Abstract
Objective:
We evaluated autonomic behavior by examining heart rate variability (HRV) in the time domain and frequency domain in pediatric patients who underwent transcatheter closure of atrial septal defect (ASD).
Methods:
A prospective study design was used. Holter ECG was performed in a control group of 30 healthy subjects and a group of 47 patients who underwent transcatheter ASD closure. ECG was taken one day before, one day after, and six months after the procedure to evaluate changes in the time domain [SDNN, rMSSD, NN, pNN50(%), and SDANN] and frequency domain (VLF, LF, HF, VHF, and LF/HF) in the patient group. Student’s t-test was used to evaluate changes prior to and after the procedure.
Results:
There were 28 females (60%) in the patient group and 21 females (70%) in the control group. The mean age and weight of the participants in the patient group were 9.61±4.72 years and 32.40±19.60 kg, respectively; the mean age and weight of the control subjects were 10.43±5.31 years and 32.83±13.00 kg, respectively. In both the time domain and frequency domain analyses, the patient group values were found to be lower than those in the control group prior to the procedure; the values in the patient group were found to approach the values in the control group following the procedure. By the sixth month, the values in the patient group reached the control levels with no statistically significant difference (SDNN: 145±0.84, 137.50±42.50; r MSSD: 72.18±48.22, 58.14±28.49; SDANN: 125.13±13.50, 122.40±41.06; VLF: 112.85±29.07, 114.41±98.39; LF: 50.40±24.09, 45.69±15.13; HF: 39.28±19.86, 44.29±13.14; VHF: 10.29±4.24, 9.99±6.47; LF/HF: 1.90±1.44, 1.24±0.81; p>0.05).
Conclusion:
The transcatheter closure of secundum ASDs was found to have a positive effect on HRV. Consequently, it may contribute to reduced mortality and morbidity. We can conclude that in children, HRV recovers approximately six months after transcatheter ASD closure.
Keywords: heart rate variability, atrial septal defect, closure, transcatheter, children
Introduction
Heart rate variability (HRV) is used to evaluate the parasympathetic and sympathetic systems on the sinus node, cardiovascular events, and mortality risk in patients (1, 2). HRV decreases in individuals with myocardial infarction, coronary heart disease, congestive heart failure, chronic mitral insufficiency, and secundum atrial septal defect (ASD) when the autonomic nervous system contributes to the pathological conditions and affects the prognosis (3, 4). HRV reduction in ASD patients is proportional to right ventricular end-diastolic pressure (4). Atrial arrhythmias in ASD patients have the effect of right atrial dilatation and tension lasting for an extensive period, which significantly contributes to cardiac morbidity and mortality (5-8).
Secundum ASDs account for 10% of congenital heart disease in neonates at birth and as much as 30%-40% of heart disease in adults (3). Transcatheter ASD closure has been safely and effectively implemented in pediatric patients for the past two decades (3). However, limited information is available regarding the effect of ASD closure on cardiac autonomic function and HRV in children. The present cross-sectional case-control study was designed to evaluate the influence of transcatheter ASD closure on cardiac autonomic functions.
Methods
Study population
The study population consisted of pediatric patients who underwent transcatheter closure of ASDs at Istanbul Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Center and Research Hospital between April 2010 and January 2013 and a control group of healthy subjects. The selection criteria for the patient group were the presence of large secundum ASDs (stretched diameter>12 mm, dilated right ventricle, and/or invasive QP/QS ratio>1.5) and transcatheter closure. The control subjects were matched to the participants in the patient group based on age and gender. This study was designed as a cross-sectional case control. Excluded from the sample were patients who did not sign the informed consent form, patients with device embolization, and patients in whom the device did not fit the septum well.
This study was designed using the HRV data obtained using a Holter ECG monitor, as presented in the previously published study, “Holter Electrocardiographic Findings and P-wave Dispersion in Pediatric Patients with Transcatheter Closure of Atrial Septal Defects.” In the previously published part of this study, the HRV data was not used (9). This current study was approved by the Medical Investigation Ethical Committee of our hospital. All the patients and control subjects provided written informed consent.
Determination of HRV via Holter monitoring
The patients admitted to our center for transcatheter ASD closure underwent a 24-h Holter electrocardiography (ECG) study on admission and on the following day, while the control subjects underwent three-channel Holter monitoring (CardioNavigator Plus Impresario, Delmar Reynolds, Paris, France). A control Holter was performed six months after the patients were discharged (9). The first electrode was placed at the right mid-clavicular, the second electrode was placed at the left mid-clavicular, and the third electrode was placed at the left anterior axillary line of the fifth rib. All the recordings were evaluated by the same specialist. Artifacts and ectopic complexes were excluded. There had to be ≥23 h of analyzable data for the 24-h recording to be accepted for the study. The HRV parameters that were used for the time domain analysis were the mean and the highest and lowest heart rates. For the time domain analysis, the following were used: SD of all normal RR intervals (SDNN) (ms), SD of the average five-minute normal RR intervals (SDANN) (ms), the square root of the mean of the sum of the squares of differences between the adjacent RR intervals (rMSSD) (ms), the average of the RR intervals (NN) (ms), the proportion of the adjacent RR intervals that differ by more than 50 ms in the 24-h recording [pNN50(%)] (ms), and the mean of all of the five-minute SDs of the normal RR intervals during the 24-h period (SDNN index) (ms). For the frequency domain analysis, the following low and high frequency values were recorded: very low frequency (VLF) {0.017-0.050 Hz [Ln(ms2)]}, low frequency (LF) {0.050–0.150 Hz[Ln(ms2)]}, high frequency (HF) {0.150-0.350 Hz[Ln(ms2)]}, and very high frequency (VHF) {0.350-0.500 Hz [Ln(ms2)]} (7, 10-15).
Statistical analysis
Statistical data were evaluated using the SPSS for Windows software package (Version 15.0, SPSS Inc., Chicago, IL, USA). The normally distributed continuous variables were assessed using the Shapiro-Wilk test. Unidirectional data in the analysis of variance was used for repeated measurement analysis performed with the same instrument before and after defect closure and in the sixth month in ASD patients. Student’s t-test was used to compare and evaluate the demographic data between the patient and control groups. The data were expressed as mean±standard deviation. A p value of <0.05 was considered statistically significant.
Results
Study population
Patient group
There were 28 females (60%) in the patient group and 21 females (70%) in the control group. The mean age and weight of the participants in the patient group were 9.61±4.72 years and 32.40±19.60 kg, respectively; the mean age and weight of the control subjects were 10.43±5.31 years and 32.83±13.00 kg, respectively. The demographic characteristics of the patients evaluated with Holter are presented in Table 1. Out of the total of 47 ASD patients, 41 had a single ASD and six had multiple ASDs. During the closure procedures, 17 AMPLATZER® septal occluders (AGA Medical Corp., Golden Valley, MN, USA) and 24 Cardi-O-Fix occluders (CSO, Starway Medical Technology Inc., Beijing, China) were used in addition to other ASD closure devices [three multi-fenestrated AMPLATZER septal occluders, two Lifetech Cera occluders, one BioSTAR occluder (NMT Medical, Inc., Boston, MA, USA)] (9).
Table 1.
The characteristics of the patient and control groups
| Patient group | Control group | P | |
|---|---|---|---|
| Number | 47 | 30 | |
| Girl/Boy | 28/19 | 21/9 | 0.662 |
| Age, year | 9.61±4.72 | 10.43±5.31 | 0.660 |
| Height, cm | 131.2±25.92 | 132.5±15.94 | 0.824 |
| Weight, kg | 32.40±19.60 | 32.83±13.00 | 0.886 |
| Body mass index, kg/m2 | 17.2±3.50 | 17.7±3.20 | 0.333 |
Student’s t-test
Control group
The control group consisted of 30 healthy individuals. The echocardiography findings of all of the control subjects were normal. There was no significant difference between the patient and control groups in terms of age, gender, weight, and body mass index (Table 1).
Holter recording results
Time domain analysis
A comparison of the time domain analysis measurements between the control and patient groups one day prior to ASD closure, one day after ASD closure, and six months later is presented in Table 2.
Table 2.
Comparisons of time domain analysis measurements of control and patient groups prior to and after the procedure and six months later
| Parameters | Control | Initial | P0 | One day after intervention | P1 | P2 | At 6. month | P3 | P4 |
|---|---|---|---|---|---|---|---|---|---|
| SDNN≠ | 137.50±42.50 | 90.25±28.14 | 0.001 | 108.30±26.23 | 0.001 | 0.01 | 145±0.84 | 0.713 | 0.001 |
| rMSSD≠ | 58.14±28.49 | 42.29±22.37 | 0.029 | 59.03±27.39 | 0.998 | 0.486 | 72.18±48.22 | 0.448 | 0.016 |
| NN≠ | 664.29±81.29 | 634.40±88.94 | 0.356 | 689.38±101.48 | 0.353 | 0.001 | 709.11±66.04 | 0.136 | 0.002 |
| pNN50%≠ | 17.90±11.25 | 10.90±7.31 | 0.011 | 18.42±11.62 | 0.987 | 0.023 | 19.9±12.94 | 0.712 | 0.001 |
| SDNN≠ index | 58.26±19.13 | 42.36±15.51 | 0.001 | 56.45±21.34 | 0.904 | 0.248 | 62.26±20.64 | 0.673 | 0.001 |
| SDANN≠ | 122.40±41.0 | 77.00±26.41 | 0.001 | 88.25±25.84 | 0.001 | 0.003 | 125.13±13.50 | 0.802 | 0.001 |
NN - the average of RR intervals; P0 - p value between the values prior to the procedure in the patient and control groups; P1-p values between the values one day after the procedure in the patient and control groups; P2 - p values between the values prior to and one day after the procedure in the patients, P3 - p values between the control group and values of patients at the sixth month; P4 - comparison of values prior to the procedure and at the sixth month; pNN50%-the proportion of adjacent RR intervals that differ by more than 50 ms in the 24-h recording; RMSSD - the square root of the mean of the sum of the squares of differences between adjacent RR intervals; SDANN - the standard deviation of the means of all the five-minute segment normal RR intervals; SDNN - the standard deviation of all the normal RR intervals; SDNN index-the mean of all the five-minute standard deviations of normal RR intervals during the 24-h period. Student’s t-test was used.
A statistically significant difference was found between the one-day pre-procedure values and control group values [SDNN: 90.25±28.14, 137.50±42.50, p=0.001; rMSSD: 42.29±22.37, 58.14±28.49, p=0.029; pNN50 (%): 10.90±7.31, 17.90±11.25, p=0.011; SDNN index: 42.36±15.51, 58.26±19.13, p=0.001; SDANN: 77.00±26.41, 122.40±41.06, p=0.001].
Between the control group and one-day post-procedure values, only SDNN and SDANN showed a significant discrepancy [SDNN: 108.30±26.23, 137.50±42.50, p=0.001; rMSSD: 59.03±27.39, 58.14±28.49, p=0.998; NN: 689.38±101.48, 664.29±81.29, p=0.353; pNN50 (%): 18.42±11.62, 17.90±11.25, p=0.987; SDNN index: 56.45±21.34, 58.26±19.13, p=0.904; SDANN: 88.25±25.84, 122.40±41.06, p=0.03].
Between the one-day pre-procedure and one-day post-procedure, SDNN, NN, pNN50 (%), and SDANN showed a statistically significant difference between the two groups (90.25±28.14, 108.30±26.23, p=0.01; 634.40±88.94, 689.38±101.48, p=0.001; 10.90±7.31, 18.42±11.62, p=0.023; 77.00±26.41, 88.25±25.84, p=0.003, respectively), whereas the rMSSD and SDNN index values showed no significant difference (42.29±22.37, 59.03±27.39, p=0.486; 42.36±15.51, 56.45±21.34, p=0.248, respectively).
There was no significant difference between the control group and sixth month values [SDNN: 137.50±42.50, 145±0.84, p=0.713; rMSSD: 58.14±28.49, 72.18±48.22, p=0.448; NN: 664.29±81.29, 709.11±66.04, p=0.136; pNN50 (%): 17.90±11.25, 19.9±12.94, p=0.712; SDNN: 58.26±19.13, 62.26±20.64, p=0.673; SDANN: 122.40±41.06, 125.13±13.50, p: 0.802].
A significant difference was observed between the one-day pre-procedure and sixth month values [SDNN: 90.25±28.14, 145±0.84, p=0.001; rMSSD: 42.29±22.37, 72.18±48.22, p=0.016; NN: 634.40±88.94, 709.11±66.04, p=0.002; pNN 50(%): 10.90±7.31, 19.9±12.94, p=0.001; SDNN index: 42.36±15.51, 62.26±20.64, p=0.001; SDANN: 77.00±26.41, 125.13±13.50, p=0.001] (Fig. 1).
Figure 1.
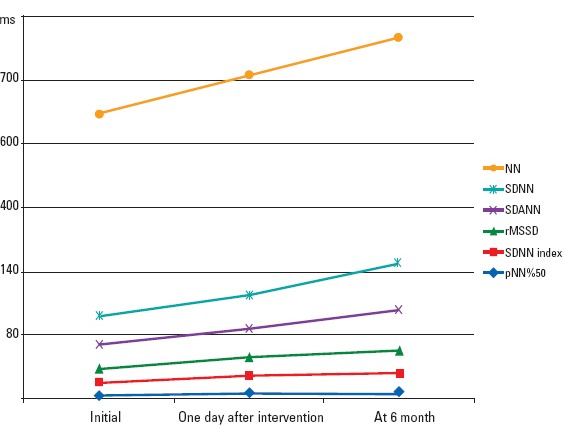
Graphic image of time domain analysis measurements of the patient group prior to and after procedure and six months later
Frequency domain analysis
A comparison of the frequency domain analysis measurements between the control and patient groups one day prior to ASD closure, one day after ASD closure, and six months later is summarized in Table 3.
Table 3.
Comparisons of frequency domain analysis measurements of control and patient groups prior to and after the procedure and six months later
| Parameters | Control | Initial | P0 | One day after intervention | P1 | P2 | At 6. month | P3 | P4 |
|---|---|---|---|---|---|---|---|---|---|
| VLF€ | 114.41±98.39 | 152.02±160.38 | 0.847 | 159.77±410.33 | 0.791 | 0.707 | 112.85±29.07 | 0.999 | 0.459 |
| LF€ | 45.69±15.13 | 58.63±13.23 | 0.001 | 46.83±14.38 | 0.878 | 0.018 | 50.40±24.09 | 0.398 | 0.064 |
| HF€ | 44.29±13.14 | 29.78±10.65 | 0.001 | 34.51±10.15 | 0.001 | 0.056 | 39.28±19.86 | 0.240 | 0.008 |
| VHFy€ | 9.99±6.47 | 11.56±6.95 | 0.760 | 18.48±11.01 | 0.001 | 0.001 | 10.29±4.24 | 0.982 | 0.732 |
| LF/HF | 1.24±0.81 | 2.55±1.58 | 0.001 | 1.70±1.54 | 0.343 | 0.966 | 1.90±1.44 | 0.238 | 0.260 |
HF - high frequency; LF - low frequency; LF/HF - low frequency/high frequency; P0-p value between the values prior to the procedure in the patient and control groups; P1-p values between the values one day after the procedure in the patient and control groups; P2-p values between the values prior to and one day after the procedure in the patients; P3-p values between the control group and values of patients at the sixth month; P4-comparison of values prior to the procedure and at the sixth month, VHF - very high frequency; VLF - very low frequency, ≠: ms
Hz [Ln (ms2)] Student’s t-test was used.
With the exception of VLF and VHF, there was a significant difference between the control group and one-day pre-procedure values (VLF: 114.41±98.39, 152.02±160.38, p=0.847; LF: 45.69±15.13, 58.63±13.23, p=0.001; HF: 44.29±13.14, 29.78±10.65, p=0.001; VHF: 9.99±6.47, 11.56±6.95, p=0.760; LF/HF: 1.24±0.81, 2.55±1.58, p=0.001).
The one-day post-procedure values increased and approached those of the control group with only HF and VHF showing a significant difference (VLF: 114.41±98.39, 159.77±410.33, p=0.791; LF: 45.69±15.13, 46.83±14.38, p=0.878; HF: 44.29±13.14, 34.51±10.15, p=0.001; VHF: 9.99±6.47, 18.48±11.01, p=0.001; LF/HF: 1.24±0.81, 1.70±1.54, p=0.343).
Between the one-day pre-procedure and one-day post-procedure values, LF and VHF varied significantly (58.63±13.23, 46.83±14.38, p=0.018; 11.56±6.95, 18.48±11.01, p=0.001, respectively), whereas VLF, HF, and LF/HF did not (152.02±160.38, 159.77±410.33, p=0.707; 29.78±10.65, 34.51±10.15, p=0.056; 2.55±1.58, 1.70±1.54, p=0.966, respectively).
There was no statistically significant difference between the control group and sixth month values (VLF: 114.41±98.39, 112.85±29.07, p=0.999; LF: 45.69±15.13, 50.40±24.09, p=0.398; HF: 44.29±13.14, 39.28±19.86, p=0.240; VHF: 9.99±6.47, 10.29±4.24, p=0.982; LF/HF: 1.24±0.81, 1.90±1.44, p=0.238) (Fig. 2).
Figure 2.
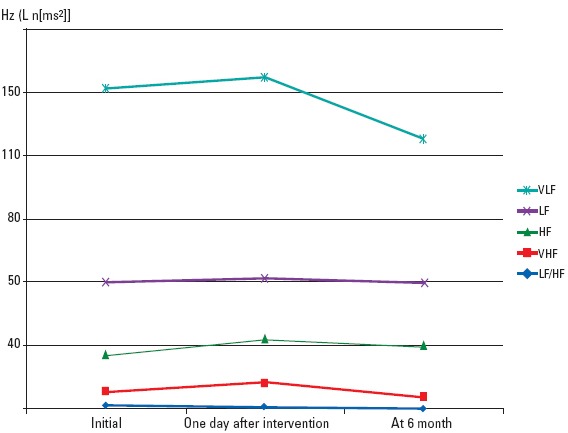
Graphic image of frequency domain analysis measurements of the patient group prior to and after procedure and six months later
Discussion
The most important results of this study are:1) In both domain analyses, the patient group values were found to be lower than those in the control group at the pre-processing time, 2) The values of the patient group were observed to approach the values of the control group at the post-treatment process, 3) The values of the patient group were reached the values of the control group within six months after the procedure, 4) These findings can be explained by increase of vagal activity and the decrease of sympathetic activity after the procedure. HRV is a parameter used for the non-invasive study of neurohumoral control of the heart. Massin and von Bernuth (10) reported reduced time domain and frequency domain measurements of HRV in children with various congenital heart diseases. This reduction was correlated with the New York Heart Association functional class of heart failure but not to any specific hemodynamic data. Our results were similar to those of Massin et al. (4) and Finley et al. (16), who found moderately reduced HRV parameters in ASD children. These findings show an increase in sympathetic control of the heart or a decrease in parasympathetic control of the heart.
In several studies of pediatric ASD patients, the HRV values are lower compared with those of healthy subjects, but the values increase following defect closure (3, 17, 18). Our study is the first to compare both time domain and frequency domain analysis values of a large patient sample before and after ASD closure with those of a control group. Frequency domain analysis data are the most important advantage of this study. Increased HRV in children is attributed to the smaller size of their right atrium and the differences in ventricular compliance (16). HRV following closure of ASD begins to return to normal owing to the anatomical changes within the heart. In healthy persons, the two most important mechanisms of sinus arrhythmia are right atrial tension, due to an increase of systemic venous return to the right atrium during inspiration, and relative tachycardia, due to inhibition of inspiration by carotid sinus reflexes (16). Respiratory fluctuation decreases because of the connection between the left atrium and right atrium in ASD patients (19, 20). Moreover, right atrial volume increases less in ASD patients during inspiration due to a larger right atrium, thereby leading to a decline in HRV.
Many studies have shown that HRV and vagal activity decrease in ASD patients due to higher right ventricular filling pressure (4-8, 21-23). Edwards et al. (24) showed atrial tension to be a crucial stimulant of atrial natriuretic peptide secretion. These findings also point to the possible positive effect of decreased volume and pressure on HRV, similar to neurohumoral factors. Atrial arrhythmias occur in ASD patients when the effects of right atrial dilation and tension persist for a long period of time (5). Atrial tachyarrhythmias, heart failure, and thromboembolic events are common in ASD patients and contribute to cardiac morbidity and mortality (6-8).
In the time domain analysis of HRV in humans, the SDNN value is more affected by the parasympathetic and sympathetic impulses associated with vagal tonus compared with the RMSSD and pNN50 (%) values (3). The HF component of frequency domain analysis and the pNN50 (%) and RMSSD values of time domain analysis reflect short-term HRV (high frequency power) (25). The LF component of frequency domain analysis and the SDNN, SDNN index, and SDANN parameters of the time domain analysis express long-term HRV (low frequency power), and both are affected by cholinergic and adrenergic activities (26).
In a study by Cansel et al. (27), parasympathetic system activity markers, such as pNN50 (%) and RMSSD, do not statistical significant change in ASD patients compared with a control group, whereas vagal activity markers, such as SDNN, SDANN, and the SDNN index, decreas. Whereas vagal activity markers, such as SDNN, SDANN, and the SDNN index, decrease. In our study, the sixth-month HRV values of patients who underwent transcatheter ASD closure approached the levels of the control group. Bakari et al. (28) compared 28 ASD patients and 32 healthy individuals and found that ASD patients have significantly reduced HRV and sympathetic dominance. Koca et al. (25) showed that HF is highly correlated with rMSSD and pNN50 (%); thus, it can be used interchangeably. HRV alteration was detected in both time domain and frequency domain parameters (28). In our study, ASD patients had lower SDNN, RMSSD, pNN50 (%), SDNN index, and SDANN values than the control subjects one day prior to ASD closure. In the study by Cansel et al. (27), the pNN50 (%) and RMSSD values were reported lower in the ASD patients although not statistically significant. The HRV values in the time domain analysis increased after the procedure compared with the initial levels, and they approached the control group levels when compared with the levels obtained before the procedure. The SDNN, pNN50 (%), and SDANN values increased in a statistically significant way one day after the procedure. The RMSSD, NN, and SDNN index values showed no change, which may be due to the fact that the measurements were taken relatively soon after the procedure. Similar to previous reports, the six-month time domain values reached the control group values (3). The procedure may have reduced sympathetic hyperactivation and increased vagal tonus. Following transcatheter ASD closure, ventricular baroreceptor dysfunction was absent because the HRV values improved after the decrease in the right ventricler size, atrial diameter, and blood volume. The sympathetic and parasympathetic balance appeared to be restored by the sixth month.
We also used spectral analysis of HRV to identify the autonomic system abnormalities responsible for the reduced HRV before the procedure and the increased HRV after the procedure. Frequency domain analysis is a method used to evaluate parasympathetic and sympathetic activities (29). Frequency domain methods study heart rate variations by breaking the heart rate signal into its constituents (frequencies) and quantifying their relative intensity (power). High and low frequency values in frequency domain analysis are determined by the amplitude in the power spectrum (28). HFVs normally meet the respiratory frequency values and reflect parasympathetic activity. LFVs are associated with both sympathetic and parasympathetic systems. A change in peak amplitude may be caused by changes in coupling between respiration and the heart or by changes in autonomic control (16, 21). The main disadvantage of this method is its complexity and dependence on respiratory rate. An increase in LF and a variable decrease in HF is seen in ASD patients with tachycardia. In addition to VLF, VHF, LF, and HF, we also measured the LF/HF ratio, which accurately reflects the autonomic balance.
Finley et al. (16) compared the power spectral values before and after surgical ASD closure in 10 healthy controls and 10 ASD patients; they showed that the high frequency vagally mediated component of variability is lower in the patients than in the controls, which points to abnormalities in the autonomic control of the heart rate. In our study, the LF values decreased in a statistically significant way one day after the procedure in comparison with the pre-procedure values, whereas the VLF, HF, and HF values significantly increased. The decrease in LF may be attributed to the fact that the measurements were performed too early after the procedure. Similarly, Finley et al. (16) found that the HF values in ASD children are lower than the LF values.
The LF/HF ratio mirrors the sympathovagal balance and reflects the sympathetic modulation of the heart, whereas the progressive reduction of other HRV parameters reflects the loss of neural control of the sinus node caused by the progressive increase of plasma norepinephrine levels (11). In our patients, the pre-procedure LF/HF ratio was higher compared with the control group because of the significant increase in the LF parameters caused by tachycardia. Other changes were not statistically significant. The LF/HF ratio was found to be approaching normal levels after the procedure.
The presence of VHF peaks is an important finding in the power spectrum of heart rate fluctuations. VHF peaks, assumed to be harmonics of the respiratory rate, originate from nonlinear coupling between the respiratory system and heart. There are two types of VHF peaks in HRV: harmonics of respiration and peaks uncorrelated with respiration. The physiological mechanism of this phenomenon is still unclear (12). In normal subjects, the fast heart rate feedback includes vagal innervation and related reflexes; they do not exhibit the higher harmonics of the respiratory frequency. It is also possible that in normal subjects, the VHF peaks in the heart rate exist but are masked by the high frequency that originates from noisy vagal innervations. Heart transplant patients are found to have reduced VHF values because of the absence of vagal innervation and respiratory frequency harmony (12-15). In our study, the VHF values increased one day after ASD closure, and they returned to the normal level found in the control group six months after ASD closure, which can be explained by the state of vagal dominance and the reduction of sympathetic dominance after the procedure.
Study limitation
In addition to the small sample size, we were unable to carry out our original plan of performing a control Holter study 12 months after transcatheter ASD closure.
Conclusion
While the HRV values showed little change one day after ASD closure in children, these values recover almost completely within six months after the procedure. In order to better demonstrate the effect that HRV has on morbidity and mortality in children after ASD closure, further studies with larger patient samples and longer follow-up times are required.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - İ.Ö., H.T.T, S.Ö., İ.B.; Design - İ.Ö., H.T.T., S.Ö., Ö.T., A.G.; Supervision - Y.E., E. Ödemiş., İ.B., E.Ö., M.G.; Materials - E.Ö., M.S., İ.C.T.; Data collection &/or processing - E.Ö., M.S., İ.C.T., Ö.T.; Analysis &/or interpretation - E.Ö., M.S., İ.C.T., M.G., Ö.T.; Literature search - S.Ö., İ.Ö., H.T.T., A.G.; Writing - İ.Ö., S.Ö., Y.E., E. Ödemiş.; Critical review - E. Ödemiş., A.G., İ.B., M.G., Y.E.
References
- 1.Malik M. Electrocardiographic and autonomic testing of cardiac risk. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: From Cell to Bedside. 5th ed. Philadelphia: WB Saunders; 2009. pp. 871–80. [Google Scholar]
- 2.Tulppo M, Huikuri HV. Origin and significance of heart rate variability. J Am Coll Cardiol. 2004;43:2278–80. doi: 10.1016/j.jacc.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Bialkowski J, Karwot B, Szkutnik M, Sredniawa B, Chodor B, Zeifert B, et al. Comparison of heart rate variability between surgical and interventional closure of atrial septal defect in children. Am J Cardiol. 2003;92:356–8. doi: 10.1016/s0002-9149(03)00648-9. [DOI] [PubMed] [Google Scholar]
- 4.Massin MM, Derkenne B, von Bernuth G. Heart rate behavior in children with atrial septal defect. Cardiology. 1998;90:269–73. doi: 10.1159/000006857. [DOI] [PubMed] [Google Scholar]
- 5.Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation. 2006;114:1645–53. doi: 10.1161/CIRCULATIONAHA.105.592055. [DOI] [PubMed] [Google Scholar]
- 6.Attie F, Rosas M, Granados N, Zabal C, Buendía A, Calderón J. Surgical treatment for secundum atrial septal defects in patients >40 years old. A randomized clinical trial. J Am Coll Cardiol. 2001;38:2035–42. doi: 10.1016/s0735-1097(01)01635-7. [DOI] [PubMed] [Google Scholar]
- 7.Konstantinides S, Geibel A, Olschewski M, Görnandt L, Roskamm H, Spillner G, et al. A comparison of surgical and medical therapy for atrial septal defect in adults. N Engl J Med. 1995;333:469–73. doi: 10.1056/NEJM199508243330801. [DOI] [PubMed] [Google Scholar]
- 8.Gatzoulis MA, Freeman MA, Siu SC, Webb GD, Harris L. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999;340:839–46. doi: 10.1056/NEJM199903183401103. [DOI] [PubMed] [Google Scholar]
- 9.Özyılmaz I, Özyılmaz S, Tola HT, Saygı M, Kıplapınar N, Tanıdır C, et al. Holter electrocardiography findings and P-wave dispersion in pediatric patients with transcatheter closure of atrial septal defects. Ann Noninvasive Electrocardiol. 2014;19:174–81. doi: 10.1111/anec.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massin M, von Bernuth G. Clinical and haemodynamic correlates of heart rate variability in children with congenital heart disease. Eur J Pediatr. 1998;157:967–71. doi: 10.1007/s004310050979. [DOI] [PubMed] [Google Scholar]
- 11.Ori Z, Monir G, Weiss J, Sayhouni X, Singer DH. Heart rate variability. Frequency domain analysis. Cardiol Clin. 1992;10:499–537. [PubMed] [Google Scholar]
- 12.Toledo E, Pinhas I, Aravot D, Akselrod S. Very high frequency oscillations in the heart rate and blood pressure of heart transplant patients. Med Biol Eng Comput. 2003;41:432–8. doi: 10.1007/BF02348086. [DOI] [PubMed] [Google Scholar]
- 13.Bernardi L, Keller F, Sanders M, Reddy PS, Griffith B, Meno F, et al. Respiratory sinus arrhythmia in the denervated human heart. J Appl Physiol. 1989;67:1447–55. doi: 10.1152/jappl.1989.67.4.1447. [DOI] [PubMed] [Google Scholar]
- 14.Bernardi L, Valle F, Leuzzi S, Rinaldi M, Marchesi E, Falcone C, et al. Non-respiratory components of heart rate variability in heart transplant recipients: evidence of autonomic reinnervation? Clin Sci. 1994;86:537–45. doi: 10.1042/cs0860537. [DOI] [PubMed] [Google Scholar]
- 15.Toledo E, Pinhas I, Aravot D, Almog Y, Akselrod S. Functional restitution of cardiac control in heart transplant patients. Am J Physiol Regul Integr Comp Physiol. 2002;282:900–8. doi: 10.1152/ajpregu.00467.2001. [DOI] [PubMed] [Google Scholar]
- 16.Finley JP, Nugent ST, Hellenbrand W, Craig M, Gillis DA. Sinus arrhythmia in children with atrial septal defect: An analysis of heart rate variability before and after surgical repair. Br Heart J. 1989;61:280–4. doi: 10.1136/hrt.61.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 18.Horner SM, Murphy CF, Coen B, Dick DJ, Harrison FG, Vespalcova Z, et al. Contribution to heart rate variability by mechanoelectric feedback. Stretch of the sinoatrial node reduces heart rate variability. Circulation. 1996;94:1762–7. doi: 10.1161/01.cir.94.7.1762. [DOI] [PubMed] [Google Scholar]
- 19.Finley JP, Nugent ST, Hellenbrand W. Heart rate variability in children: spectral analysis of developmental changes between 5 and 24 years. Can J Physiol Pharmacol. 1987;65:2048–52. doi: 10.1139/y87-320. [DOI] [PubMed] [Google Scholar]
- 20.Melcher A. Respiratory sinus arrhythmia in man: A study in heart regulating mechanisms. Acta Physiol Scand. 1976;435:1–31. [PubMed] [Google Scholar]
- 21.Pomeranz B, Mac Aulay RJB, Caudill MA, Kutz I, Adam D, Gordon D, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:151–3. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 22.Levin AR, Spach MS, Boineau JP, Canent RV Jr, Capp MP, Jewett PH. Atrial pressure-flow dynamics in atrial septal defects (secundum type) Circulation. 1968;37:476–88. doi: 10.1161/01.cir.37.4.476. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson JJ, 3rd, Miller MJ, Aroesty JM, Sahagian P, Grossman W, McKay RG, et al. Assessment of right atrial pressure–volume relations in patients with and without an atrial septal defect. J Am Coll Cardiol. 1989;13:630–6. doi: 10.1016/0735-1097(89)90604-9. [DOI] [PubMed] [Google Scholar]
- 24.Edwards BS, Zimmerman RS, Schwab TR, Heublein DM, Burnett JC Jr. Atrial stretch, not pressure, is the principal determinant controlling in the acute release of atrial natriuretic factor. Circ Res. 1988;62:191–5. doi: 10.1161/01.res.62.2.191. [DOI] [PubMed] [Google Scholar]
- 25.Koca B, Bakari S, Öztunç F. Correlation among the heart rate variability indices in healthy children and those with atrial septal defect. Turk Kardiyol Dern Ars. 2013;41:193–8. doi: 10.5543/tkda.2013.37676. [DOI] [PubMed] [Google Scholar]
- 26.Massin M, Von Bernuth G. Normal ranges of heart rate variability during infancy and childhood. Pediatr Cardiol. 1997;18:297–302. doi: 10.1007/s002469900178. [DOI] [PubMed] [Google Scholar]
- 27.Cansel M, Yağmur J, Ermiş N, Açıkgöz N, Taşolar H, Ataş H, et al. Effects of transcatheter closure of atrial septal defects on heart rate variability. J Int Med Res. 2011;39:654–61. doi: 10.1177/147323001103900235. [DOI] [PubMed] [Google Scholar]
- 28.Bakari S, Koca B, Öztunç F, Abuhandan M. Heart rate variability in patients with atrial septal defect and healthy children. J Cardiol. 2013;22:436–9. doi: 10.1016/j.jjcc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Miyakoshi M, Ikeda T, Miwa Y, Sakaki K, Ishiguro H, Abe A, et al. Quantitative assessment of cibenzoline administration for vagally mediated paroxysmal atrial fibrillation using frequency-domain heart rate variability analysis. J Cardiol. 2009;54:86–92. doi: 10.1016/j.jjcc.2009.04.009. [DOI] [PubMed] [Google Scholar]


