Abstract
Objective:
Digitalis preparations are commonly used by children and adults with heart diseases worldwide, although excessive doses may cause cardiac effects. The aim of the study is to evaluate the antiarrhythmic effect of Crataegus oxyacantha extract on digoxin-induced arrhythmias in anesthetized Wistar rats.
Methods:
Control and experimental groups were evaluated for arrhythmias induced by digoxin. Fifteen rats (7 as controls and 8 as the experimental group) were included in the study. The dry fruits of 100 mg Crataegus oxyacantha were extracted by percolation method. Digoxin, at a dose of 40 μg/kg/min, was infused to form the arrhythmias in all rats. Simultaneously, the extract was infused into the experimental group, while 0.9% NaCl was infused into control group. Electrocardiographic QRS prolongation and arterial blood pressure changes were analyzed.
Results:
The experimental group lived longer (62.13±2.20 min) than the controls (p=0.002). On the other hand, the time to beginning of QRS prolongation did not differ between the two groups (p=0.812). Bradycardia was significant in the control group (288.01±10.54 beat/min and p=0.01). The maximum QRS duration was observed in the control group during the digoxin and 0.9% NaCl infusion period (53.29±3.99 ms and p=0.001). Also, the durations of atrial and ventricular arrhythmias were shorter in the experimental group. However, arterial blood pressure dipping was significant in the experimental group (23.67±10.89 mm Hg and p<0.001).
Conclusion:
Crataegus oxyacantha alcoholic extract produced an antiarrhythmic effect that was induced by digoxin in Wistar rats. However, in the clinical use of this extract, the hypotensive effect should be considered. Also, the alcoholic extract of Crataegus oxyacantha may be an alternative treatment medication for arrhythmias induced by digoxin toxicity in humans.
Keywords: crataegus oxyacantha, digoxin, arrhythmia, antiarrhythmic effect, hypotension
Introduction
Digitalis preparations are widely prescribed for children and adults with heart diseases worldwide (1, 2), although these preparations are potent medications and excessive doses may cause cardiac, gastrointestinal, neurologic, and metabolic effects. Today, digitalis toxicity continues to be a problem for pediatric patients undergoing therapy with cardiac glycosides for heart failure or arrhythmias, as well as in accidental ingestions. There are several reports presenting acute or chronic digitalis intoxications in children in the literature (3, 4). Deaths from digitalis toxicity are usually because of the lethal rhythm disturbances. Additionally, these cardiac arrhythmias significantly affect heart function, posing a risk to the patient’s life if not suitably treated. Thus, different antiarrhythmic medications and digoxin-specific antibody Fab fragments are commonly used in the treatment. Also, digoxin-specific antibody Fab fragments are effective in ameliorating the signs of digitalis poisoning in children. Not only can Fab fragments rapidly eradicate potentially life-threatening arrhythmias and conduction defects, they are also effective in treating hyperkalemia and other noncardiac manifestations of digitalis toxicity (5). However, alternative and effective medications should be evaluated.
Several species of the genus Crataegus have been reported to possess a wide range of pharmacological actions (6-12). Preparations of Crataegus are used in minor forms of coronary heart disease, heart failure, and cardiac arrhythmia. Crataegus has antioxidant (13, 14), positive inotropic (15), anti-inflammatory (16, 17), anti-cardiac remodeling (18), antiplatelet aggregation (19, 20), vasodilating (21-23), endothelial protective (24), smooth muscle cell migration and proliferation-reducing (25), ischemia/reperfusion injury protective (26, 27), lipid-lowering (28, 29), arterial blood pressure-decreasing (30, 31), and antiarrhythmic effects (8-10). Although some species of Crataegus have been shown to prevent cardiac arrhythmias induced by aconitine, calcium chloride, adrenaline (32), and the ischemic process (10), its antiarrhythmic effect on digoxin-induced arrhythmias has not yet been demonstrated. The aim of this study is to evaluate the antiarrhythmic effect of Crataegus oxyacantha extract on digoxin-induced arrhythmias in Wistar rats.
Methods
Study design
A total of 15 rats (7 as controls and 8 as the experimental group) were included in the study. Wistar rats were taken from Kombassan Experimental Medicine Research and Application Center.
Extract preparation
The dry fruits of 100 mg Crataegus oxyacantha were extracted (1:10) with 100% ethanol at 25°C for 3 hours, the particle size was 2-3 mm, the production method was percolation (32), and the flow speed of the extract was 0.5 mL/min (32). The suspension was centrifuged at 9000 rpm for 20 min. The supernatant was filtered through 0.20-μm pores. After the filtration, the extract was concentrated under reduced pressure to remove the ethanol. The dried substance was dissolved in 0.9% NaCl with pH 7.4 to form an extract of 5 mg/mL.
Surgical procedure
A total of 15 rats (7 as controls and 8 as the experimental group) were included in the study. Male Wistar rats (280-300 g) were anesthetized with 29 mg/kg xylazine 2% (Rompun, Bayer, MISSISSAUGA, Canada) and 39 mg/kg ketamine (Ketalar, 50 mg/mL, (Ketalar, 50 mg/mL, Pfizer, İstanbul, Turkey). The systemic arterial blood pressure was recorded from a catheter inserted into the left carotid artery. The right and left jugular veins were cannulated for administration of anesthetic or extract solutions.
Digoxin-induced arrhythmias
Before the beginning of the study, 6 rats were used to determine the arrhythmic dose of digoxin infusion. The intravenous form of digoxin (0.50 mg/2 mL, Novartis) was used. Digoxin was infused from the left jugular vein of the rats, and the starting dose was 5 μg/kg/min during 60 min. If no arrhythmic effect (QRS prolongation, atrial and ventricular arrhythmias) was observed, the dose was increased gradually up to 40 μg/kg/min (33-35). Finally, the arrhythmic effect (QT prolongation) was stored at an infusion dose of 40 μg/kg/min after about 10 min. Multiple arrhythmias, such as supraventricular arrhythmias, ventricular tachycardia, and multiple premature ventricular extrasystole, were observed in different rats during the first step of the study. To standardize the arrhythmias in all rats, QRS prolongation, the duration of premature atrial contractions, ventricular extrasystole, ventricular tachycardia, and ventricular fibrillation were measured in all rats.
Extract administration
The rats were randomly divided into 2 groups: 7 rats for controls and 8 rats for extract infusion (experimental group). After stabilization of the rats (30 min), digoxin infusion was started into all rats at a dose of 40 μg/kg/min into the left jugular vein. Also, simultaneously, 0.9% NaCl infusion was started at a dose of 1 ml/kg/min in the control group, while Crataegus oxyacantha extract was infused into the right jugular vein at a dose of 4 mg/kg/min in the experimental group (9, 10). The drug infusions (digoxin, 0.9% NaCl, Crataegus oxyacantha extract) were continued for 60 min.
Data measurements and arrhythmias analysis
The standard extremity electrocardiography leads were placed into four extremities near the thoracic and abdominal regions, where the electrocardiography waves were recorded best. Electrocardiograms were monitored (MP36, BIOPAC Systems, Inc., California, USA) continuously during the experiment. The heart rate was calculated from the electrocardiographic records. Ventricular arrhythmias and QT prolongation were analyzed according to the guidelines of the Lambeth Conventions for the determination of experimental arrhythmias (36). Also, arterial blood pressure was continuously recorded by a transducer (TSD104A, BIOPAC Systems, Inc., California, USA) from a catheter that was inserted into the left carotid artery. All data were analyzed by a computer program (AcqKnowledge BIOPAC Systems, Inc., California, USA).
Statistical analysis
All statistical analyses were performed by SPSS for Windows, version 16.0 (SPSS Inc, Chicago, Illinois, USA). All results were given as mean±SD. Normal assumptions were assessed before using parametric tests. Wilcoxon test was used to compare the QT prolongation time and time to death in rats. Also, Kruskal-Wallis and ANOVA tests were used for other parametric measurements and intergroups, respectively. A value of p<0.05 was used to indicate statistical significance.
Results
After the stabilization period (heart rate and arterial blood pressures were stabilized in 30 min.) was completed, digoxin infusion was started in the two groups. Digoxin infusion in anesthetized rats (all control and experimental groups) resulted in QRS prolongation, bradycardia, and asystole. Table 1 summarizes the effects of Crataegus oxyacantha extract on electrocardiography. Bradycardia started at 21.03±1.67 min. and 27.12±2.61 min. in the control and experimental groups, respectively. The experimental group lived longer (62.13±2.20 min) than the controls (49.86±2.34 min), and the statistical difference was significant (p=0.002) (Fig. 1). Also, only one rat was alive in the experiment group after digoxin infusion. On the other hand, the time to beginning of QRS prolongation did not differ between the two groups (p=0.812) (Fig. 2). Although the heart rate was similar between the two groups during the stabilization period, it decreased gradually in all rats by digoxin infusion. However, bradycardia was significant in the control group (288.01±10.54 beat/min and p=0.01). Also, heart rate decreased in the experimental group during the digoxin and extract infusion periods (306.31±12.79 beat/min and p=0.01). The maximum QRS duration was observed in the control group during the digoxin and 0.9% NaCl infusion period (53.29±3.99 ms vs. 43.25±1.98 and p=0.001). Additionally, QRS duration was longer during the digoxin and extract infusion period than in the stabilization period in the experimental group (43.25±1.98 ms and p=0.001) (Fig. 3).
Table 1.
Comparison of the data measured from control and experimental groups
| Control group (n: 7) | Experimental group (n: 8) | P | |||
|---|---|---|---|---|---|
| Survival, minutes | 49.86±2.34 | 62.13+2.20 | 0.002 | ||
| Time to beginning of QRS prolongation, minutes | 28.01±1.99 | 29.94+2.47 | 0.812 | ||
| Stabilization period | Digoxin with 0.9% NaCl infusion | Stabilization period | Digoxin with extract infusion | ANOVA | |
| Heart rate, beats/minute | 366.41±10.28 | 288.01+10.54* | 345.51+9.41 | 306.31+12.79** | 0.01 |
| Maximum QRS duration, milliseconds | 32.71±1.34 | 53.29+3.99* | 31.38+0.90 | 43.25+1.98** | 0.001 |
| Blood pressure, mm Hg | 125.12+11.54 | 45.34+12.45* | 123.09+14.05 | 23.67+10.89** | <0.001 |
Digoxin with 0.9% NaCl infusion vs. all groups;
Digoxin with extract infusion vs. all groups
Figure 1.
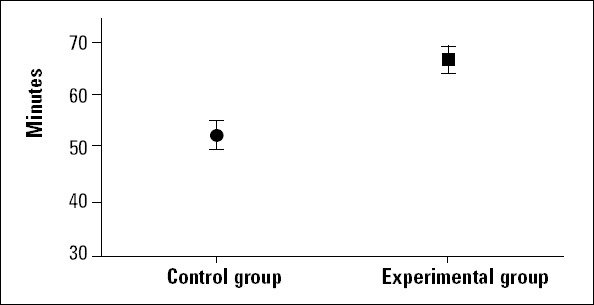
The survival time of rats in the control and experimental groups
Figure 2.
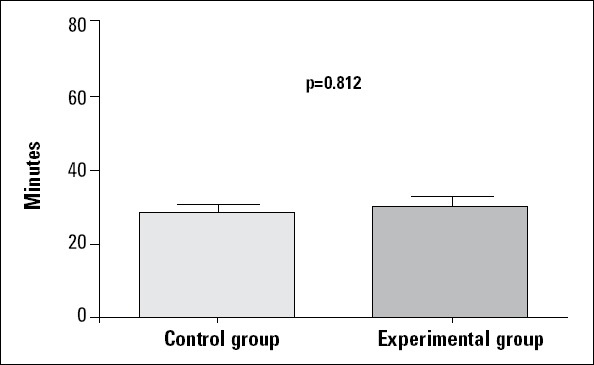
Diagram of time to beginning of QRS prolongation in the control and experimental groups Kruskal-Wallis test
Figure 3.
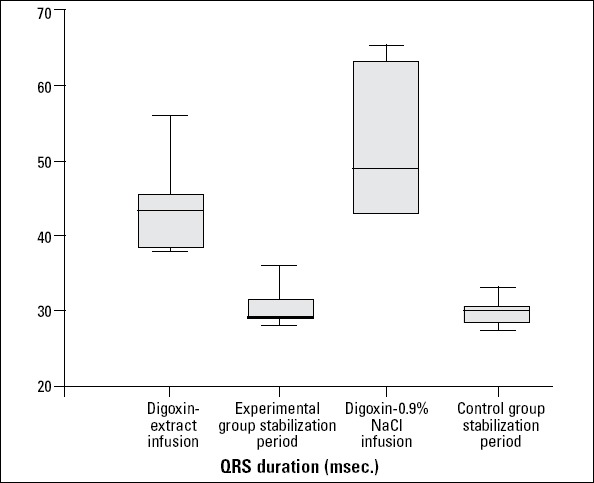
The durations of QRS prolongations in the study population during the experimental period
In the experimental group, the durations of premature atrial contractions (6.23±2.53 min), ventricular extrasystole (29.25±1.98 min), ventricular tachycardia (39.19±8.02 min), and ventricular fibrillation (11.94±4.46 min) were significantly shorter than in the control group (Table 2).
Table 2.
Effects of ethanol extract of Crataegus oxyacantha on digoxin-induced arrhythmias in study groups
| Arrhythmia type | Duration of arrhythmia, minutes | ||
|---|---|---|---|
| Control group (n: 7) Digoxin with 0.9% NaCl infusion | Experimental group (n: 8) Digoxin with extract infusion | P | |
| Premature atrial contractions | 13.45±5.21* | 6.23±2.53 | <0.001 |
| Ventricular extrasystole | 48.56±7.19* | 29.25±1.98 | <0.001 |
| Ventricular tachycardia | 41.25±7.03* | 39.19±8.02 | <0.001 |
| Ventricular fibrillation | 23.12±6.71* | 11.94±4.46 | <0.001 |
Arterial blood pressures decreased gradually in all rats. However, blood pressure dipping was significant in the experimental group (23.67±10.89 mm Hg and p<0.001). Additionally, arterial blood pressure decreased in the control group during the digoxin and extract infusion period (45.34±12.45 mm Hg and p<0.001) (Fig. 4).
Figure 4.
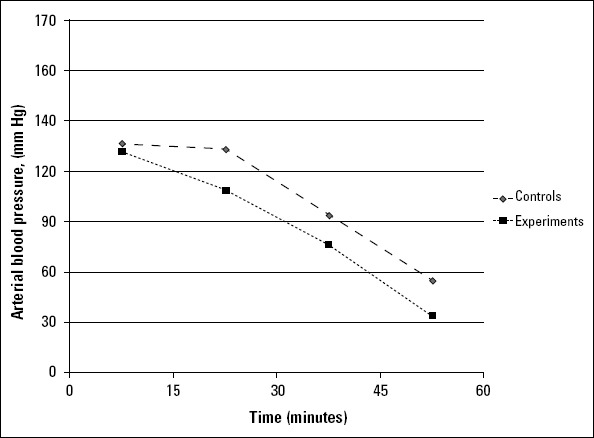
Arterial blood pressure variations in the study population during the experimental period
Discussion
In the present study, the alcoholic extract of Crataegus oxyacantha produced a protective effect against digoxin-induced arrhythmias. In our study, we used QRS prolongation and durations of premature atrial contractions, ventricular extrasystole, ventricular tachycardia, and ventricular fibrillation as predictive markers of arrhythmias because of the observation of multiple arrhythmia types in different rats. Also, our study demonstrated that these atrial and ventricular arrhythmias were decreased in the experimental group, in which the ethanol extract of Crataegus oxyacantha was infused with digoxin. The studies that evaluated digoxin-induced cardiac toxicity in animal models revealed that multiple ventricular and supraventricular arrhythmias were observed during the study periods (33-35). However, QRS prolongation starts earlier than these cardiac arrhythmias. Animal studies showed that digitalis-induced Na+ accumulation results in an increase in Ca2+, via the Na+/Ca2+ exchanger, leading to enhanced sarcoplasmic reticulum Ca2+ load, responsible for the positive inotropic and toxic arrhythmogenic effects of glycosides (37). Also, a recent study of Gonano et al. (38) demonstrated that active calcium-calmodulin kinase II mediates ouabain-induced arrhythmic and toxic effects by phosphorylation of the ryanodine receptor, resulting in Ca2+ leakage from the sarcoplasmic reticulum. The exact mechanism underlying the antiarrhythmic effect of Hawthorn species remains unclear. However, in a study, it was shown that Crataegus extract prolonged the action potential duration and delayed the recovery of Vmax (39). On the other hand, concerns have been raised regarding the blocking of repolarizing potassium currents in ventricular myocytes, which is similar to the action of class III antiarrhythmic drugs (40). The extract of different Hawthorn species may affect these pathways to produce their antiarrhythmic effects. Also, it was found that especially saponin and flavonoid fractions from different Hawthorn species produce a decrease in arterial blood pressure and an antiarrhythmic effect (32, 37).
Hawthorn (Crataegus species) plant extracts and medications have been recognized internationally as source of medicine worldwide. The source material contains a range of pharmacologically active substances, including flavonoids, triterpenic acids, and phenol carboxylic acids (32, 37). Despite a number of preclinical and clinical studies, the mechanisms of the protective effects of Hawthorn extracts remain poorly understood (6-12). Moreover, there are limited findings on its action in protecting arrhythmias in animal studies (8-10).
The preliminary study of Thompson et al. (9) revealed that extract of the bark and leaves of Crataegus monogyna possessed prophylactic antiarrhythmic activity in rabbits. Also, they used aconitine to induce the arrhythmias in their study. Similarly, Garjani et al. showed that the extract of Crataegus meyeri has a hypotensive and potential antiarrhythmic effect on ischemic myocardium in rats (10). They included hydroalcohol, chloroform, and ethylacetate extracts in the study. Also, the study revealed that each form of extract had different reducing effects on ventricular arrhythmias (10). On the other hand, a recent study of Salehi et al. (8) revealed that Crataegus monogyna extract decreased the contraction frequency of neonatal murine cardiomyocytes via muscarinic receptor activation. So, this finding suggests the atropine-sensitive activity of Hawthorn extracts. Additionally, Swaminattan et al. (12) reported that Crataegus oxyacantha extract reduced oxidative stress in reperfused myocardium, and it played a significant role in the inhibition of apoptotic pathways, leading to cardioprotection.
Koçyıldız et al. (30) showed that the hyperoside fraction of Crataegus prevented L-NAME-induced hypertension in rats and had beneficial effects on the cardiovascular system. Crataegus, administered at escalating doses, produced a dose- and time-dependent decrease in heart rate and mean arterial pressure. Also, higher doses produced the most significant reduction in both heart rate and mean arterial pressures. Shatoor et al. (31) suggested that the underlying mech anism that leads to hypotension appeared to be related to direct stimulation of the muscarinic receptor M2 and possible blockade of beta-receptors. In our study, in the experimental group, maximal arterial blood dipping was observed. This may be due to the total effect of digoxin toxicity and the effect of Crataegus oxyacantha extract.
Study limitations
We did not use other extracts of Crataegus oxyacantha, such as hydroalcohol or chloroform, for analysis in the study. Also, a standard infusion dose (40 mcg/kg/min) was used for digoxin; it was not increased. Additionally, a small number of selected groups of rats were used in the study.
Conclusion
Finally, as demonstrated in the present study, Crataegus oxyacantha alcoholic extract produced antiarrhythmic effects against arrhythmia induced by digoxin in Wistar rats. However, in the clinical use of this extract, the hypotensive effect should be considered. Alcoholic extract of Crataegus oxyacantha may be an alternative treatment medication for arrhythmias induced by digoxin toxicity in humans. So, further studies are needed for this suggestion.
Footnotes
Conflict of interest: None declared.
Peer-review: Partially external peer-reviewed.
Ethical Standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals (rats) and has been approved by the Institutional Committee. Also, the study was supported by Necmettin Erbakan University Scientific Research Projects.
Authorship contributions: Concept - H.A.; Design - H.A., B.C.S.; Supervision - H.A., B.C.S.; Resource - B.C.S., A.S.Ş.; Materials - H.A., B.C.S.; Data collection &/or processing - H.A., B.C.S.; Analysis &/or interpretation - H.A.; Literature search - H.A., T.B.; Writing - H.A., B.C.S.; Critical review - T.B., A.S.Ş.
References
- 1.Smith TW. Should digoxin be the drug of first choice after diuretics in chronic heart failure? II. Protagonist’s viewpoint. J Am Coll Cardiol. 1988;12:267–71. doi: 10.1016/0735-1097(88)90385-3. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 2.Doherty JE. Clinical use of digitalis glycosides:an update. Cardiology. 1985;72:225–54. doi: 10.1159/000173883. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Flowler RS, Rathi L, Keith JD. Accidental digitalis intoxication in children. J Pediatr. 1964;64:188–200. doi: 10.1016/s0022-3476(64)80262-6. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Aarnoudse AL, Dieleman JP, Stricker BH. Age- and gender-specific incidence of hospitalisation for digoxin intoxication. Drug Saf. 2007;30:431–6. doi: 10.2165/00002018-200730050-00006. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 5.Woolf AD, Wenger TL, Smith TW, Lovejoy FH., Jr Results of multicenter studies of digoxin-specific antibody fragments in managing digitalis intoxication in the pediatric population. Am J Emerg Med. 1991;9:16–20. doi: 10.1016/0735-6757(91)90162-d. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Xiong X, Feng B. Effect of Crataegus usage in cardiovascular disease prevention:an evidence-based approch. Evid Based Complement Alternat Med. 2013;2013:1–16. doi: 10.1155/2013/149363. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barros L, Carvalho AM, Ferreira IC. Comparing the composition and bioactivity of Crataegus Monogyna flowers and fruits used in folk medicine. Phytochem Anal. 2011;22:181–8. doi: 10.1002/pca.1267. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Salehi S, Long SR, Proteau PJ, Filtz TM. Hawthorn (Crataegaus monogyna jacq.) extract exhibits atropine-sensitive activity in a cultured cardiomyocyte assay. J Nat Med. 2009;63:1–8. doi: 10.1007/s11418-008-0278-4. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 9.Thompson EB, Aynilian GH, Gora P, Farnworth NR. Preliminary study of potential antiarrhythmic effects of Crataegus monogyna. J Pharm Sci. 1974;63:1936–7. doi: 10.1002/jps.2600631222. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Garjani A, Nazemiyeh H, Maleki N, Valizadeh H. Effect of extraxt from flowering tops of Crataegus meyeri A. Pojark. On isch- aemic arrhythmias in anaesthetized rats. Phytother Res. 2000;14:428–31. doi: 10.1002/1099-1573(200009)14:6<428::aid-ptr618>3.0.co;2-l. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.Chang Q, Zou Z, Ho WK, Chow MS. Comparison of the pharmacokinetics of Hawthorn phenolics in extract versus individual pure compound. J Clin Pharmacol. 2005;45:106–12. doi: 10.1177/0091270004270500. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.Swaminattan JK, Khan M, Mohan IK, Selvendiran K, Niranjali Devaraj S, et al. Cardioprotective properties of Crataegus oxycan- tha extract against ischemia-reperfusion injury. Pyhotomedicine. 2010;17:744–52. doi: 10.1016/j.phymed.2010.01.009. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayalakshmi R, Niranjali Devaraj S. Cardioprotective effect of tincture of Crataegus on isoproterenol-induced myocardial infarction in rats. J Pharm Pharmacol. 2004;56:921–6. doi: 10.1211/0022357023745. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.Jayalakshmi R, Thirupurasundari CJ, Devaraj SN. Pretreatment with alcoholic extract of shape Crataegus oxyacantha (AEC) activates mitochondrial protection during isoproterenol-induced myo- cardial infarction in rats. Mol Cell Biochem. 2006;292:59–67. doi: 10.1007/s11010-006-9218-3. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez ME, Poindexter BJ, Bick RJ, Dasgupta A. A comparison of the effects of commercially available hawthorn preparations on calcium transients of isolated cardiomyocytes. J Med Food. 2008;11:680–6. doi: 10.1089/jmf.2008.0080. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 16.Li C, Wang MH. Anti-inflammatory effect of the water fraction from hawthorn fruit on LPS-stimulated RAW 264.7 cells. Nut Res Pract. 2011;5:101–6. doi: 10.4162/nrp.2011.5.2.101. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vijayan NA, Thiruchenduran M, Devaraj SN. Anti- inflammatory and anti-apoptotic effects of Crataegus oxyacan- tha on isoproter- enol-induced myocardial damage. Mol Cell Biochem. 2012;367:1–8. doi: 10.1007/s11010-012-1251-9. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Hwang HS, Bleske BE, Ghannam MJ, Converso K, Russell MW, Hunter JC, et al. Effects of hawthorn on cardiac remodeling and left ventricular dys- function after 1 month of pressure overload- induced cardiac hypertrophy in rats. Cardiovasc Drugs Ther. 2008;22:19–28. doi: 10.1007/s10557-008-6082-2. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 19.Mayer TO, Biller J. Antiplatelet prescribing patterns for TIA and ischemic stroke:the Indiana University experience. J Neurol Sci. 2003;207:5–10. doi: 10.1016/s0022-510x(02)00348-9. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Shatoor AS, Soliman H, Al-Hashem F, Gamal BE, Othman A, El-Menshawy N. Effect of hawthorn (Crataegus aronia syn. Azarolus (L)) on platelet function in albino Wistar rats. Thrombos Res. 2012;130:75–80. doi: 10.1016/j.thromres.2012.01.001. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 21.Busse R, Edwards G, Fèlètou M, Fleming I, Vanhoutte PM, Weston AH. EDHF:bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–80. doi: 10.1016/s0165-6147(02)02050-3. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 22.Topal G, Koç E, Karaca C, Altuğ T, Ergin B, Demirci C, et al. Effects of Crataegus microphylla on vascular dysfunction in streptozoto- cin-induced diabetic rats. Phytother Res. 2013;27:330–7. doi: 10.1002/ptr.4726. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Rieckeheer E, Schwinger RHG, Bloch W, Brixius K. Hawthorn special extract WS 1442 increases red blood cell NO-formation without altering red blood cell deformability. Phytomedicine. 2011;19:20–4. doi: 10.1016/j.phymed.2011.08.059. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.Elisabeth AW, Roland M, Alexander IB. The vascular barrier-protecting hawthorn extract WS 1442 raises endothelial calcium levels by inhibition of SERCA and activation of the IP 3 pathway. J Mol Cell Cardiol. 2012;53:567–77. doi: 10.1016/j.yjmcc.2012.07.002. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 25.Furst S, Zirrgiebel U, Totzke F, Zahler S, Vollmar AM, Koch E. The Crataegus extract WS1442 inhibits balloon catheter induced inti- mal hyperplasia in the rat carotid artery by directly influencing PDGFR-ß. Atherosclerosis. 2010;211:409–17. doi: 10.1016/j.atherosclerosis.2010.04.003. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 26.Veveris M, Koch E, Chatterjee SS. Crataegus special extract WS 1442 improves cardiac function and reduces infarct size in a rat model of prolonged coronary ischemia and reperfusion. Life Sci. 2004;74:1945–55. doi: 10.1016/j.lfs.2003.09.050. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 27.Al Makdessi S, Sweidan H, Dietz K, Jacob R. Protective effect of Crataegus oxyacantha against reperfusion arrhythmias after global no-flow ischemia in the rat heart. Basic Res Cardiol. 1999;94:71–7. doi: 10.1007/s003950050128. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 28.Fan C, Yan J, Qian Y, Wo X, Gao L. Regulation of lipoprotein lipase expression by effect of hawthorn flavonoids on peroxisome prolif- erator response element pathway. J Pharmacol Sci. 2006;100:51–8. doi: 10.1254/jphs.fp0050748. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 29.Akila M, Devaraj H. Synergistic effect of tincture of Crataegus and Mangifera indica L. extract on hyperlipidemic and antioxidant status in atherogenic rats. Vascul Pharmacol. 2008;49:173–7. doi: 10.1016/j.vph.2008.07.007. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 30.Koçyıldız ZÇ, Birman H, Olgaç V, Akgün-Dar K, Melikoğlu G, Meriçli AH. Crataegus tanacetifolia leaf extract prevents L-NAME-induced hypertension in rats:a morphological study. Phytother Res. 2006;20:66–70. doi: 10.1002/ptr.1808. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 31.Shatoor AS. In vivo hemodynamic and electrocardiographic changes following Crataegus aronia syn. Azarolus L administration to normotensive Wistar rats. Saud Med J. 2013;34:123–34. [PubMed] [Google Scholar]
- 32.Ammon HPT, Handel M. Crataegus toxicology and pharmacology part 3:Pharmacodynamics. Planta Med. 1981;43:209–39. [CrossRef] [PubMed] [Google Scholar]
- 33.Weinhouse E, Kaplanski J, Posner J. Comparison of digoxin- induced cardiac toxicity in resistant and sensitive species. J Pharm Pharmacol. 1983;35:580–3. doi: 10.1111/j.2042-7158.1983.tb04337.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 34.Weinhouse E, Kaplanski J, Warszawski D, Danon A, Gorodischer R. Cardiac toxicity of digoxin in newborn and adult rats. Pediatr Pharmacol (New York) 1980;1:97–103. [PubMed] [Google Scholar]
- 35.Oubaassie R, Bibault P, Roegel JC, Alexandre E, Sigrist S, Lavaux T, et al. Cardio protective effect of glucose-insulin infusion on acute digoxin toxicity in rats. Toxicology. 2006;224:238–43. doi: 10.1016/j.tox.2006.04.035. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 36.Walker MJA, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, et al. The Lambeth conventions:Guidelines fort he study of arrhythmias in ischaemic infarction and reperfusion. Cardiovasc Res. 1988;22:447–55. doi: 10.1093/cvr/22.7.447. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 37.Chang Q, Zou Z. Hawthorn. J Clin Pharmacol. 2002;42:605–12. doi: 10.1177/00970002042006003. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 38.Gonano LA, Sepúlveda M, Rico Y, Kaetzel M, Valverde CA, Dedman J, et al. Calcium-calmoduline kinase II mediates digitalis-induced arrhythmias. Circ Arrhythm Electrophysiol. 2011;4:947–57. doi: 10.1161/CIRCEP.111.964908. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 39.Müller A, Linke W, Zhao Y, Klaus W. Crataegus extract prolonges action potential duration in guinea-pig papillary muscle. Phytomedicine. 1996;3:257–61. doi: 10.1016/S0944-7113(96)80063-8. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 40.Müller A, Linke W, Klaus W. Crataegus extract blocks potassium currents in guinea pig ventricular cardiac myocites. Planta Medica. 1999;65:335–9. doi: 10.1055/s-1999-13997. [CrossRef] [DOI] [PubMed] [Google Scholar]


