Abstract
Objective:
There are several studies showing an association between an increase in the plasma levels of homocysteine and the pathogenesis of hypertension. In this study, we assessed normotensive children of hypertensive adult parents to determine whether there is any change in homocysteine levels prior to the onset of hypertension.
Methods:
A total of 79 normotensive children of essential hypertensive parents who were followed-up at the cardiology department and 72 healthy children of normotensive parents who presented to the department of pediatrics at our clinic with complaints such as nonspecific chest pain and innocent murmur were included in the study. The participants’ complete blood count and low-density lipoprotein, high-density lipoprotein, triglyceride, total cholesterol, folic acid, vitamin B12, and homocysteine levels were noted.
Results:
No statistically significant differences were noted between the two groups in terms of age, gender, height, weight, body mass index, or levels of fasting lipids, folic acid, and vitamin B12 (p>0.05). Although the mean systolic and diastolic blood pressures were within the normal limits in both groups, they were significantly higher in children with a family history of hypertension than in controls (p<0.05). Similarly, homocysteine levels of children with a family history of hypertension were significantly higher than those of controls (p<0.01).
Conclusion:
Homocysteine levels of normotensive children of hypertensive parents are elevated before they develop hypertension. Homocysteine levels may be predictive of the subsequent development of hypertension in normotensive children of hypertensive parents.
Keywords: homocysteine level, children, hypertensive parents
Introduction
Hypertension, an important contributor to morbidity and mortality, is a common cardiovascular system disease (1, 2). The pathogenesis of hypertension is not clearly understood; various environmental and genetic aspects are believed to be responsible for its etiology. Because numerous factors determine blood pressure, no single pathophysiological mechanism can be considered as the cause of hypertension. In addition to the genetic background, lifestyle, socioeconomic status, environmental factors, and demographic and metabolic features affect blood pressure changes (3). Genetic factors are considered to be responsible for 30%-50% of hypertension pathogenesis (4). A family history of hypertension is known to be an important risk factor for essential hypertension during childhood (5). Recent studies have shown that children of hypertensive parents have higher blood pressure than children of normotensive parents (5-7).
Homocysteine is an independent risk factor for the pathogenesis of peripheral vascular disease, coronary artery disease, cerebral stroke, acute myocardial infarction, and atherosclerosis (8-10). Several studies have reported an association between an increase in the plasma levels of homocysteine and pathogenesis of hypertension (11, 12). Strong oxidizing substances, present during oxidation of homocysteine, damage the cellular structure and function. Decreasing arterial elasticity, resulting from the changes in the arterial wall structure and function, is an important factor in the pathogenesis of hypertension. Homocysteine increases the proliferation of vascular smooth muscle cells. Therefore, arterial medial layer smooth muscle cells increase in number and decrease in compliance. Homocysteine can lead to an imbalance between blood endothelin and nitric oxide levels or it can increase the level of calcium ions in vascular smooth muscle cells, resulting in increased systolic pressure (13). These findings demonstrate the potential important role of homocysteinemia in the pathogenesis of hypertension. However, the pathogenic mechanism underlying hypertension remains unclear (8, 13).
Dietary deficiency of folic acid and vitamin B12, malabsorption, renal failure, and malnutrition have been proposed to result in increased plasma total homocysteine levels (14). Vitamin B12 and folic acid levels are among the important determinants of plasma homocysteine; plasma homocysteine levels have been reported to be negatively correlated with vitamin B12 and folic acid levels. In addition, previous studies failed to detect a significant association between homocysteine levels and increased cholesterol and triglyceride (TG) levels, although homocysteine emerged as an independent risk factor for coronary disease (15).
Vascular factors and functional abnormalities, such as endothelial dysfunction, increased oxidative stress, vascular remodeling, and decreased compliance, can occur before hypertension and contribute to the pathogenesis of hypertension (16).
Adult and pediatric studies have shown the coexistence of hypertension and elevated homocysteine levels (17, 18). However, no previous study has assessed normotensive children of hypertensive parents.
In the etiopathogenesis of hypertension, it is important to know whether there is any change in homocysteine levels prior to the onset of hypertension. Therefore, in this study, we assessed normotensive children of hypertensive parents to determine whether there is any change in homocysteine levels prior to the onset of hypertension and development of end-organ damage.
Methods
In this study, we assessed homocysteine levels in normotensive children of hypertensive adult parents to determine whether there is any change prior to the onset of hypertension.
A total of 79 normotensive children of essential hypertensive parents who were followed up at the cardiology department and 72 healthy children of normotensive parents who presented to the department of pediatrics at our clinic with complaints such as nonspecific chest pain and innocent murmur were included in the study. Consent for the study protocol was attained from the Ethical Committee of Eskişehir Osmangazi University Medical School (consent number April 4, 2013/03).
Detailed medical, personal, and family histories were recorded. Children who had chronic disease or anemia and those using medication were excluded from the study. Physical examination was completed, and children with normal findings were included. Complete blood count and low-density lipoprotein (LDL), TG, total cholesterol (TC), folic acid, vitamin B12, and homocysteine levels were noted.
Children of parents who were diagnosed with essential hypertension and followed-up at the cardiology department with the use of regular antihypertensives were included in the study. We considered that the parents had no cardiovascular or other chronic disease.
Height was measured using a calibrated scale, and height was determined with a standard height scale while the child was in an upright position without wearing shoes. Body mass index (BMI) was calculated as weight (kg)/height (m2). Blood pressure was measured 3 times with a mercury sphygmomanometer using an appropriate cuffs while in the seated position after 10 min of rest, and the mean blood pressure was noted. Blood samples were collected from the antecubital vein following 12-h fasting. Serum homocysteine and vitamin B12 levels were measured with the chemiluminescent method by IMMULITE 2000 (Siemens Healthcare Diagnostics AG, Erlangen, Germany) using a SIEMENS homocysteine kit. Venous blood samples were drawn into BD Vacutainer Z tubes (2 mL) for analysis of folic acid levels. The analysis was performed in the hematology department using a HITACHI E170 modular device on the basis of Roche Diagnostic criteria. For TC, TG, high-density lipoprotein (HDL), and LDL analysis, following 12-h fasting, 5-mL venous blood samples were drawn into standard biochemical tubes. The analysis was performed in the biochemistry department with the enzymatic colorimetric method using a Roche modular device (Mainheim, Germany) on the basis of Roche Diagnostic criteria.
Statistical analysis
Data were statistically analyzed using SPSS 13.0 (SPSS Inc., Chicago, Illinois, USA). The Shapiro–Wilk test was performed to evaluate the consistency of variables with normal distribution. Variables with normal distribution were analyzed using the independent samples t-test and the results were expressed as mean±standard deviation (SD). The Mann–Whitney U test was performed for variables with non-normal distribution, and the results were expressed as median and percentile values (25%- 75%). The Pearson chi-square test was employed for cross-table analysis. To demonstrate the power and type (positive or negative) of the relationship between the variables, Spearman correlation analysis was performed for variables with non-normal distributions. A value of p<0.05 was considered statistically significant for all tests.
Results
A total of 79 normotensive children (40 females and 39 males) between the age of 5–20 years with hypertensive family member were included in the study, and 72 normotensive children (35 females and 37 males) between the age of 5-20 years with normotensive family members were included as controls. Anthropometric measurements, blood pressure, and lipid and homocysteine levels of the groups are shown in Table 1. No statistically significant difference was noted between the two groups in terms of age, gender, height, weight, BMI, and fasting lipid, folic acid, and vitamin B12 levels (p>0.05) (Table 1).
Table 1.
Comparison of the study and control groups on the basis of anthropometric measures, blood pressure, and echocardiographic variables
| Study group (n=79) | Control group (n=72) | P | |
|---|---|---|---|
| Age, years | 12.4±4.0 | 12.1±4.2 | 0.976 |
| Female/male | 40/39 | 35/37 | 0.804 |
| Weight | 47.5±16.7 | 43.0±5.4 | 0.641 |
| Height | 151.2±18.8 | 147.5±18.4 | 0.503 |
| Body mass index | 19.9±3.2 | 18.9±2.9 | 0.753 |
| SBP mm Hg | 109.6±9.2 | 106.4±7.9 | 0.023 |
| DBP mm Hg | 68.7±8.9 | 66.3±6.3 | 0.013 |
| Total cholesterol, mg/dL | 149 (88-196) | 152 (95-198) | 0.763 |
| Triglyceride, mg/dL | 76 (33-188) | 69 (39-152) | 0.486 |
| HDL, mg/dL | 52 (24-79) | 55 (23-101) | 0.687 |
| LDL, mg/dL | 86 (34-130) | 84 (43-158) | 0.535 |
| Folic acid, mg/dL | 8.7 (3.6-17.5) | 8.9 (3.5-17.21) | 0.766 |
| Vitamin B12, mg/dL | 288(141-806) | 303 (120-961) | 0.920 |
| tHcy, mg/dL | 9.5 (4.8-31.7) | 7.5 (4.4-13.4) | 0.002 |
DBP - diastolic blood pressure; HDL - high-density lipoprotein; LDL - low-density lipoprotein; SBP - systolic blood pressure; tHcy - total homocysteine Parameters with normal distribution are shown as mean±SD, parameters with nonnormal distribution are shown as median (25%-75%). Variables with normal distribution were analyzed using the independent samples t-test and the Mann-Whitney U test was performed for variables with non-normal distribution
When blood pressure was evaluated, the mean systolic blood pressure of children with family members with essential hypertension was 109.6±9.2 mm Hg, and the mean diastolic blood pressure was 68.7±8.3 mm Hg; for the control group, the mean systolic blood pressure was 106.4±7.9 mm Hg and the mean diastolic blood pressure was 66.3±6.3 mm Hg. Although the mean systolic and diastolic blood pressures were within the normal limits in both groups, they were significantly higher in children with a family history of hypertension than in controls (p<0.05) (Table 1). The mean homocysteine levels were 9.5 (4.8-31.7) μmol/L in children with hypertensive family members and 7.5 (4.4-13.4) μmol/L in controls, and the difference was statistically significant (p<0.01) (Table 1).
Evaluation of the systolic and diastolic blood pressures of the subjects according to age revealed no statistically significant differences between the study and control groups for children below the age of 10 years, although the blood pressures were significantly higher in children >10 years of age (p<0.001) (Table 2).
Table 2.
Comparison of blood pressure and homocysteine levels according to age groups
| Study group 5-9 years | Control group 5-9 years | Study group 10-14 years | Control group 10-14 years | Study group 15-19 years | Control group 15-19 years | |
|---|---|---|---|---|---|---|
| SBP mm Hg | 99.7±7.4 | 100.4±7.4 | 110.5±7.5 (p=0.013) | 106.8±6.4 (p=0.013) | 115.2±4.6 (p=0.02) | 112.0±5.6 (p=0.02) |
| DBF; mm Hg | 64.1±4.4 | 63.4±6.3 | 68.2±7.2 (p=0.011) | 64.4±5.8 (p=0.011) | 72.5±6.7 (p=0.02) | 69.8±6.5 (p=0.02) |
| tHcy, mg/dL | 7.0 (4.8-9.9) | 6.91 (4.7-9.6) | 9.8 (6.1-26.7) (p=0.002) | 7.5 (4.4-11.9) (p=0.002) | 12.0 (6.7-31.7) (p=0.003) | 9.5 (5.2-13.4) (p=0.003) |
DBP - diastolic blood pressure; SBP - systolic blood pressure; tHcy - total homocysteine
Homocysteine levels was higher than 15 μmol/L in 11 of 79 (13.92%) children with a family history of hypertension; of these, 3 (27.27%) were in the 10-14-year age group and 8 (72.72%) were in the 15-19-year age group. Homocysteine levels were not higher than 15 μmol/L in any of the children aged 5-9 years (Fig. 1, Table 2). Similarly, children in the control group had homocysteine levels lower than 15 μmol/L.
Figure 1.
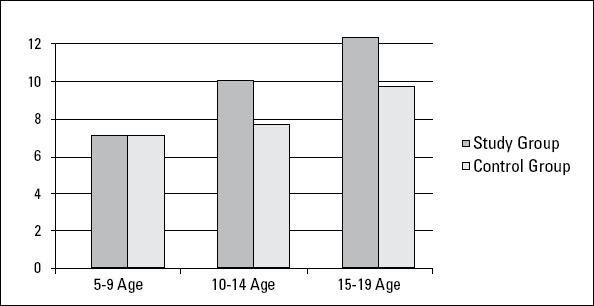
Comparison of the groups according to homocysteine levels
The Pearson chi-square test was employed for cross-table analysis.
p=0.768 betwenn under 10 years of age
p=0.002 betwenn 10-14 years of age
p=0.003 betwenn 15-19 years of age
There was a positive correlation between homocysteine levels and age, weight, and BMI (r=0.630, p<0.001; r=0.620, p<0.001; and r=0.556, p<0.001, respectively). Plasma homocysteine levels showed a positive correlation with systolic and diastolic blood pressures (r=0.458, p<0.001 and r=0.389 p<0.001, respectively), and they were negatively correlated with vitamin B12 and folic acid levels (r=-0.433, p<0.001 and r=-0.380, p=0.001, respectively). Although the plasma homocysteine levels of children with a family history of hypertension were positively correlated with TG levels (r=0.324, p<0.01), no significant correlation was noted with TC, HDL, and LDL levels (p>0.05).
Discussion
In the current study, homocysteine levels were significantly increased in children of hypertensive parents compared with the control group. When different age groups were analyzed, no significant difference was noted in homocysteine levels in the 5-9-year age group; however, the 10-14-year and 15-19-year age groups of children with a family history of hypertension had significantly higher homocysteine levels than the control group.
In the current study, homocysteine levels were significantly increased in children of hypertensive parents compared with the control group. When different age groups were analyzed, no significant difference was noted in homocysteine levels in the 5-9-year age group; however, the 10-14-year and 15-19-year age groups of children with a family history of hypertension had significantly higher homocysteine levels than the control group.
Hypertension is one of the most common cardiovascular diseases across the world. In previous studies, children of hypertensive parents were shown to have significantly higher blood pressure than children of normotensive parents as adolescents (6, 7). A study of children with hypertension by Robinson et al. (19) reported a 51% incidence of positive family histories, and Flynn et al. (5) reported an incidence of 86.2%. Longitudinal studies have revealed that blood pressure in childhood could be predictive of adult blood pressure (20). Prediction and prevention of adult hypertension before it develops may be possible by identifying children who can potentially develop hypertension (21).
Although systolic and diastolic blood pressures were within the normal limits in the subjects, the blood pressure of children with a family history of essential hypertension was significantly higher. When the subjects were reclassified according to age, no significant difference was found in the 5-9-year age group; however, both systolic and diastolic pressures were higher in subjects older than 10 years compared with controls. Xu et al. (22), Ravogli et al. (23), and Kucerova et al. (24) have demonstrated similar findings in young adults and Aglony et al. (6) have demonstrated similar findings in normotensive children with a family history of hypertension. The same investigators emphasized the significance of a family history of hypertension as an important risk factor. Ravogli et al. (23) showed that blood pressure was higher in subjects with a family hypertension than in those without a history. In the same study, echocardiographic evaluation showed that interventricular septum and posterior wall thicknesses were increased in young adults with hypertensive parents before an increase in blood pressure. They reported that a family history of hypertension is an important risk factor for the development of end-organ damage.
Sutton et al. (17) assessed 179 patients over the age of 60 years with isolated systolic hypertension and observed a significant relationship between systolic hypertension and plasma homocysteine levels. That finding was attributed to the damaging effect of homocysteine on the vascular endothelium by destruction of elastin. Joseph et al. (25) claimed that hyperhomocysteinemia can lead to diastolic pressure dysfunction. They attributed the mechanism to an increase in peripheral vascular collagen levels. Xiao et al. (26) performed a study of 1680 hypertensive subjects and showed an independent and strong association between total homocysteine levels and arterial stiffness. In another study, Zheng et al. (27) showed that left ventricular diastolic dysfunction is more common in hypertensive patients with hyperhomocysteinemia. Araki et al. (28) evaluated the relationship between plasma homocysteine levels and hypertension and demonstrated an increase in both systolic and diastolic blood pressures in subjects with high plasma total homocysteine levels.
Homocysteine is a non-essential sulfur-containing amino acid that is produced through demethylation of dietary methionine. Genetic metabolic disorders; chronic diseases (chronic renal failure, leukemia, diabetes, and hypothyroidism); deficiency of vitamin B6, B12, or folic acid; advanced age; and certain drugs may lead to the development of hyperhomocysteinemia (29, 30). Thus, patients with chronic conditions, malnutrition, and cigarette smoking and those using certain medications known to be associated with hyperhomocysteinemia were excluded from our study. In 11 children with above-normal blood homocysteine levels, blood folic acid and vitamin B12 levels were normal. In addition, blood folic acid, vitamin B12, and homocysteine levels were normal in the parents of these 11 children. In children with normal blood homocysteine levels, these tests were not performed in the family members. Further studies with a larger sample size examining homocysteine, vitamin B12, and folic acid levels in patients and family members may provide further insights.
A positive correlation was found between homocysteine levels and both age and blood pressure in children of hypertensive parents. Chang et al. (31) performed a randomized study in Taiwan and studied homocysteine, vitamin B12, and folic acid levels in 1234 children in the 12-15-year age group. Homocysteine levels were higher and folic acid and vitamin B12 levels were lower in males than in females, and an increase in homocysteine levels was observed with aging. The same study showed a positive correlation between homocysteine levels and BMI as well as a negative correlation between folic acid and vitamin B12 levels. In a study by Rauh et al. (32), 257 children in the 6-17-year age group and their parents were assessed. Homocysteine levels were higher than 15 μmol/L in 7% of the adults, and none of the children had homocysteine levels higher than 15 μmol/L. Males were demonstrated to have significantly higher homocysteine levels than females, and there were negative correlations between homocysteine levels and vitamin B12 and folic acid levels as well as positive correlations with BMI and serum creatinine levels. Our study also showed a positive correlation between plasma homocysteine levels and both body weight and BMI.
Vitamin B12 and folic acid levels are among the important factors that determine plasma homocysteine levels, and there is a negative correlation between plasma homocysteine levels and vitamin B12 and folic acid levels (33). In our study, vitamin B12 and folic acid levels were not significantly different between the patient group with a family history of hypertension and the control group. In the group with a family history of hypertension, there was a negative correlation between homocysteine levels and both folic acid and vitamin B12 levels. As the vitamin B12 and folic acid levels decreased, homocysteine levels increased.
Venn et al. (34) treated patients with homocysteine levels >10 μmol/L with folic acid for 4 weeks and demonstrated an increase in serum folic acid levels as well as a decrease in homocysteine levels compared with the control group. While the increase in folic acid levels correlated with the dose of folic acid, the decrease in homocysteine levels was similar and independent of the folic acid dose. Mayer et al. (35) noted a significant decrease in homocysteine levels when patients with a high coronary artery disease risk and homocysteine levels >20 μmol/L were treated with folic acid at a dose of 10 mg/day for 2 months.
In our study, homocysteine levels were significantly higher in children of hypertensive parents than in the control group; however, there were no significant differences in TC, TG, HDL, and LDL levels. No significant correlation was observed between homocysteine levels and TC, HDL, and LDL levels. There was a significant positive correlation between plasma homocysteine and TG levels in children of hypertensive parents.
Taqi et al. (36) performed a study in 60 adults who were over 35 years of age and showed that total plasma homocysteine, TC, TG, and LDL cholesterol levels were significantly elevated and HDL levels were significantly lower in the hypertensive group than in the control group. However, there was no significant difference in homocysteine levels between males and females in that study. Eren et al. (37) assessed homocysteine levels in 25 hypertensive normolipidemic, 25 hypertensive hyperlipidemic, and 21 control patients. Plasma homocysteine levels were significantly elevated in the hypertensive patients, and lipid levels did not affect homocysteine levels.
Knekt et al. (15) demonstrated a lack of correlation between homocysteine levels and cholesterol and TG levels, supporting homocysteine as an independent risk factor for coronary artery disease.
Study limitations
The present study has some limitations. One of these is the small number of participants. Second, we investigated only homocysteine levels and did not examine other cardiac risk factors that may play a major role in endothelial function and atherosclerosis. In addition, absence of blood folic acid, vitamin B12, and homocysteine measurements in the family members of children with normal homocysteine levels may be considered as a potential limitation. Further longitudinal studies involving larger patient series and family members with vitamin B12 and folic acid measurements are warranted. Therefore, additional prospective studies that include larger series with long-term followup evaluation are necessary to clarify homocysteine levels, hypertension, and cardiac risk factors, such as arterial stiffness, carotid intima-media thickness, or elevated C-reactive protein levels. Our study contributes to the literature because it is the first to investigate the changes in homocysteine levels in normotensive children of hypertensive parents.
Conclusion
Homocysteine levels are elevated in normotensive children of hypertensive parents before the development of hypertension. Children of hypertensive parents should be evaluated regularly with homocysteine levels prior to the development of hypertension. In the setting of increased homocysteine levels in normotensive children of hypertensive parents, diet and lifestyle modifications may be recommended in the absence of other cardiovascular risk factors. Further longitudianol studies are neccessary for evaluation this corelation.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - Z.K., B.U., A.Y.; Design - Z.K., B.U, F.K.; Supervision - B.U., A.Y., F.K.; Resource - Eskişehir Osmangazi University Scientific Research Commitee; Materials - P.K., G.Ö.; Data collection &/or processing - P.K., G.Ö.; Analysis &/or interpretation - F.K., A.Y., Ö.A.; Literature search - F.K., A.Y.; Writing -F.K., A.Y.; Critical review - Z.K., A.Y., G.Ö., P.K.
References
- 1.Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension:a systematic review. J Hypertens. 2004;22:11–9. doi: 10.1097/00004872-200401000-00003. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 2.Collins R, Peto R, Macmahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Short term reduction in blood pressure. Overview of randomized drug trials in their epidemiologic context. The Lancet. 1990;335:827–38. doi: 10.1016/0140-6736(90)90944-z. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Luma GB, Spiotta RT. Hypertension in children and adolescents. Am Fam Physician. 2006;73:1158–68. [PubMed] [Google Scholar]
- 4.Coy V. Genetics of essential hypertension. J Am Acad Nurse Pract. 2005;17:219–24. doi: 10.1111/j.1041-2972.2005.00036.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 5.Flynn JT, Alderman MH. Characteristics of children with primary hypertension seen at a referral center. Pediatr Nephrol. 2005;20:961–6. doi: 10.1007/s00467-005-1855-3. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 6.Aglony IM, Arnaiz GP, Acevedo BM, Barja YS, Marquez US, Guzman AB, et al. Blood pressure and family history of hypertension in children from Santiago, Chile. Rev Med Chil. 2009;137:39–45. [PubMed] [Google Scholar]
- 7.Staessen JA, Wang J, Bianchi G. Essential Hypertension. Lancet. 2003;261:1629–41. doi: 10.1016/S0140-6736(03)13302-8. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Ingrid S, Ding Y, Liu Y, Qi JG, Tang CS, et al. Imbalance of endogenous homocysteine and hydrogen sulfide metabolic pathway in essential hypertensive children. Chin Med J. 2007;120:389–93. [PubMed] [Google Scholar]
- 9.Sato Y, Kaji M, Kondo M, Yoshida H, Satoh K, Metoki N. Hyperhomocysteinemia in Japanese patients with convalescent stage ischemic stroke:Effect of combined therapy with folic acid and mecobalamine. J Neur Sci. 2002;202:65–8. doi: 10.1016/s0022-510x(02)00210-1. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Ubhink JB, Delport R, Vermaak WJ. Plasma homocysteine concentrations in a population with a low coronary heart disease prevalence. J Nutr. 1996;126:1254–7. doi: 10.1093/jn/126.suppl_4.1254S. [DOI] [PubMed] [Google Scholar]
- 11.Yan H, Du JB, Tang CS. The regulatory role of the gasotransmitter hydrogen sulfide upon the aortic structural remodeling in spontaneous hypertensive rats. J Peking Univ. 2004;36:234–7. [PubMed] [Google Scholar]
- 12.Kahleova R, Palyzova D, Zvara K, Zvarova J, Hrach K, Novakova I. Essential hypertension in adolescents:Association with insulin resistance and with metabolism of homocysteine and vitamins. Am J Hypertens. 2002;15:857–64. doi: 10.1016/s0895-7061(02)02984-9. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.Sun XN, Li YM, Guo H. An approach on correlated factors of genetic polymorphism of the key enzyme in metabolism of homo- cysteine in patients with simple systolic hypertension. Chin Cardiovasc Dis J. 2003;31:269–73. [Google Scholar]
- 14.Norlund L, Grubb A, Fex G, Leksell H, Nilsson JE, Schenck H, et al. The increase of plasma homocysteine concentrations with age is partly due to the deterioration of renal functions as determined by plasma cystatin C. Clin Chem Lab Med. 1998;36:175–8. doi: 10.1515/CCLM.1998.032. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Knekt P, Reunanen A, Alfthan G, Heliövaara M, Rissanen H, Marniemi J, et al. Hyperhomocysteinemia;A risk factor or consequence of coronary heart disease. Arch Inter Med. 2001;161:1589–94. doi: 10.1001/archinte.161.13.1589. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 16.Oparil S, Zaman A, Calhoun DA. Pathogenesis of Hypertension. Physiology in Medicine. 2003;139:761–76. doi: 10.7326/0003-4819-139-9-200311040-00011. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Sutton K, Bostom A, Selhub J, Zeigler-Johnson C. High homocysteine levels are independently related to isolated systolic hypertension in older adults. Circulation. 1997;96:1745–9. doi: 10.1161/01.cir.96.6.1745. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Fakhrzadeh H, Ghotbi S, Pourebrahim R, Heshmat R, Nouri M, Shafaee A, et al. Plasma homocysteine concentration and blood pressure in healthy Iranian adults:the Tehran Homocysteine Survey (2003-2004) J Hum Hypertens. 2005;19:869–76. doi: 10.1038/sj.jhh.1001911. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 19.Robinson RF, Batisky DL, Hayes JR, Nahata MC, Mahan JD. Body mass index in primary and secondary pediatric hypertension. Pediatr Nephrol. 2004;19:1379–84. doi: 10.1007/s00467-004-1588-8. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Reid C, Chantler C. Systemic hypertension. In: Anderson EJ, Macertney FJ, Rigby ML, Shinebourne EA, Tynon M, editors. Pediatric Cardiology. 2th ed. London: 2002. pp. 1809–24. [Google Scholar]
- 21.Urbina EM, Srinivasan SR, Berenson GS. Epidemiology of essential Hypertension in children. In: Portman RJ, Sorof JM, Ingelfinger JR, editors. Pediatric Hypertension. Totowa, New Jersey: Riverview Brive; 2004. pp. 121–41. [Google Scholar]
- 22.Xu L, Wang S, Wang YX, Jonas JB. Prevelance of arterial hypertension in the adult population in rural and urban China:the Beijing eye study. Am J Hypertens. 2008;21:1117–23. doi: 10.1038/ajh.2008.247. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Ravogli A, Trazzi S, Villani A, Mutti E, Cuspidi C, Sampieri L, et al. Early 24-Hour Blood Pressure Elevation in Normotensive Subjects With Parental Hypertension. Hypertension. 1990;16:491–7. doi: 10.1161/01.hyp.16.5.491. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.Kucerova J, Filipovsky J, Staessen JA, Cwynar M, Wojciechowska W, Stolarz K, et al. Arteial characteristics in normotensive offspring of parents with or without a history of hypertension. Am J Hypertens. 2006;19:264–9. doi: 10.1016/j.amjhyper.2005.09.015. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 25.Joseph J, Washington A, Joseph L, Koehler L, Fink LM, Hauer-Jensen M, et al. Hyperhomocysteinemia leads to adverse cardiac remodeling in hypertensive rats. Am J Physiol Heart Circ Physiol. 2002;283:H2567–74. doi: 10.1152/ajpheart.00475.2002. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 26.Xiao W, Bai Y, Ye P, Luo L, Liu D, Wu H, et al. Plasma homocysteine is associated with aortic arterial stiffness but not wave reflection in Chinese hypertensive subjects. PLoS One. 2014;27:9. doi: 10.1371/journal.pone.0085938. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng H, Li Y, Xie N, Xu H, Huang J, Luo M. Echocardiographic assessment of hypertensive patients with or without hyperhomo- cysteinemia. Clin Exp Hypertens. 2014;36:181–6. doi: 10.3109/10641963.2013.804542. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 28.Araki A, Sako Y, Fukushima Y, Matsumo M, Asada T, Kita T. Plasma sulfydryl-containing amino acids in patients with cerebral infarction and in hypertensive subjects. Aterosclerosis. 1989;79:139–46. doi: 10.1016/0021-9150(89)90118-4. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 29.Norlund L, Grubb A, Fex G, Leksell H, Nilsson JE, Schenck H, et al. The increase of plasma homocysteine concentrations with age is partly due to the deterioration of renal functions as determined by plasma cystatin C. Clin Chem Lab Med. 1998;36:175–8. doi: 10.1515/CCLM.1998.032. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 30.Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr. 1994;60:2–11. doi: 10.1093/ajcn/60.1.2. [DOI] [PubMed] [Google Scholar]
- 31.Chang JB, Chu NF, Shen MH, Wu DM, Liang YH, Shieh SM. Determinants and distributions of plasma total homocysteine concentrations among school children in Taiwan. Eur J Epidemiol. 2003;18:33–8. doi: 10.1023/a:1022504602101. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 32.Rauh M, Verwied S, Knerr I, Dörr HG, Sönnichsen A, Koletzko B. Homocysteine concentrations in a German chort of 500 individuals:reference ranges and determinants of plasma levels in healthy children and their parents. Amino Acids. 2001;20:409–18. doi: 10.1007/s007260170037. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 33.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr. 1993;57:182–9. doi: 10.1093/ajcn/57.2.182. [DOI] [PubMed] [Google Scholar]
- 34.Venn BJ, Mann JI, Williams SM, Riddell LJ, Chisholm A, Harper MJ, et al. Assesment of three levels of folic acid on serum folate and plasma homocysteine:a randomised placebo-controlled doubleblind dietary intervention trial. Eur J Clin Nutr. 2002;56:748–54. doi: 10.1038/sj.ejcn.1601388. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 35.Mayer O Jr, Simon J, Rosolova H, Hromádka M, Subrt I, Vobrubová I. The effects of folate supplementation on some coagulation parameters and oxidative status surrogates. Eur J Clin Pharmacol. 2002;58:1–5. doi: 10.1007/s00228-001-0421-6. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 36.Al-Baldawi AT. Evaluation of Amino acid Homocysteine in Hypertensive Patients. The Iraqi Postgraduate Medical Journal. 2006;5:151–4. [Google Scholar]
- 37.Eren ŞH, Yılmaz AK, Aktaş C, Korkmaz I, Güven FMK. Plazma homocysteine levels in hypertensive patients and it correlation with low density lipoprotein cholesterol. JAEM. 2008;7:30–3. [Google Scholar]


