Abstract
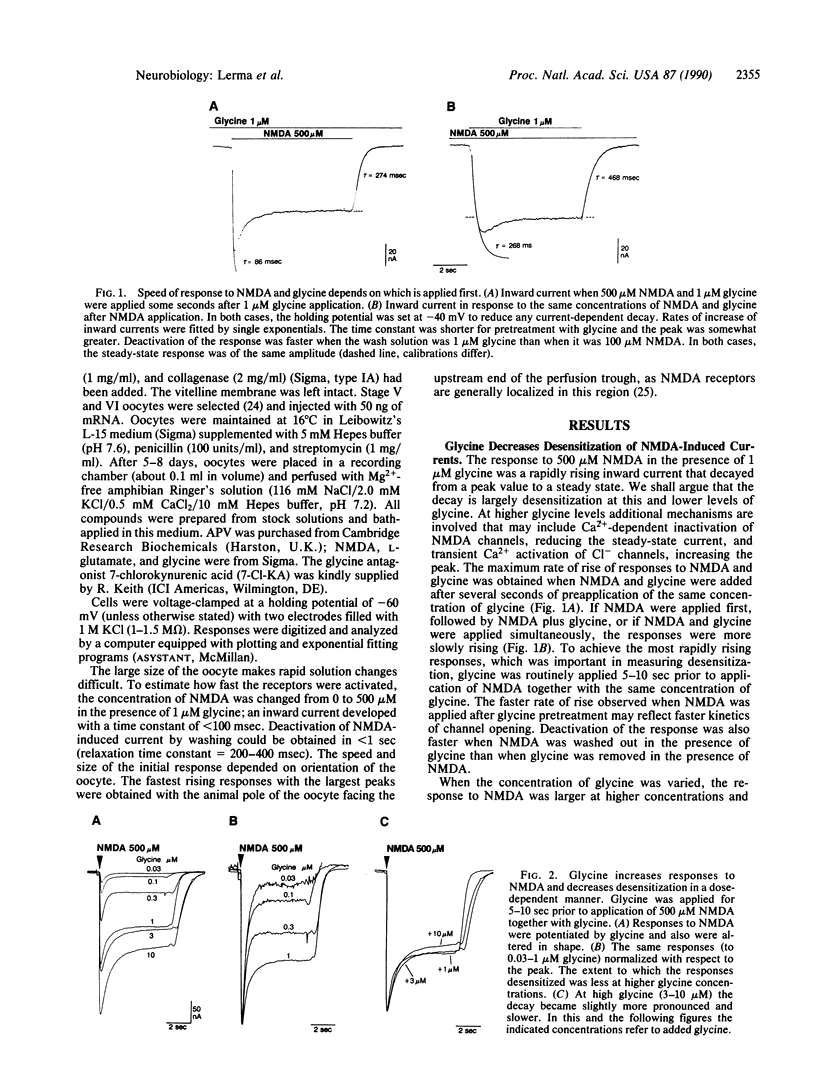
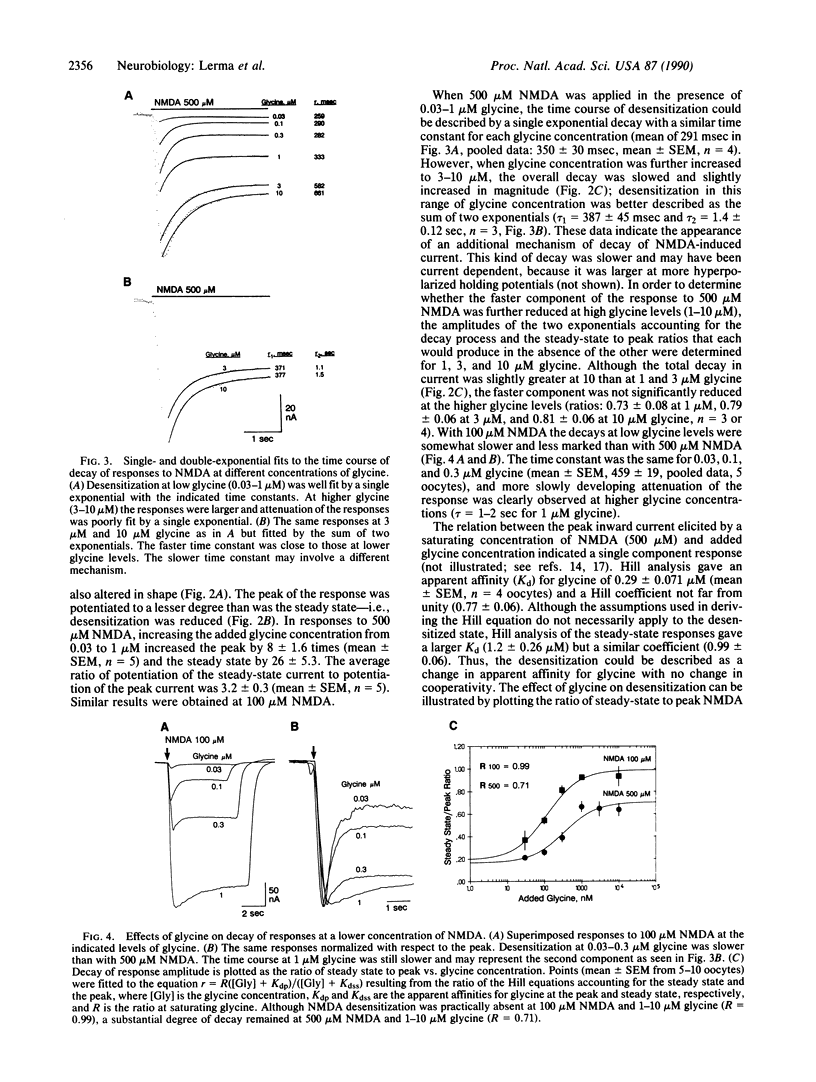
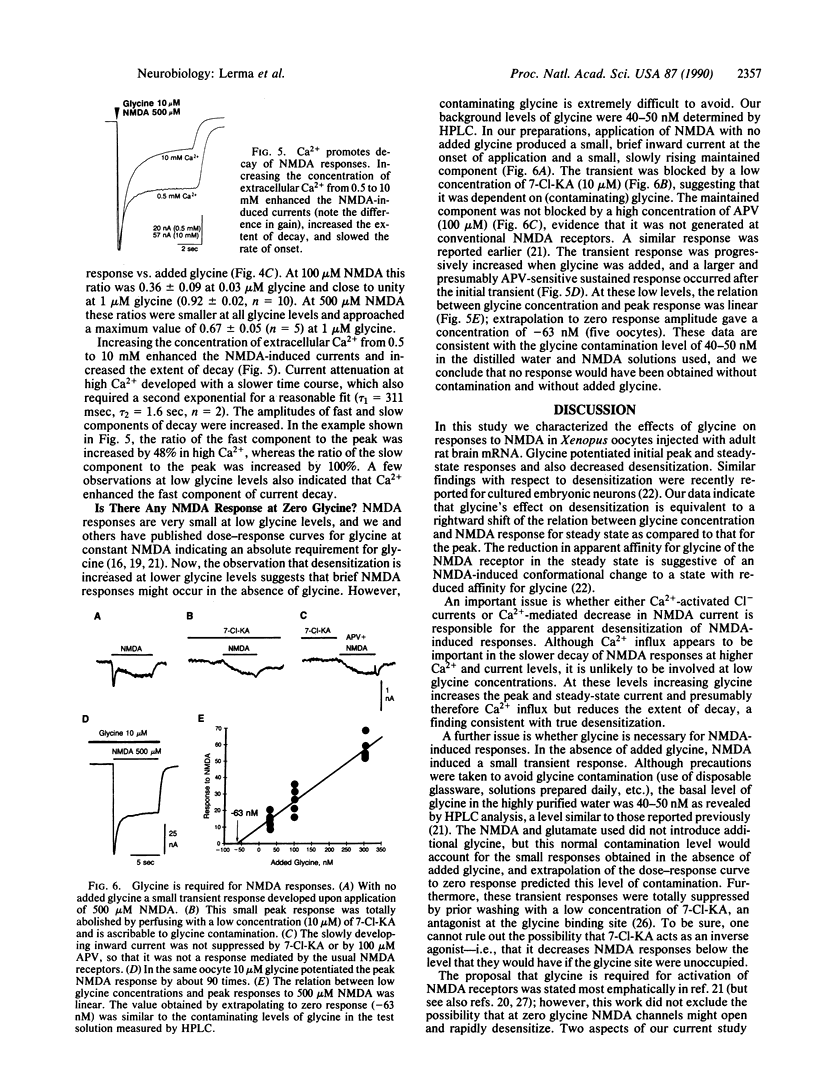
In Xenopus oocytes injected with rat brain mRNA, as in neurons, glycine greatly potentiated responses of the N-methyl-D-aspartate (NMDA) type of excitatory amino acid receptor. Injected oocytes generated a partially desensitizing inward current in response to NMDA with 30 nM added glycine. As the added glycine concentration was increased from 30 nM to 1 microM, the NMDA response was increased and exhibited less desensitization. The relationship between the NMDA peak response and added glycine concentration indicated a single component response with apparent affinity of 0.29 microM and a Hill coefficient of 0.77. The desensitized response was also fit by the Hill relation with a lower affinity but similar coefficient. The time course of desensitization at 500 microM NMDA was exponential with a time constant (350 msec) that was independent of glycine concentration between 0.03 and 0.3 microM. At higher glycine concentration a slower component of decay (tau = 1.4 sec) was observed. This component was enhanced by increasing the extracellular Ca2+. NMDA without added glycine evoked a small transient response. However this response was suppressed completely by prewashing with the glycine antagonist 7-chlorokynurenic acid, suggesting that it may have been due to glycine contamination. The dose-response relation for low concentrations of glycine indicated that the measured level of glycine contamination accounted for these responses. These results indicate that glycine has at least two actions at the NMDA receptor: it enables channel opening by the agonist and decreases desensitization.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anis N. A., Berry S. C., Burton N. R., Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983 Jun;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G. J., Cotman C. W. NMDA receptor regulation of neuronal morphology in cultured hippocampal neurons. Neurosci Lett. 1989 May 8;99(3):268–273. doi: 10.1016/0304-3940(89)90458-8. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R., Hynes M. A., King G. L. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986 Nov;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Fiekers J. F., Spannbauer P. M., Scubon-Mulieri B., Parsons R. L. Voltage dependence of desensitization. Influence of calcium and activation kinetics. J Gen Physiol. 1980 May;75(5):511–529. doi: 10.1085/jgp.75.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Foster A. C., Leeson P. D., Priestley T., Tridgett R., Iversen L. L., Woodruff G. N. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. W., Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988 Aug 12;241(4867):835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Kushner L., Lerma J., Zukin R. S., Bennett M. V. Coexpression of N-methyl-D-aspartate and phencyclidine receptors in Xenopus oocytes injected with rat brain mRNA. Proc Natl Acad Sci U S A. 1988 May;85(9):3250–3254. doi: 10.1073/pnas.85.9.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J., Kushner L., Spray D. C., Bennett M. V., Zukin R. S. mRNA from NCB-20 cells encodes the N-methyl-D-aspartate/phencyclidine receptor: a Xenopus oocyte expression study. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1708–1711. doi: 10.1073/pnas.86.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J., Kushner L., Zukin R. S., Bennett M. V. N-methyl-D-aspartate activates different channels than do kainate and quisqualate. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2083–2087. doi: 10.1073/pnas.86.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Miljkovic Z., Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987 Aug;58(2):251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Webb G. D. The effects of external Ca++ and Mg++ on the voltage sensitivity of desensitization in Electrophorus electroplaques. J Gen Physiol. 1980 Jun;75(6):693–708. doi: 10.1085/jgp.75.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Koh J., Choi D. W. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987 May 1;236(4801):589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., Hahn S. Ketamine-xylazine anaesthesia blocks consolidation of ocular dominance changes in kitten visual cortex. Nature. 1987 Mar 12;326(6109):183–185. doi: 10.1038/326183a0. [DOI] [PubMed] [Google Scholar]
- Siara J., Ruppersberg J. P., Rüdel R. Human acetylcholine receptors desensitize much faster than rat acetylcholine receptors. Neurosci Lett. 1989 Sep 11;103(3):298–302. doi: 10.1016/0304-3940(89)90116-x. [DOI] [PubMed] [Google Scholar]
- Simon R. P., Swan J. H., Griffiths T., Meldrum B. S. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984 Nov 16;226(4676):850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Fischbach G. D. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989 Aug;3(2):209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Kleckner N. W., Dingledine R. Rat brain N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Science. 1987 Nov 20;238(4830):1114–1116. doi: 10.1126/science.2825347. [DOI] [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B. A possible correlate of the postsynaptic condition for long-lasting potentiation in the guinea pig hippocampus in vitro. Neurosci Lett. 1984 Feb 24;44(3):327–332. doi: 10.1016/0304-3940(84)90044-2. [DOI] [PubMed] [Google Scholar]
- Zorumski C. F., Yang J., Fischbach G. D. Calcium-dependent, slow desensitization distinguishes different types of glutamate receptors. Cell Mol Neurobiol. 1989 Mar;9(1):95–104. doi: 10.1007/BF00711446. [DOI] [PMC free article] [PubMed] [Google Scholar]



