes. Chromogen solution A and B were added, and the plate was incubated at 37°C for 10 minutes. Stop solution was added, and optical density was measured at 450 nm within 15 minutes.
Heart tissue catalase activity
Catalase activity was measured in the heart tissue homogenate at 25°C by using a Rat Catalase Kit (sensitivity: 2-35 nmoL/min/mL; Cayman Chemical Company, Ann Arbor, Michigan, USA) according to the manufacturer’s instructions. A well plate was divided into different parts as follows: formaldehyde standard, positive control, and sample. Thereafter, experimental buffer, methanol, and standard were added to each well in the formaldehyde standard group. Experimental buffer, methanol, and catalase were added into the positive control well. Experimental buffer, methanol, and sample were added to the sample wells. The reaction was started after diluted hydrogen peroxide was added to all wells. The plate cover was closed, and the plate was incubated at room temperature for 20 minutes. Potassium hydroxide was added to each well in order to terminate the reaction, and catalase was added. The plate cover was closed and the plate was incubated at room temperature for 10 minutes. The plate was then incubated at room temperature for five minutes after the addition of catalase potassium periodate. Absorbance was measured at 540 nm on a plate reader.
Heart tissue superoxide dismutase activity
Superoxide dismutase (SOD) activity was measured by using a Rat Superoxide Dismutase Kit (sensitivity: 0.025-0.25 unit/mL; Cayman Chemical Company, Ann Arbor, Michigan, USA). Diluted radical detector and standard were added to each well reserved for SOD Standards according to the manufacturer’s instructions. Diluted radical detector and sample were added to the wells reserved for samples. The reaction was started after diluted xanthine oxidase was added to all wells. The plate was carefully shaken to allow mixing and the plate cover was closed. It was the incubated at room temperature for 20 minutes. Absorbance was measured at 440-460 nm on a plate reader.
Heart tissue glutathione peroxidase activity
Glutathione peroxidase (GPx) activity was measured using a Rat Glutathione Peroxidase Kit ranging between 50-344 nmoL/min/mL (Cayman Chemical Company, Ann Arbor, Michigan, USA). According to the manufacturer’s instructions, experimental buffer and co-substrate mixture were added to the non-enzymatic wells; experimental buffer, co-substrate mixture, and diluted glutathione peroxidase were added to the positive control wells; experimental buffer, co-substrate mixture, and sample were added to the sample wells. The reaction was started after hydroperoxide was added. The plate was carefully shaken to allow mixing. Absorbance was measured at 340 nm on a plate reader.
Tumor necrosis factor-alpha activity in heart tissue
Tumor necrosis factor-alpha (TNF-alpha) activity was measured using a Rat TNF Alfa Kit with a minimum sensitivity of <4 pg/mL (Invitrogen Corporation, Camarillo, USA). According to the manufacturer’s instructions, standards were prepared and added to the wells. Next, samples were added to the wells. The plate cover was closed, and the plate was incubated at room temperature for two hours. It was washed in an automatic washer. Biotinylated Rt-TNF-alpha biotin conjugate solution was then added to the plate, and it was incubated at room temperature for one hour. Re-washed tissues were incubated at room temperature for 30 minutes after added streptavidin-HRP solution. Next, stabilized chromogen was added. The plate was incubated at room temperature in the dark for 30 minutes. Stopping solution was added. Absorbance was read at 450 nm.
Histological evaluation
Tissue samples were fixed with a 4% formaldehyde solution, placed in fixation solution for 48 hours, and in water for one night. Next, the samples were dehydrated by passing through increasing concentrations of alcohol, cleared by passing through xylol, and finally embedded in paraffin blocks. Five µm sections were cut from the paraffin blocks and these were the placed on poly-lysine coated slides. Standard histological methods (xylol) were used to remove the paraffin, and the samples were passed through a gradual alcohol series and hydrated. Hematoxylin-eosin (H&E) staining was used to demonstrate the general histological structure.
TUNEL method
Apoptosis in heart tissue was investigated by TUNEL method, which provides detection of deoxyribonucleic acid fragments in situ. An in situ Cell Death Detection Kit, ‘’Fluorescein” (Roche Diagnostics GmbH, Penzberg, Germany) was used for this purpose. Heart tissues (5-6 µm) were deparaffinized and rehydrated, then washed with phosphate buffered saline. After being washed, the heart tissues were stored in sodium citrate buffer in a microwave at 350 W for 5 minutes for antigen recovery. They were left to cool at room temperature for 20 minutes. Tissues were washed with phosphate buffered saline, and incubated with the TUNEL reaction mixture at 37°C in a dark, humid environment for 60 minutes. Tissue samples were washed with phosphate buffered saline, and counterstained with 4,6-diamidine-2¢-phenylindole. The samples were then covered with a mounting solution (glycerol) and examined at 450-500 nm wavelength under a fluorescent microscope (Olympus BX-51). For each section, images were acquired from 10 different sections at 40´ magnification. Apoptotic cell number and total cell number were determined in each section. Apoptotic cell number/total cell number percentage was taken as the apoptotic index.
Statistical analysis
Data was analyzed by IBM SPSS Statistics 22.0 statistical programme (IBM Corporation, New York, USA). The assumptions of equality of variances and normal distribution were checked for all variables (Shapiro-Wilk test and Q-Q graphics). To identify intergroup differences, one-way variant analysis or the Kruskal-Wallis test was used. A p<0.05 value was accepted as statistically significant.
Results
Echocardiographic evaluation of left ventricular function
Regarding left ventricular functions, the decrease in IVSTs, EF, and FS values and the increase in LVESD were significant in the doxorubicin group compared with that in the control group (p<0.05). The cardioprotective effect of metformin against left ventricular systolic dysfunction was observed in the doxorubicin + metformin group (Table 1).
Table 1.
Echocardiographic evaluation of left ventricular function in the control, doxorubicin, metformin, and doxorubicin + metformin groups
| Control (n=10) | DOX (n=10) | MET (n=10) | DOX + MET (n=9) | |
|---|---|---|---|---|
| LVEDD, cm # median (25%-75%) |
0.57±0.06 0.57 (0.50-0.61) |
0.61±0.05 0.63 (0.59-0.64) |
0.55±0.09 0.53 (0.49-0.55) |
0.55±0.08 0.55 (0.49-0.62) |
| LVESD, cm | 0.33±0.04 | 0.44±0.04* | 0.34±0.08 | 0.35±0.05 |
| IVSTd, cm | 0.12±0.02 | 0.11±0.01 | 0.13±0.03 | 0.12±0.01 |
| IVSTs, cm | 0.19±0.01 | 0.15±0.01* | 0.19±0.03 | 0.20±0.02 |
| EF (%) | 77.76±4.95 | 60.92±9.25* | 75.71±6.13 | 71.62±5.40 |
| FS (%) | 41.32±4.47 | 28.76±6.16* | 39.60±5.57 | 36.03±4.31 |
DOX - doxorubicin group; MET - metformin group; DOX + MET-doxorubicin + metformin group. EF - ejection fraction; FS - fractional shortening; IVSTs - interventricular septum thickness in systole; IVSTd - interventricular septum thickness in diastole; LVEDD - left ventricle end-diastolic diameter; VESD - left ventricle end-systolic diameter. Data represent mean ± SD. n - number of rats.
indicates a significant difference compared to the control group, P<0.05 one-way Variant analysis or
the Kruskal-Wallis test
One of the rats from the doxorubicin + metformin group died on the third day of the study during drug administration for the doxorubicin cardiotoxicity experimental model. This death was associated with a technical gavage error.
Serum BNP and CNP levels and catalase, SOD, GPx, and TNF-a levels in heart tissue
There was no change in BNP levels with the relevant dose schema of doxorubicin. However, doxorubicin and doxorubicin + metformin group caused a significant decrease in CNP levels (p<0.05) (Table 2).
Table 2.
BNP and CNP levels in control, doxorubicin, metformin and doxorubicin + metformin groups
| Control (n=10) | DOX (n=10) | MET (n=10) | DOX + MET (n=9) | |
|---|---|---|---|---|
| BNP, ng/L | 29.49±6.27 | 28.50±4.50 | 33.43±5.70 | 34.65±7.40 |
| CNP, ng/L | 38.06±3.74 | 29.21±5.81* | 39.37±5.94 | 30.11±5.07* |
DOX - doxorubicin group; MET - metformin group; DOX + MET-doxorubicin + metformin group. BNP - brain natriuretic peptide; CNP-C - type natriuretic peptide. Data represent mean ± SD. n - number of rats
indicates a significant difference compared to the control group, P<0.05 one-way Variant analysis
Doxorubicin treatment did not cause a significant difference in catalase, SOD, GPx, or TNF-a levels in the heart tissue (Table 3).
Table 3.
Catalase, SOD, GPx, and TNF-α levels in the heart tissue in control, doxorubicin, metformin, and doxorubicin + metformin groups
| Control (n=10) | DOX (n=10) | MET (n=10) | DOX + MET (n=9) | |
|---|---|---|---|---|
| Catalase, nmoL/mL | 16.13±2.66 | 20.17±2.26 | 18.64±3.85 | 19.20±3.76 |
| SOD, IU/L # median (25%-75%) | 0.30±0.04 | 0.27±0.06 | 0.30±0.06 | 0.27±0.09 |
| 0.30 (0.30-0.31) | 0.28 (0.21-0.34) | 0.30 (0.28-0.36) | 0.31 (0.20-0.36) | |
| GPx, nmoL/mL | 61.24±42.19 | 51.07±48.27 | 44.04±45.11 | 17.93±19.27 |
| TNF-alpha, pg/mL | 112.4±49.43 | 150.82±53.05 | 121.95±38.22 | 124.81±26.08 |
DOX - doxorubicin group; MET - metformin group; DOX + MET-doxorubicin + metformin group. GPx - glutathione peroxidase; SOD - superoxide dismutase; TNF - alpha-tumor necrosis factor-alpha Data represent mean ± SD. n-number of animals. one-way Variant analysis or
the Kruskal-Wallis test
Histopathological evaluation by H&E staining of heart tissue
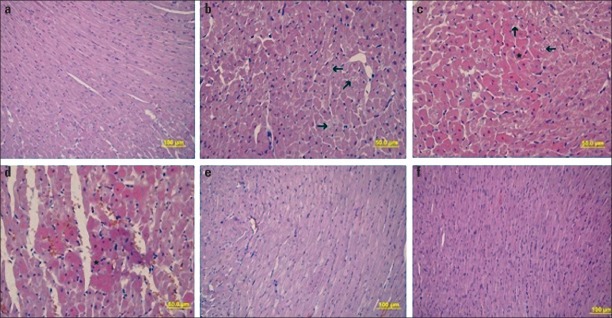
The control group showed normal tissue (Fig. 1a). During the light microscopic examination, doxorubicin was observed to cause organization disorder between myocardium muscle fibers, myofibril loss in some cells (Fig. 1b). In the Figure 1c, doxorubicin increased in eosinophilic staining in certain areas of the myocardium layer and intracytoplasmic vacuole formation, and congestive and hemorrhagic areas in some veins were detected in the Figure 1d. The metformin group, histopathological examination with H&E staining showed normal tissue (Fig. 1e), and the doxorubicin + metformin group revealed almost normal histologic heart tissue appearance (Fig. 1f).
Figure 1.
a-f. H&E staining of heart tissue. (a) control group, (b-d) doxorubicin group, e) metformin group, f) doxorubicin + metformin group. The arrows show disorganization between myocardium muscle fibers, and loss of myofibrils in some cells, respectively. (*) indicates the myocardium layer and intracytoplasmic vacuole formation
Apoptotic index
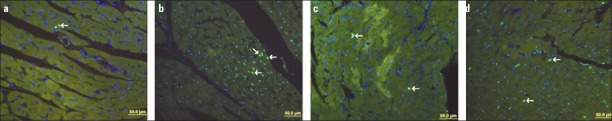
The apoptotic index increased in the doxorubicin group (0.71±0.27) compared with that of the control group (0.43±0.26) and the metformin group (0.35±0.08). However, it was not statistically significant. When the doxorubicin + metformin group (0.48±0.25) was compared with the doxorubicin group, cotreatment metformin reduced apoptosis. However, it was not statistically significant (Fig. 2).
Figure 2.
a-d. Apoptotic cell nuclei (arrow) are represented with a green fluorescent signal, while other cells are represented with a blue fluorescent signal. (a-control group, b-doxorubicin group, c-metformin group, d-doxorubicin + metformin group)
Discussion
Our data revealed that doxorubicin caused left ventricle systolic dysfunction and enlargement in the left ventricle cavity in rats. Metformin showed protective effects against left ventricle dysfunction caused by doxorubicin. In addition, doxorubicin caused histopathological changes in the myocardium and metformin protected the heart tissue from the histopathological changes caused by doxorubicin. Doxorubicin treatment caused an increase in the apoptotic index, although it was not statistically significant. Metformin application had cardioprotective effects against apoptosis on cardiomyocytes, although it was not statistically significant. Doxorubicin did not cause a significant change in serum BNP and catalase, superoxide dismutase, glutathione peroxidase, and TNF-a levels in the heart tissue. However, doxorubicin reduced serum CNP level and cotreatment with metformin did not prevent CNP level reduction.
It was reported that doxorubicin enters into cardiomyoctes by passive diffusion and stimulates the free radicals that cause cell damage. At the same time doxorubicin directly or indirectly inhibits gene transcription, mitochondrial function, and energy production (1). Production of free radicals, enzymatic pathways, non-enzymatic pathways, and redox related damage caused by iron accumulation were suggested to be the responsible for cardiotoxicity. It was also suggested that the main factor was the negative disturbance of the balance between free oxygen radicals and antioxidant systems (9).
Given that doxorubicin is a strong antineoplastic agent, it appears that it will continue to be used until strong chemotherapeutic agents with low side effects are developed. Thus, treatment strategies are being developed to prevent doxorubicin cardiotoxicity. Previous studies have shown that various pharmacological agents have protective effects against doxorubicin cardiotoxicity (1).
Dexrazoxane is the only agent that has been proven to reduce acute antracycline cardiotoxicity in adults, and is recommended by the American Clinical Oncology Society to prevent cardiotoxicity in specific adult cancer treatment protocols (10). However, some researchers are hesitant to use dexrazoxane as it can exert protective effects not only on cardiomyocytes, but also on cancer cells. Moreover, dexrazoxane has certain disadvantages including bone marrow toxicity and high cost (11).
Metformin is commonly used as an oral antihyperglycemic drug in type 2 diabetes mellitus patients. In addition to its role in decreasing blood glucose levels, metformin also leads to weight loss, decreases plasma lipid levels, and prevents certain vascular complications (12). Various studies have provided evidence that metformin has cardioprotective effects on myocardial ischemia in experimental animal models (13-15). It is hypothesized that metformin exerts cardioprotective effects during ischemia-reperfusion injury by reducing adenosine monophosphate. Adenosine monophosphate-activated protein kinase activation and activation of the phosphatidylinositol-3-kinase pathway by increased adenosine receptor stimulation lead to endothelial nitric oxide synthase phosphorylation. Endothelial nitric oxide synthase phosphorylation prevents the opening of the mitochondrial permeability transit pore during reperfusion. The inhibition of pore opening with the above-mentioned methods is suggested to increase the tolerance of the myocardium against ischemia and reperfusion (16, 17).
In the last few years, several studies regarding the cardioprotective role of metformin against the doxorubicine cardiotoxicity has been published (8, 11). Ashour et al. (8) established a doxorubicin cardiotoxicity model in rats by administering doxorubicin (3 mg/kg, every second day, cumulative: 18 mg/kg) intraperitoneally for 11 days. The authors reported that oral administration of metformin (50 mg/kg and 500 mg/kg) eliminated biochemical and histopathological changes, thereby exerting a cardioprotective effect against doxorubicin cardiotoxicity. Asensio-Lopez et al. (18) demonstrated that metformin protects cardiomyocytes from doxorubicin-induced damage and that the cardiac adiponectin system plays an important role in this protective action. In their study, Asensio-Lopez et al. (11) reported that the protective effect of metformin against doxorubicin cardiotoxicity is exerted through the ferritin heavy chain, which has been identified as the major mediator. Kobashigawa et al. (19) demonstrated that metformin has protective effect against doxorubicin cardiotoxicity through activation of 5-adenosine monophosphate-activated protein kinase in invitro study.
Early detection of doxorubicin cardiotoxicity can allow preventive measures to be taken before the development of irreversible damage. Thus, it is important to define cardiotoxic risk factors. Elevation of serum levels of cardiac troponin T ve NT Pro-BNP at the beginning of doxorubicin treatment was observed to predict clinical cardiotoxicity (20-22).
Although detecting the systolic dysfunction relatively late, the evaluation of left ventricle systolic functions and the measurement of left ventricle cavity diameter is a safe and commonly practiced method. These measurements allow determination of the effects of chemotherapy on myocardium performance and structure and enable monitoring due to its easy reproducibility (23).
According to the study by Richard et al. (24), echocardiographic examination of doxorubicin-treated rats showed a significant increase in left ventricle end-diastole diameter. Bertinchant et al. (25) similarly reported that doxorubicin increases left ventricle end-diastole diameter in rats. Chen et al. (26) reported that doxorubicin treatment in rats led to an increase in left ventricle end-diastole diameter and left ventricle end-systole diameter, and a decrease in EF and FS. In the present study, we observed an increase in both end-diastole and end-systole diameter, but only the latter was significant.
Natriuretic peptides play a critical role in a cardiovascular homeostasis by coordination of fluid/electrolyte balance and vascular tone. The natriuretic peptide family consists of three principal members: atrial natriuretic peptide (ANP), BNP, and CNP. ANP, BNP, and the amino terminal fragment of its precursor (NT-proBNP) are rapidly produced and secreted by the heart in response to atrial and ventricular distention as congestive heart failure (27, 28). These natriuretic peptides are also useful markers of left ventricular dysfunction in patients undergoing anthracycline chemotheraphy (29). Hayakawa et al. (30) reported that 34 children previously treated with anthracycline showed inverse correlation between ANP and BNP levels and cardiac systolic function. In the present study, serum BNP level did not change in comparison with the levels of control rats. Richard et al. (24) used cumulative doxorubicin (10 mg/kg) administration in rats, and did not detect an increase in troponin I or BNP levels. Chen et al. (31) reported that doxorubicin inhibits BNP gene expression in newborn rat myocytes. Bernardini et al. (32) treated Wistar albino rats with single-dose (10 mg/kg) intravenous doxorubicin, and found a significant decrease in plasma atrial natriuretic peptide (ANP) levels 3-6 hours after drug administration. The authors also tested a chronic drug treatment regime (3 mg/kg, intravenous, single dose/week), and found a significant increase in plasma ANP levels 21 and 31 days after drug administration. The reason for this was interpreted as early suppression, which would reflect acute myocyte toxicity, and stimulation by subsequent heart failure.
On the other hand, CNP is primarily released by the vascular endothelial cells in addition to the heart, where it plays a role in the local regulation of vascular tone. Exogenous CNP is a potent arterial and venous dilator in mammalian blood vessels. CNP plasma level is increased in heart failure (33). Positive effects of CNP on cardiac contractility have been described in several studies using experimental animal heart (34). Furthermore, in vivo administration of CNP has been shown to have cardioprotective role after myocardial infarction in rats (35). In the present study, cardioproventive yerine cardioprotective serum CNP level significantly decreased compared with those of control rats. Our study is the first one that evaluates CNP level in doxorubicin cardiotoxicity in an experimental rat model. We believe that similar to the reduction of ANP gene expression in acute phase in Chen et al. (31) study, CNP serum levels decreased because of the early suppression of production and secretion.
We did not detect an increase in antioxidant enzyme levels in the current study. Similarly, Richard et al. (24) did not detect a significant change in antioxidant enzyme levels, and the authors concluded that free radicals that were induced with anthracycline were inhibited by the endogenous antioxidant defense mechanism. However, Özdoğan et al. (36) showed that superoxide dismutase, glutathione peroxidase, and catalase activities were depressed by doxorubicin. Ashour et al. (8) established an experimental doxorubicin cardiotoxicity model in rats by cumulative 18 mg/kg intraperitoneal doxorubicin administration, and found an increase in serum LDH and CK-MB levels.
Chen et al. (26) investigated the results of cumulative 15 mg doxorubicin treatment in an experimental rat model, and observed that two weeks after doxorubicin treatment, the histopathological examination of myocardial tissue showed myocyte degeneration, myocyte loss, myocyte atrophy, hypertrophy in residual myocytes, and an increase in interstitial fibrosis. Barçın et al. (37) reported cytoplasmic vacuolization and myofibrillar loss in histopathological examination of myocardium by the application of cumulative 20 mg/kg doxorubicin treatment in an experimental rabbit model. In the current study, the histopathological findings caused by doxorubicin are similar to the results of the studies (22, 37).
Previous experimental animal models have reported different apoptotic indices [Hou et al. (38): 13.64±2.46; Chen et al. (26): 0.93±0.08; Nakamura et al. (39): 0.86±0.11; Fisher et al. (40): 0.61±0.09)]. The apoptotic index in the current study (0.71±0.27) is generally consistent with the literature. These findings suggest that cell death in doxorubicin cardiotoxicity not only occurs with apoptosis, but also through other mechanisms including necrosis and autophagy. Combination treatment of metformin and doxorubicin caused a decrease in apoptosis, although this decrease was not significant.
Study limitations
The limitation of the current study is that biochemical and histopathological examinations were performed only one day after the last dose of doxorubicin; this prevented us from determining delayed cardiotoxicity, as well as the long-term cardioprotective effect of metformin.
Conclusion
The current study provided experimental evidence that metformin has a protective effect against doxorubicin cardiotoxicity. Regarding rapid increase in the incidence of cancer around the world, it is important to consider the possible use of metformin to attenuate the doxorubicin cardiotoxicity; perhaps, it could be used as protective prevention strategy against doxorubicin cardiotoxicity in patients with cancer. However, further studies are required to investigate the long-term cardioprotective effect of metformin against doxorubicin cardiotoxicity.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - M.A., K.Ü., M.F.S., A.Ü., Z.S., K.T.C., S.U., Ö.P., AB., F.N., F.E., N.N.; Design - M.A., K.Ü., M.F.S., A.Ü., Z.S., K.T.C., S.U., Ö.P., AB., F.N., F.E., N.N.; Supervision - M.A., K.Ü., M.F.S., A.Ü., Z.S., K.T.C., S.U., Ö.P., AB., F.N., F.E., N.N.; Resource - M.A., K.Ü., M.F.S., D.K., Z.S., K.T.C., S.U.; Materials - M.A., K.Ü., M.F.S.; Data collection &/or processing - M.A., K.Ü., M.F.S., F.E., N.N.; Analysis &/or interpretation - M.F., K.Ü., M.F.S., N.N.; Literature search - M.A., K.Ü., M.F.S.; Writing - M.A., K.Ü., M.F.S.; Critical review - M.A., K.Ü., M.F.S., N.N.
References
- 1.Hareke D, Franco VI, Henkel JM, Miller TL, Lipshultz SE. Cardiotoxicity in childhood cancer survivors: strategies for prevention and management. Future Cardiol. 2012;8:647–70. doi: 10.2217/fca.12.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh ET. Cardiotoxicity induced by chemotherapy and antibody therapy. Annu Rev Med. 2006;57:485–98. doi: 10.1146/annurev.med.57.121304.131240. [DOI] [PubMed] [Google Scholar]
- 3.Mertens AC, Yasui Y, Neglia JP, Potter JD, Nesbit ME, Jr, Ruccione K, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–72. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 4.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Colan SD. Cardiovascular trials in long-term survivors of childhood cancer. J Clin Oncol. 2004;22:769–73. doi: 10.1200/JCO.2004.12.937. [DOI] [PubMed] [Google Scholar]
- 6.Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmocokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22:820–7. doi: 10.1097/FPC.0b013e3283559b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Messaoudi S, Rongen GA, de Boer RA, Riksen NP. The cardioprotective effects of metformin. Curr Opin Lipidol. 2011;22:445–53. doi: 10.1097/MOL.0b013e32834ae1a7. [DOI] [PubMed] [Google Scholar]
- 8.Ashour AE, Sayed-Ahmed MM, Abd-Allah AR, Korashy HM, Maayah ZH, Alkhalidi H, et al. Metformin rescues the myocardium from doxorubicin-induced energy starvation and mitochondrial damage in rats. Oxid Med Cell Longev. 2012;2012:434195. doi: 10.1155/2012/434195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tocchetti CG, Carpi A, Coppola C, Quintavalle C, Rea D, Campesan M, et al. Ranolazine protects from doxorubicin-induced oxidative stress and cardiac dysfunction. Eur J Heart Fail. 2014;16:358–66. doi: 10.1002/ejhf.50. [DOI] [PubMed] [Google Scholar]
- 10.Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131:561–78. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 11.Asensio-Lopez MC, Sanchez-Mas J, Pascual-Figal DA, Abenza S, Perez-Martinez MT, Valdes M, et al. Involvement of ferritin heavy chain in the preventive effect of metformin against doxorubicin-induced cardiotoxicity. Free Radic Biol Med. 2013;157:188–200. doi: 10.1016/j.freeradbiomed.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 12.van Staa TP, Patel D, Gallagher AM, de Brui ML. Glucose-lowering agents and the patterns of risk for cancer: a study with the General Practice Research Database and secondary care data. Diabetologia. 2012;55:654–65. doi: 10.1007/s00125-011-2390-3. [DOI] [PubMed] [Google Scholar]
- 13.Charlon V, Boucher F, Mouhieddine S, de Leiris J. Reduction of myocardial infarct size by metformin in rats submitted to permanent left coronary artery ligation. Diabetes Metab. 1988;14:591–5. [Google Scholar]
- 14.Paiva M, Riksen NP, Davidson SM, Hausenloy DJ, Goncalez L, Providencia L, et al. Metformin prevents myocardial reperfusion injury by activating the adenosine receptor. J Cardiovasc Pharmacol. 2009;53:373–8. doi: 10.1097/FJC.0b013e31819fd4e7. [DOI] [PubMed] [Google Scholar]
- 15.Solskov L, Lofgren B, Kristiansen SB, Jessen N, Pold R, Nielsen TT, et al. Metformin induces cardioprotection against ischaemia/reperfusion injury in the rat heart 24 h after administration. Basic Clin Pharmacol Toxicol. 2008;103:82–7. doi: 10.1111/j.1742-7843.2008.00234.x. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, et al. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009;119:2568–77. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 17.Kewalramani G, Puthenveetil P, Wang F, Kim MS, Deppe S, Abrahani A, et al. AMP-activated protein kinase confers protection against TNF-a-induced cardiac cell death. Cardiovasc Res. 2009;84:42–53. doi: 10.1093/cvr/cvp166. [DOI] [PubMed] [Google Scholar]
- 18.Asensio-López MC, Lax A, Pascual-Figal DA, Valdés M, Sánchez-Más J. Metformin protects against doxorubicin-induced cardiotoxicity: involvement of the adiponectin cardiac system. Free Radic Biol Med. 2011;51:1861–71. doi: 10.1016/j.freeradbiomed.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Kobashigawa LC, Xu YC, Padbury JF, Tseng YT, Yano N. Metformin protects cardiomyocyte from doxorubicin induced cytotoxicity through an AMP-activated protein kinase dependent signaling pathway: an in vitro study. PLoS One. 2014;9:e104888. doi: 10.1371/journal.pone.0104888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipshultz SE, Sambatakos P, Maguire M, Karnik R, Ross SW, Franco VI, et al. Cardiotoxicity and cardioprotection in childhood cancer. Acta Haematol. 2014;132:391–9. doi: 10.1159/000360238. [DOI] [PubMed] [Google Scholar]
- 21.Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64:938–45. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 22.Desai VG, C Kwekel J, Vijay V, Moland CL, Herman EH, Lee T, et al. Early biomarkers of doxorubicin-induced heart injury in a mouse model. Toxicol Appl Pharmacol. 2014;281:221–9. doi: 10.1016/j.taap.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Pınarlı FG, Oğuz A, Tunaoğlu FS, Karadeniz C, Gökçora N, Elbeg S. Late cardiac evaluation of children with solid tumors after anthracycline chemotherapy. Pediatr Blood Cancer. 2005;44:370–7. doi: 10.1002/pbc.20281. [DOI] [PubMed] [Google Scholar]
- 24.Richard C, Lauzier B, Delemasure S, Talbot S, Ghibu S, Collin B, et al. Effects of angiotensin-1 converting enzyme inhibition on oxidative stress and bradykinin receptor expression during doxorubicin-induced cardiomyopathy in rats. J Cardiovasc Pharmacol. 2008;52:278–85. doi: 10.1097/FJC.0b013e3181865f28. [DOI] [PubMed] [Google Scholar]
- 25.Berctinchant JP, Polge A, Juan JM, Oliva-Lauraire MC, Giuliani I, Marty-Double C, et al. Evaluation cardiac troponin I and T levels as markers of myocardial demage in doxorubicin-induced cardiomyopathy rats, and their relationship with echocardiographic and histological findings. Clin Chim Acta. 2003;329:39–51. doi: 10.1016/s0009-8981(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Chen Y, Bi Y, Fu N, Shan C, Wang S, et al. Preventive cardioprotection of erythropoetin against doxorubicin-induced cardiomyopathy. Cardiovasc Drug Ther. 2007;21:367–74. doi: 10.1007/s10557-007-6052-0. [DOI] [PubMed] [Google Scholar]
- 27.Volpe M, Rubattu S, Burnett J. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35:419–25. doi: 10.1093/eurheartj/eht466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sellitti DF, Koles N, Mendonça MC. Regulation of C-type natriuretic peptide expression. Peptides. 2011;32:1964–71. doi: 10.1016/j.peptides.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Kittiwarawut A, Vorasettakarnkij Y, Tanasanvimon S, Manasnayakorn S, Sriuranpong V. Serum NT-proBNP in the early detaection of doxorubicin-induced cardiac dysfunction. Asia Pac J Clin Oncol. 2013;9:155–61. doi: 10.1111/j.1743-7563.2012.01588.x. [DOI] [PubMed] [Google Scholar]
- 30.Hayakawa H, Komada Y, Hirayama M, Hori H, Ito M. Plasma levels of natriuretic peptides in relation to doxorubicin-induced cardiotoxicity and cardiac function in children with cancer. Med Pediatr Oncol. 2001;37:4–9. doi: 10.1002/mpo.1155. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Garami M, Gardner DG. Doxorubicin selectively inhibits brain versus atrial natriuretic peptide gene expression in cultured neonatal rat myocytes. Hypertension. 1999;34:1223–31. doi: 10.1161/01.hyp.34.6.1223. [DOI] [PubMed] [Google Scholar]
- 32.Bernardini N, Agen C, Favilla S, Danesi R, Tacca MD. Doxorubicin cardiotoxicity is associated with alterations of plasma levels of atrial natriuretic factor. J Endocrinol Invest. 1992;15:79–84. doi: 10.1007/BF03348668. [DOI] [PubMed] [Google Scholar]
- 33.Ry SD. C-type natriuretic peptide: A new cardiac mediator. Peptides. 2013;40:93–8. doi: 10.1016/j.peptides.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Beaulieu P, Cardinal R, Page P, Francoeur F, Tremblay J, Lambert C. Positive chronotropic an inotropic effects of C-type natriuretic peptide in dogs. Am J Physiol. 1997;273:1933–40. doi: 10.1152/ajpheart.1997.273.4.H1933. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Waard MC, Sterner-Kock A, Stepan H, Schultheiss HP, Duncker DJ, et al. Cardiomyocyte-restricted over-expression of C-type natriuretic peptide cardiac hyperthrophy induced by mypcardial infarction in mice. Eur J Heart Fail. 2007;9:548–57. doi: 10.1016/j.ejheart.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Özdoğan K, Taşkın E, Dursun N. Protective effect of carnosine on adriamycin-induced oxidative heart demage in rats. Anatol J Cardiol. 2011;11:3–10. doi: 10.5152/akd.2011.003. [DOI] [PubMed] [Google Scholar]
- 37.Barçın C, Kuşaklıoğlu H, Safalı M, İyisoy A, Köse S, Barındık N, et al. Effect of octreotide in the prevention of doxorubicin cardiotoxicity. Anatol J Cardiol. 2005;5:18–23. [PubMed] [Google Scholar]
- 38.Hou XW, Son J, Wang Y, Ru YX, Lian Q, Majiti W, et al. Granulocyte colony-stimulating factor reduces cardiomyocyte apoptosis and improves cardiac function in adriamycin-induced cardiomyopathy in rats. Cardiovasc Drug Ther. 2006;20:85–91. doi: 10.1007/s10557-006-7652-9. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura T, Ueda Y, Juan Y, Katsuda S, Takhashi H, Koh E. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: in vivo study. Circulation. 2000;102:572–80. doi: 10.1161/01.cir.102.5.572. [DOI] [PubMed] [Google Scholar]
- 40.Fisher PW, Saloum F, Das A, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–10. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]