Abstract
Objective:
Vitamin D deficiency is an independent risk factor for cardiovascular mortality. The relationship between vitamin D level and left ventricle (LV) myocardial performance index (MPI=Tei index), which incorporates both LV systolic function and diastolic function, was not investigated in previous studies. We hypothesized that vitamin D level may be associated with LV function and geometry. We aimed to investigate the association between serum 25-hydroxyvitamin D (25 [OH] D) levels and MPI and LV hypertrophy in hypertensive patients with newly diagnosed and preserved ejection fraction.
Methods:
We studied 151 sequential newly diagnosed hypertensive subjects who lived in the Çukurova region without known cardiovascular risk factors or overt heart disease (mean age: 62.8±10.4 years). Serum 25 (OH) D was measured using a direct competitive chemiluminescent immunoassay. The patients were divided into two groups according to serum 25 (OH) D level: vitamin D-non-deficient group (vitamin D> 20.00 ng/mL, n=53) and vitamin D-deficient group (vitamin D< 20.00 ng/mL, n=98). MPI was defined as the sum of isovolumic contraction and relaxation times divided by the ejection time. LV mass index (LVMI) was calculated by using the Devereux formula and body surface area.
Results:
MPI and LVMI values were lower and low-density lipoprotein (LDL) levels were higher in patients who were vitamin D-non-deficient than patients who were vitamin D-deficient (p<0.05 for all). Multivariate linear regression analysis showed that serum 25 (OH) D was indepen-dently associated with MPI (β=-0.426, p<0.001), LVMI (β=-0.345, p=<0.001), and LDL (β=0.140, p<0.026).
Conclusion:
Lower serum 25 (OH) D levels are significantly associated with impaired myocardial performance and LVMI.
Keywords: vitamin D, Tei index, myocardial performance index, hypertension
Introduction
Hypertension is an established major independent risk factor for cardiovascular disease (1). Serum 25 (OH) D deficiencies or insufficiency is a substantially prevalent condition in the general population (2). Furthermore, vitamin D deficiency is independently associated with cardiovascular morbidity and mortality in the general population and hypertensive patients (3, 4). Lower serum 25 (OH) D levels are associated with a higher prevalence of hypertension (5, 6). Moreover, vitamin D suppresses the renin-angiotensin-aldosterone system (RAAS), which has an effect on blood pressure (7).
Vitamin D receptors are found in many cells of the cardio-vascular system (8). Vitamin D affects left ventricular structure by increasing left ventricle mass and myocardial contractility by affecting calcium flux and calcium homeostasis (9). Left ventricle (LV) myocardial performance index (MPI) combines the indices of contract ion and relaxation in an overall index of LV function (10, 11). We hypothesized that vitamin D level may be associated with LV function and geometry. In present study, we aimed to investigate the association between 25 (OH) D and MPI and LV hypertrophy in newly diagnosed hypertensive patients.
Methods
Study population
Between January 2013 and July 2013, 151 sequential patients (mean age: 62.8±10.4 years) who were admitted to the Adana Numune Training and Research Hospital Cardiology Department outpatient clinic with newly diagnosed essential hypertension (without evidence of secondary causes of hypertension) were included the study. All patients in this study lived in the Çukurova region (average latitude 23 meters), located in the southern part of Turkey. Hypertensive patients had three clinic blood pressure measurements (>140/90 mm Hg) taken at 1-week intervals in the absence of any previous antihypertensive treatment to exclude the pharmacological effects on hemodynamic or ventricular hypertrophy and function. None of the patients was taking calcium or vitamin D supplements. The patients were divided into two groups according to serum 25 (OH) D level: vitamin D-non-deficient group (vitamin D≥20.00 ng/mL, n=53) and vitamin D-deficient group (vitamin D<20.00 ng/mL, n=98). Exclusion criteria were malignant HT (high blood pressure with acute impairment of one or more organ systems), evidence of secondary HT (diabetic nephropathy, polycystic kidney disease, renovascular hypertension, Cushing syndrome, thyroid diseases, hyperparathyroidism, coarctation of the aorta, obesity etc.), diabetes mellitus, systolic heart failure (left ventricle ejection fraction (LVEF) <55%), positive history or clinical signs of ischemic heart disease, cerebrovascular disease, valve disease, atrial fibrillation, smoking, receiving any drugs (lithium, NSAİD, prednisolone, etc.), moderate and severe renal insufficiency (eGFR formulated by MDRD <60 mL/min/1.73 m2), evidence of hypercalcemia (normal range: 9-10.5 mg/dL or 2.2-2.6 mmol/L), severe hepatic dysfunction, and major non-cardiovascular diseases, such as autoimmune disease, cancer, and systemic inflammatory conditions. The institutional Ethics Committee approved the study, and written informed consent for participation in the study was obtained from all individuals.
Blood pressure measurements used in this study were taken with a mercury sphygmomanometer. Body mass index (BMI) was computed as weight divided by height squared (kg/m2). The body surface area of all subjects was computed [BSA (m2) = [height (cm) x weight (kg)]/3600]½
Blood samples
Blood samples were drawn in the morning after a 20-min rest following a fasting period of 12 hours. Blood samples and echocardiographic examinations were performed on the same day in all patients. Glucose, creatinine, triglyceride, and total and high-density lipoprotein (HDL)-cholesterol levels were measured for each patient. The low-density lipoprotein (LDL) value was calculated with Friedewald’s formula. High-sensitivity C-reactive protein (hs-CRP) was measured using a BN2 nephe-lometer (Scil Diagnostics GmbH, Viernheim, Germany). Serum calcium (8.2-10.2 mg/dL) and parathyroid hormone levels (15-65 pg/mL) were measured on a Cobas c501 analyzer (Roche Diagnostics, Japan). Serum and plasma from EDTA and citrate blood were separated by centrifugation and stored as aliquots at -40°C. The level of serum 25 (OH) D was measured by using a direct competitive chemiluminescent immunoassay (Elecsys; Roche Diagnostics, Mannheim, Germany). We measured vit-D level two times and averaged them in all patients. Intra- and inter-assay coefficients of variation (CVs) were below 4.3% and 7.2%, respectively. We also recorded the dates of the 25-hydroxyvitamin D measurements and categorized them into 2 seasons: winter (Jan-Mar) and spring-summer (April-July). In this study, 20 ng/mL 25-hydroxyvitamin D was used as a cut-off for vitamin D sufficiency according to the recommendations from the WHO and, more recently, the Institute of Medicine (12, 13).
Echocardiography
Transthoracic echocardiographic examination was performed in the left lateral position. Standard 2-dimensional and Doppler echocardiographies were performed using a commercially available echocardiography machine (Vivid 7R GE Medical System, Horten, Norway). LV end-diastolic diameter (LVDd), LV end-systolic diameter, and left atrial (LA) diameter were measured according to established standards of the American Society of Echocardiography (14). LVEF was calculated by Simpson’s method (14). All measurements were calculated from an average of 5 consecutive cardiac cycles.
LV mass (LVM) was calculated according to the Devereux formula (15): LVM=1:04 [(LVDd+IVSth+PWT)3-(LVDdH-13.6. Thereafter, LV mass index (LVMI) was obtained by the following formula: LVM/body surface area (g/m2) (13). Intra- and inter-assay CV were below 4.8% and 6.5%, respectively. Relative wall thickness (RWT) was measured at end-diastole as the ratio of (2xPWT)/LVEDD.
Tissue Doppler measurements and calculation of the tissue Doppler-derived myocardial performance index
The tissue Doppler (TD) of the mitral annulus movement was obtained from the apical 4-chamber view. A 2-mm sample volume was placed sequentially at the lateral and septal annular sites.
Pulse wave tissue Doppler was performed with a 2-mm sample volume placed at the lateral and septal corner of the mitral annulus from the apical 4-chamber view. Filters were set to exclude high-frequency signals and gain minimized. Doppler ultrasound scanning intervals were measured from the mitral annular velocity intervals. The isovolumetric relaxation time (IVRT) was measured from the onset of closure of the aortic valve until the onset of opening of the mitral valve. The isovolumetric contraction time (ICT) was measured from the onset of closure of the mitral valve until the onset of the opening of the aortic. The ejection time (ET) was calculated from the opening until the closure of the aortic valve (10). All parameters were measured from the same cardiac cycle. Tissue Doppler measurements were calculated from an average of 5 consecutive cardiac cycles.
The TD-derived MPI value was calculated by adding ICT and IVRT and dividing the sum by the ET: (ICT + IVRT)/ET (10). We used the averages of the septal and lateral annulus of the mitral ICT, IVRT, and ET for calculating the MPI. The method of MPI is shown in Figure 1. The intra-assay CV was less than 5%, and the inter-assay CV less than 6% for measurements of the MPI.
Figure 1.
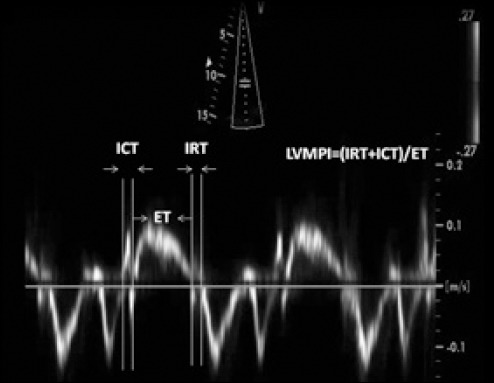
Representation of measurements of Doppler time intervals for the calculation of MPI (according to Tei et al.16). MPI is calculated by the formula [(ICT + IRT)/ET]
ET - ejection time; ICT - isovolumic contraction time; IRT - isovolumic relaxation time
Statistical analyses
All analyses were conducted using SPSS 17.0 (SPSS for Windows 17.0, Chicago, IL, USA). Continuous variables were expressed as mean±SD, and categorical variables were expressed as percentages. Analysis of normality was performed with the Kolmogorov-Smirnov test. Comparison of categorical variables between the groups was performed using the chi-square (χ2) test. Independent-samples t-test was used in the analysis of continuous variables. Non-normally distributed variables (hs-CRP and triglyceride) were compared with Mann-Whitney U test. The correlations between 25 (OH) D and laboratory, hemodynamic, and echocardiographic parameters were assessed by Pearson correlation analysis. Multivariate, stepwise linear regression analysis was performed to identify the independent associations of serum 25 (OH) D. All significant parameters in the univariate analysis were selected in the multivariate analysis. To avoid overfitting and collinearity in assessing the multivariate model, independent variables were tested for intercorrelation. Collinearity between variables was excluded before modeling. Finally, MPI, LDL, LVMI, LVID, Cr, and parathyroid hormone levels were selected in the multivariate model. A p <0.05 was considered statistically significant.
Results
Comparison of baseline characteristics
The median serum 25 (OH) D value was 14.35 ng/mL. The vitamin D concentrations measured in winter were similar to in spring-summer (13.91±5.9 ng/mL vs. 14.79±7.4 ng/mL, p=0.132) in patients.
The comparison of baseline characteristics of patients is shown in Table 1. Age, gender, BMI, SBP DBF, heart rate, fasting blood glucose, triglyceride, HDL, hs-CRP and calcium were similar between groups (p>0.05 for all). However, total cholesterol and LDL level were significantly higher and creatinine and parathyroid hormone levels were significantly lower in patients who were vitamin D-non-deficient compared with patients who were vitamin D-deficient (p<0.05).
Table 1.
Comparison of baseline charateristics of patients
| Variables | Vitamin D-non-deficient (n=53) | Vitamin D-deficient (n=98) | P |
|---|---|---|---|
| Age, years | 60.9±9.7 | 63.9±10.7 | 0.09 |
| Gender, malea | 28 (52.8%) | 48 (49.0%) | 0.734 |
| BMI, kg/m2 | 30.5±4.4 | 29.0±4.7 | 0.07 |
| SBP, mm Hg | 147.8±18.5 | 145.9±12.6 | 0.509 |
| DBP, mm Hg | 92.0±9.8 | 91.5±8.4 | 0.721 |
| Heart rate, beats/minute | 76.3±14.3 | 76.4±12.3 | 0.978 |
| Glucose, mg/dL | 93.1±12.5 | 94.9±13.8 | 0.424 |
| Total cholesterol, mg/dL | 205.94±42.64 | 187.87±41.18 | 0.012 |
| TRG, mg/dL median (25th-75th) | 168 (123-228) | 148 (102-202) | 0.325 |
| HDL, mg/dL | 42.9±9.3 | 42.2±11.5 | 0.657 |
| LDL, mg/dL | 136.3±32.7 | 117.4±34.6 | 0.001 |
| hs-CRP, mg/dL median (25th-75th) | 0.4 (0.2-0.8) | 0.4 (0.2-1.1) | 0.1 |
| Creatinine, mg/dL | 0.74±0.18 | 0.79±0.19 | 0.044 |
| MDRD, mL/min/1.73 m2 | 108.1±28.8 | 97.8±26.7 | 0.034 |
| Vitamin D, ng/mL | 25.9±4.3 | 11.8±3.9 | <0.001 |
| Parathyroid hormone, pg/mL | 40.2±15.7 | 49.4±25.4 | 0.006 |
| Calcium, mg/dL | 9.3±0.6 | 9.2±0.5 | 0.439 |
Chi-square
BMI - body mass index; DBP - diastolic blood pressure; HDL - high-density lipoprotein cholesterol; hs-CRP - high-sensitivity C-reactive protein; LDL - low-density lipoprotein cholesterol; SBP - systolic blood pressure; TRG - triglyceride; Vit D - vitamin D Data are n (%) for categorical variables, means±SD for continuous variables, or median (25% and 75% interquartiles) for non-normally distributed variables
Comparison of echocardiographic parameters
The comparison of echocardiographic parameters of patients is demonstrated in Table 2. LVEF and LA diameter were similar between groups (p>0.05). However, patients who were vitamin D-deficient had higher values of IVSth, PWT, LVMI, TD-IVRT, TD-IVCT, TD-ET, and MPI values compared with patients who were vitamin D-non-deficient (p<0.05 for all).
Table 2.
Comparison of echocardiographic parameters
| Variables | Vitamin D-non-deficient (n=53) | Vitamin D-deficient (n=98) | P |
|---|---|---|---|
| LVID, cm | 4.31±0.63 | 4.55±0.53 | 0.020 |
| LA, cm | 3.55±0.32 | 3.61±0.33 | 0.212 |
| LVEF, % | 64.7±3.6 | 63.2±5.1 | 0.501 |
| IVSth, cm | 0.98±0.20 | 1.13±0.19 | <0.001 |
| PWth, cm | 1.00±0.15 | 1.12±0.20 | <0.001 |
| LVMI, g/m2 | 89.1±32.9 | 125.9±46.9 | <0.001 |
| TD-IVRT, msec | 84.3±9.5 | 95.8±15.8 | <0.001 |
| TD-IVCT, msec | 45.9±6.3 | 55.0±10.4 | <0.001 |
| TD-ET, msec | 258.1±25.6 | 266.3±37.9 | <0.045 |
| TD-MPI | 0.47±0.11 | 0.63±0.16 | <0.001 |
Values are means±SD.
ET - ejection time; IVCT - isovolumic contraction time; IVRT - isovolumic relaxation time; IVSth - interventricular septal thickness; LA - left atrial diameter; LVEF - left ventricular ejection fraction; LVID - left ventricle internal diameter; LVMI - left ventricle mass index; MPI - myocardial performance index; PWth - posterior wall thickness; TD - tissue Doppler
Bivariate and multivariate relationships of vitamin D
On Pearson’s correlation analysis, serum 25 (OH) D level was significantly positively associated with LDL-cholesterol (r=0.234, p=0.004) and significantly negatively associated with LVMI (r=-0.500, p<0.001), MPI (r=-0.562, p<0.001), and LVID (r=-0.282, p<0.001). The relationships between 25 (OH) D with MPI and LVMI are shown in Figures 2 and 3.
Figure 2.
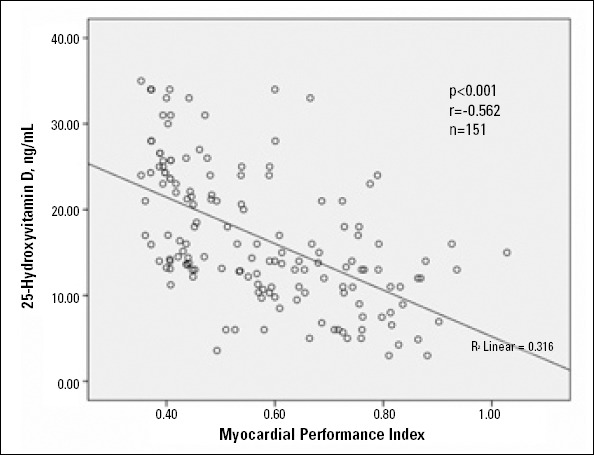
Scattergraph showing strong inverse correlation between 25-hydroxyvitamin D (ng/mL) and myocardial performance index
Figure 3.
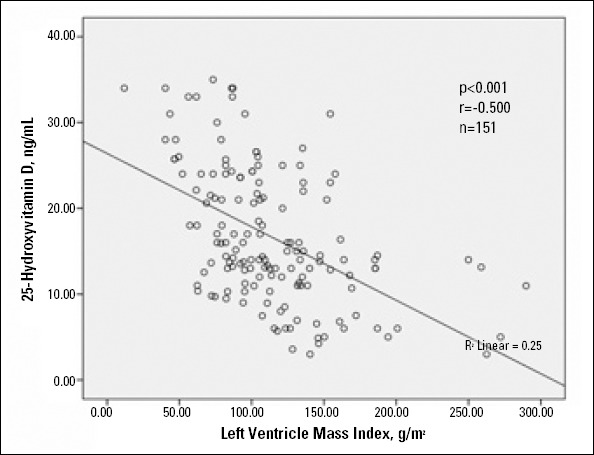
Scattergraph showing relationship between 25- hydroxyvitamin D (ng/mL) and left ventricle mass index (g/m2)
Multivariate relationships of vitamin D are shown in Table 3. Multivariate linear regression analysis showed that 25 (OH) D was independently associated with MPI (β=-0.426, p<0.001), LVMI (β=-0.345, p=<0.001), and LDL (β=0.140, p<0.026).
Table 3.
Multivariate Relationships of Vit D
| Variables | Standardized β-regression coefficients | P |
|---|---|---|
| MPI | -0.426 | <0.001 |
| LDL, mg/dL | 0.140 | 0.026 |
| LVMI, g/m2 | -0.345 | <0.001 |
LDL - low-density lipoprotein cholesterol; LVMI - left ventricle mass index; MPI - myocardial performance index
Discussion
The main findings of our study were as follows: (1) patients who were vitamin D-deficient had higher creatinine value, parathyroid hormone level, MPI, and LVMI and lower total cholesterol and LDL compared with patients who were vitamin D-non-deficient and (2) serum 25 (OH) D level was independently associated with MPI, LVMI, and LDL values. The study findings confirmed our hypothesis.
Recent clinical studies showed that low levels of vitamin D are associated with hypertension and cardiovascular diseases, such as myocardial infarction and congestive heart failure (5, 6, 16-19). The antihypertensive effects of vitamin D include suppression of renin and parathyroid hormone levels and renoprotective, antiinflammatory, and vasculoprotective properties (20, 21).
Low vitamin D and high PTH level have been associated with clinical cardiovascular disease (CVD) outcomes, including incident hypertension, myocardial hypertrophy, heart failure, and CVD death (22, 23). Left ventricular mass and LVEF are both important predictors of heart function decline and mortality (24).
Improvements in LVEF have been associated with improved prognosis, and this may point to a potential role for PTH as a determinant of cardiac remodeling (24). According to the ICELAND-MI substudy of AGES-Reykjavik, PTH was associated with measures of cardiac structure and function by using cardiac MRI (25). In our study, PTH level was significantly higher in patients with vitamin D deficiency but was not independently associated with MPI. We think that one of the most important reasons of the difference between our results and the previous study results is the technical difference of evaluating left ventricle function.
Vitamin D deficiency is prevalent in chronic heart failure and is associated with poor outcomes (16). However, the effect of vitamin D on myocardial performance in patients with preserved left ventricular systolic function (LVEF>50%) has not been studied previously. Our results showed that 25 (OH) D levels were independently associated with impaired MPI. The MPI has been widely used to quantitatively assess myocardial performance (26). MPI is more reflective of overall cardiac function than systolic or diastolic function alone and predicts both worsened morbidity and mortality (10, 27, 28). There are limited numbers of studies investigating the association between vitamin D and LV systolic and diastolic functions. But, these studies have controversial results (29-33). Fall et al. (29) found that higher circulating vitamin D concentrations are associated with better LV systolic function and smaller LV diameter. Ameri et al. (30) reported that congestive heart failure patients with 25 (OH) D <25 nmol/L LV have significantly higher end-diastolic and end-systolic diameters, larger LV end-diastolic and end-systolic volumes, and lower fractional shortening than patients with 25 (OH) D ≤25 nmol/L. Pilz et al. (33) reported that serum levels of vitamin D were not significantly associated with LV structure and function. In the studies mentioned above, the relation between 25 (OH) D and MPI was not investigated. It has been well known that the MPI value is more reflective of overall cardiac function than systolic or diastolic function alone (10, 26-28). In the present study, we showed that a lower level of 25 (OH) D was independently associated with impaired MPI.
The pathophysiology related to impaired myocardial performance in patients with lower vitamin D is not yet fully clear. Calcitriol (1, 25-dihydroxyvitamin D3) may modulate key processes involved in the pathogenesis of left ventricle dysfunction, including calcium flux and calcium homeostasis, the renin-angiotensin system, cardiomyocyte proliferation, myocardial fibrosis and proliferation, and arterial stiffness (7, 9, 34, 35). Calcium flux and calcium homeostasis are crucial for the electro-physiology and contractility of the heart (9). Calcitriol has been shown to increase the Ca2+ uptake in cardiac ventricular cells. The effects of calcitriol on cardiac contractility and intracellular calcium handling were studied in an experimental study (34). The relationship between lower vitamin D and increased arterial stiffness is well known (36). Increased arterial stiffness affects both LV systolic and diastolic functions (36). Therefore, a lower vitamin D level may be effective on impaired myocardial performance via increased arterial stiffness. On the other hand, a lower vitamin D level is associated with increased activation of the renin-angiotensin-aldosterone system (RAAS) (6). Therefore, increased RAAS activation may stimulate myocardial fibrosis and hypertrophy, leading to LV systolic and diastolic dysfunction (37).
The present study demonstrated that lower 25 (OH) D levels were also independently associated with left ventricle hypertrophy. The association of low 25 (OH) D and LVMI seen in our study is consistent with prior studies (31). Prior studies have shown a relationship between vitamin D deficiency and adverse cardiac changes, especially left ventricular hypertrophy in patients with chronic kidney disease (31). Vitamin D-mediated regulation of the RAAS is one of the postulated mechanisms responsible for left ventricular hypertrophy (7-31). Moreover, nutritional supplementation with vitamin D has been shown to reverse left ventricular hypertrophy in chronic kidney disease patients receiving hemodialysis (38).
Vitamin D supplementation is significantly associated with better survival, specifically in patients with documented vitamin D deficiency (39). Moreover, vitamin D supplementation regresses both LV hypertrophy and LV dysfunction (38-42). In our study, we found an inverse relationship between 25 (OH) D and MPI. Vitamin D supplementation is simple, safe, and inexpensive. However, larger trials are needed to confirm our results and to determine whether vitamin D interventions prevent the development of impaired myocardial performance in hypertensive patients with newly diagnosed and preserved LVEF.
Study limitations
The present study has some limitations. Firstly, our study is a cross-sectional design and does not provide any insight into the mechanisms that are responsible for the observed associations. Secondly, it has been known that serum vitamin D levels vary with region, seasonality, and altitude due to possible effects of sunlight. Thirdly, the half-life of vitamin D is approximately 3 weeks, but serum vitamin D levels may change throughout the day and season of the year. So, a single measurement may not reflect the actual vitamin D status. Lastly, the Tei index is dependent on heart rate (43). In our study, the heart rates of the groups were similar.
Conclusion
The present study showed that lower serum 25 (OH) D levels were significantly associated with impaired myocardial performance, as well as LV hypertrophy and LDL level, in hypertensive patients with newly diagnosed and preserved EF. Lower 25 (OH) D levels may play a role in the pathogenesis of myocardial dysfunction, even if EF is normal.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - M.G., T.Ş.; Design - M.K.; Supervision - M.Ç., M.G.; Materials - H.H., B.Ö.; Data collection &/or processing - A.O.B., O. Ka., Ö.Ş.; Analysis &/or interpretation - M.Ç., M.G.; Literature search - T.Ş., G.Y.K.; Writing - T.Ş., M.G.; Critical review - C.T., H.U., O. Ku.
References
- 1.Grundy SM, Balady GJ, Criqui MH, Fletcher G, Greenland P, Hiratzka LF, et al. Guide to primary prevention of cardiovascular diseases. A statement for healthcare professionals from the Task Force on Risk Reduction, American Heart Association Science Advisory and Coordinating Committee. Circulation. 1997;95:2329–31. doi: 10.1161/01.cir.95.9.2329. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 2.Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N, Kochupillai N. Prevalence and significance of low 25-hydroxyvita-min D concentrations in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472–5. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 3.Feneis JF, Arora RR. Role of vitamin D in blood pressure homeostasis. Am J Ther. 2010;17:221–9. doi: 10.1097/MJT.0b013e3181d16999. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Semba RD, Houston DK, Ferrucci L, Cappola AR, Sun K, Guralnik JM, et al. Low serum 25-hydroxyvitamin D concentrations are associated with greater all-cause mortality in older community-dwelling women. Nutr Res. 2009;8:525–30. doi: 10.1016/j.nutres.2009.07.007. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2011;29:636–45. doi: 10.1097/HJH.0b013e32834320f9. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 7.Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. 2003;88:327–31. doi: 10.1002/jcb.10343. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, et al. Synthesis of 1,25- dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–9. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 9.Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV. Calcitriol modulation of cardiac contractile performance via protein kinase. J Mol Cell Cardiol. 2006;41:350–9. doi: 10.1016/j.yjmcc.2006.05.019. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Fernandes JM, Rivera IR, de Oliveira Romao B, Mendonça MA, Vasconcelos ML, Carvalho AC, et al. Doppler-derived myocardial performance index in patients with impaired left ventricular relaxation and preserved systolic function. Echocardiography. 2009;26:907–15. doi: 10.1111/j.1540-8175.2009.00896.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.Alihanoğlu YI, Kaya Z, Arı H, Karaarslan S, Yıldız BS, Karanfil M, et al. Assessment of left ventricular systolic and diastolic function with conventional and tissue Doppler echocardiography imaging techniques in patients administered tyrosine kinase inhibitor. Turk Kardiyol Dern Arş. 2012;40:597–605. doi: 10.5543/tkda.2012.53896. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.WHO Scientific Group on the Prevention and Management of osteoporosis. Prevention and management of osteoporosis: report of a WHO scientific group. Geneva, Switzerland: WHO; 2003. [Google Scholar]
- 13.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–8. doi: 10.1161/01.cir.55.4.613. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 16.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Körfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–12. doi: 10.1016/s0735-1097(02)02624-4. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weishaar RE, Simpson RU. Vitamin D3 and cardiovascular function in rats. J Clin Invest. 1987;79:1706–12. doi: 10.1172/JCI113010. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akın F, Ayca B, Köse N, Duran M, Sarı M, Uysal OK, et al. Serum vitamin D levels are independently associated with severity of coronary artery disease. J Investig Med. 2012;60:869–73. doi: 10.2310/JIM.0b013e31825457cb. [DOI] [PubMed] [Google Scholar]
- 20.Pilz S, Tomaschitz A. Role of vitamin D in arterial hypertension. Expert Rev Cardiovasc Ther. 2010;8:1599–608. doi: 10.1586/erc.10.142. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 21.McGreevy C, Williams D. New insights about vitamin D and cardiovascular disease: a narrative review. Ann Intern Med. 2011;155:820–6. doi: 10.7326/0003-4819-155-12-201112200-00004. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 22.van Ballegooijen AJ, Snijder MB, Visser M, van den Hurk K, Kamp O, Dekker JM, et al. Vitamin D in relation to myocardial structure and function after eight years of follow-up: the Hoorn study. Ann Nutr Metab. 2012;60:69–77. doi: 10.1159/000336173. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Saleh FN, Schirmer H, Sundsfjord J, Jorde R. Parathyroid hormone and left ventricular hypertrophy. Eur Heart J. 2003;24:2054–60. doi: 10.1016/j.ehj.2003.09.010. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–82. doi: 10.1016/s0735-1097(99)00630-0. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 25.van Ballegooijen AJ, Visser M, Cotch MF, Arai AE, Garcia M, Harris TB, et al. Serum Vitamin D and parathyroid hormone in relation to cardiac structure and function: The ICELAND-MI substudy of AGES-Reykjavik. J Clin Endocrinol Metab. 2013;98:2544–52. doi: 10.1210/jc.2012-4252. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function-a study in normal and dilated cardiomyopathy. J Cardiol. 1995;26:357–66. [PubMed] [Google Scholar]
- 27.Arnlöv J, Lind L, Andrén B, Risérus U, Berglund L, Lithell H. A Doppler-derived index of combined left ventricular systolic and diastolic function is an independent predictor of cardiovascular mortality in elderly men. Am Heart J. 2005;149:902–7. doi: 10.1016/j.ahj.2004.07.022. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 28.Şahin DY, Gür M, Elbasan Z, Uysal OK, Özaltun B, Şeker T, et al. Relationship between myocardial performance index and severity of coronary artery disease assessed with SYNTAX score in stable coronary artery disease. Echocardiography. 2013;30:385–91. doi: 10.1111/echo.12077. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 29.Fall T, Shiue I, Bergeâaf Geijerstam P, Sundström J, Ärnlöv J, Larsson A, et al. Relations of circulating vitamin D concentrations with left ventricular geometry and function. Eur J Heart Fail. 2012;14:985–91. doi: 10.1093/eurjhf/hfs091. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 30.Ameri P, Ronco D, Casu M, Denegri A, Bovio M, Menoni S, et al. High prevalence of vitamin D deficiency and its association with left ventricular dilation: an echocardiography study in elderly patients with chronic heart failure. Nutr Metab Cardiovasc Dis. 2010;20:633–40. doi: 10.1016/j.numecd.2010.01.002. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 31.Patange AR, Valentini RP, Gothe MP, Du W, Pettersen MD. Vitamin D deficiency is associated with increased left ventricular mass and diastolic dysfunction in children with chronic kidney disease. Pediatr Cardiol. 2013;34:536–42. doi: 10.1007/s00246-012-0489-z. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 32.van Ballegooijen AJ, Snijder MB, Visser M, van den Hurk K, Kamp O, Dekker JM, et al. Vitamin D in relation to myocardial structure and function after eight years of follow-up: the Hoorn study. Ann Nutr Metab. 2012;60:69–77. doi: 10.1159/000336173. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 33.Pilz S, Henry RM, Snijder MB, van Dam RM, Nijpels G, Stehouwer CD, et al. Vitamin D deficiency and myocardial structure and function in older men and women: the Hoorn study. J Endocrinol Invest. 2010;33:612–7. doi: 10.1007/BF03346658. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 34.Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. 2011;58:186–92. doi: 10.1016/j.jacc.2011.02.051. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connell TD, Berry JE, Jarvis AK, Somerman MJ, Simpson RU. 1,25-Dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol. 1997;272:1751–8. doi: 10.1152/ajpheart.1997.272.4.H1751. [DOI] [PubMed] [Google Scholar]
- 36.Gür M, Yılmaz R, Demirbağ R, Yıldız A, Özdoğru I, Baş MM, et al. Relationship between myocardial performance index and aortic distensibility in patients with essential hypertension. Int J Clin Pract. 2008;62:138–42. doi: 10.1111/j.1742-1241.2006.01202.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 37.Patel BM, Mehta AA. Aldosterone and angiotensin: Role in diabetes and cardiovascular diseases. Eur J Pharmacol. 2012;697:1–12. doi: 10.1016/j.ejphar.2012.09.034. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 38.Lemmilä S, Saha H, Virtanen V, Ala-Houhala I, Pasternack A. Effect of intravenous calcitriol on cardiac systolic and diastolic function in patients on hemodialysis. Am J Nephrol. 1998;18:404–10. doi: 10.1159/000013384. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 39.Vacek JL, Vanga SR, Good M, Lai SM, Lakkireddy D, Howard PA. Vitamin D deficiency and supplementation and relation to cardiovascular health. Am J Cardiol. 2012;109:359–63. doi: 10.1016/j.amjcard.2011.09.020. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 40.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, et al. 1,25 (OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–98. doi: 10.1161/CIRCULATIONAHA.109.856070. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, et al. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol. 2010;5:905–11. doi: 10.2215/CJN.06510909. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 43.Karatzis EN, Giannakopoulou AT, Papadakis JE, Karazachos AV, Nearchou NS. Myocardial performance index (Tei Index): evaluating its application to myocardial infarction. Hellenic J Cardiol. 2009;50:60–5. [PubMed] [Google Scholar]


