Abstract
Objective:
Available evidence suggests that inflammation may be associated with atrial fibrillation (AF). This prospective and observational study aimed to assess whether plasma neopterin (NPT) and interleukin-6 (IL-6) levels before and after electrical cardioversion (CV) predict AF recurrence.
Methods:
The study was designed as a prospective observational trial. Blood samples were collected (24 hours before, 24 h after CV, and 7 days after CV) in 60 patients with a dual-chamber pacemakar and preserved left ventricular systolic function who underwent successful CV of persistent AF. All significant parameters associated with AF recurrence lasting ≥30 min and detected by pacemaker data logs were evaluated in multivariate logistic regression analysis. Echocardiography was performed 7 days after CV in patients with sinus rhythm. The control group included 17 subjects without AF.
Results:
The analysis included 51 patients who remained in sinus rhythm 7 days after CV. During 12 months of follow-up, AF recurred in 46 patients. Baseline IL-6 levels did not differ between the two groups, but baseline NPT levels were higher in the study group than in the control group (19±7 vs. 11±5 nmol/mL, p<0.001). NPT levels of ≥14.6 nmol/L at baseline and ≥13.3 nmol/L 7 days after CV separated the patients with AF recurrence from those without arrhythmia after CV. Only left atrial emptying fraction <38% was an independent predictor of AF recurrence (p=0.03), whereas NPT levels of ≥13.3 nmol/L 7 days after CV showed borderline statistical significance (p=0.07).
Conclusion:
Increased NPT level was observed in patients with persistent AF. Neither baseline IL-6 and NPT levels nor their changes within 7 days after CV were predictive of AF recurrence. Further studies are needed to establish the prognostic significance of NPT in patients with AF. (Anatol J Cardiol 2016; 16: 563-71) 0000; 00: 000–000
Keywords: atrial fibrillation, recurrence, neopterin, interleukin-6
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice. Because of its high prevalence and the lack of fully effective therapy, AF remains a challenge in modern medicine. Better understanding of the mechanisms responsible for the occurrence and maintenance of this arrhythmia would be helpful to establish effective treatment strategies.
Available evidence suggests that systemic inflammation may be associated with AF and its pathogenesis (1, 2). However, it is still unclear whether the inflammatory response is a cause or a consequence of AF. In addition, these pathways may be interrelated. High-grade systemic inflammation can trigger AF by activation of atrial foci in patients with anatomically or electrically susceptible atrial substrate. Alternatively, rapid atrial activation during AF induces calcium overload that may cause atrial cardiomyocyte apoptosis. This cardiomyocyte loss is accompanied by replacement fibrosis within the atria, with subsequent anatomical and electrical remodeling. However, the precise mechanism by which inflammation contributes to the development of AF remains unclear.
C-reactive protein (CRP) and interleukin-6 (IL-6) are the most frequently evaluated inflammatory markers in AF research (1, 3–10). Other markers of inflammation, such as tumor necrosis factor-α (TNF-α) levels, transforming growth factor-β (TGF-β) levels, interleukin-8 (IL-8) levels, and white blood cell count (7, 8, 10–13), were also investigated; however, the reported results are equivocal.
Neopterin (NPT) is an immune modulator produced by monocytes/macrophages upon activation by proinflammatory stimuli like Th1-type cytokine interferon-gamma. Increased serum NPT levels have been reported in patients with coronary artery disease and have been shown to be a predictor of future cardiovascular events (14–18). Elevated serum NPT levels have also been demonstrated in patients with heart failure, and they have been shown to be associated with left ventricular (LV) enlargement (19, 20) and a high incidence of cardiac events (21). Currently, no data are available regarding the role and predictive significance of NPT levels in patients with AF.
The aims of this prospective and observational study were as follows: (a) to evaluate plasma NPT and IL-6 levels before and after electrical cardioversion (CV) in patients with persistent AF, preserved LV systolic function, and an implanted dual-chamber pacemaker; (b) to assess whether these inflammatory markers predicted arrhythmia recurrence during 12 months of follow-up after CV.
Methods
The study conducted by the two cardiology centers and was designed as a prospective observational trial. We studied patients with persistent non-valvular AF referred to Department of Cardiology and Electrotherapy Medical University of Gdańsk in Poland for CV who had an implanted dual-chamber pacemaker equipped with AF detection function. Atrial fibrillation was considered persistent if it lasted 7 days or longer, or if CV was performed within 7 days of its occurrence. The exclusion criteria were as follows: impaired global LV systolic function (LV ejection fraction <45% in the echocardiographic study before CV), clinically significant valvular disease or prosthetic valve, hyperthyroidism, New York Heart Association (NYHA) class-III or class-IV heart failure, history of malignancy or connective tissue disease, anti-inflammatory or immunosuppresive treatment, active inflammatory process (based on clinical assessment and/or CRP levels of >10 mg/L), myocardial infarction, unstable angina, and percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) within 3 months before the enrollment. The control group consisted of 17 subjects of similar age and gender as the study group, in whom we excluded AF and any cardiovascular disease on the basis of their history and medical records. Chronic kidney disease was recognized if the glomerular filtration rate, estimated by the Cockroft–Gault formula, was <60 mL/min on two consecutive examinations. AF episode duration before CV was calculated on the basis of intracardiac recordings obtained from the pacemaker data logs, as well as ECG documentation received from the patients.
Adequate anticoagulation was confirmed in all patients included in the study. Electrical cardioversion was performed under conscious sedation using a direct-current biphasic shock (Medtronic Physio-Control Lifepack 20E, Redmond, USA) applied with paddles placed in the anterolateral position at least 10–15 cm away from the pacemaker can. The shock sequence was 100 J followed by 200 J. In case of unsuccessful CV, another 200-J shock was applied. Pacemaker parameters (including sensing and pacing settings and battery status assessment) were checked before and directly after CV. CV was considered successful if sinus rhythm persisted for 24 h.
Estimated required sample sizes was 46 (β=0.8, α=0.05). Sample size for repeated measures ANOVA for neopterin was calculated on the basis of the assumption that the change in the mean levels during consecutive measurements was at least 3 units, standard deviation was 7, and the correlation between follow-up measurements was 0.7. The study protocol was approved by the local Ethics Committee, and all patients gave their written consent to participation in the study.
Study protocol
Patient evaluation included history, physical examination, electrocardiogram (ECG), pacemaker interrogation with detailed analysis of its data logs and stored ECGs, and blood sampling for NPT and IL-6 level measurement. CRP levels were measured using the latex-enhanced immunoturbidimetric method (high-sensitivity CRP assay was not available). On the 7th day after CV, echocardiography was performed in patients in sinus rhythm.
Sinus rhythm control and device data logs were evaluated during follow-up visits, scheduled at 1, 3, 6, and 12 months after CV. The study endpoint was AF recurrence lasting at least 30 min and confirmed by ECG and/or pacemaker data logs. Depending on the timing of AF occurrence, patients were divided into three groups: Group 1–AF recurrence within 7–30 days after CV, Group 2–AF recurrence between 31 days and 1 year after CV, and Group 3–no AF during 1 year follow-up after CV.
Transthoracic echocardiography was performed using a 3.5-MHz phased array transducer (Vivid 3, GE Healthcare, USA). Echocardiographic parameters included LV dimensions measured at end-systole and end-diastole in the parasternal long-axis view. LV end-systolic and end-diastolic volumes were calculated from the 2-chamber and 4-chamber apical views. LV ejection fraction (LVEF) was calculated by the biplane Simpson’s method (22). Left atrial (LA) diameter (in the parasternal long-axis view) and LA and right atrial (RA) area (in the apical 4-chamber view at end-systole) were measured. LA and RA end-systolic and end-diastolic volumes were calculated from the apical 4-chamber view, and LA and RA emptying fractions (LAEF and RAEF, respectively) were determined.
Measurement of plasma NPT and IL-6 levels
Blood samples were collected within 24 h before, 24 h after, and 7 days after CV in fasting patients after 30-min rest. Samples were centrifuged for 20 min at 4°C and the plasma was stored at –70°C until assays. Plasma NPT and IL-6 levels were determined by the immunoenzymatic method using standard laboratory techniques according to manufacturers’ specifications. We used Neopterin Brahms No. 99.1 kits, Thermo Scientific, Germany (values expressed in nmol/L) and Quantikine Human IL-6 No. D6050 kits, R&D Systems, USA (values in pg/mL). Patients with an AF episode within the first 7 days after CV were excluded from the analysis.
Statistical analysis
Data are presented as mean values±standard deviation and range, patient numbers, and percentages, or median values with the 1st and 3rd quartile [QI; QIII] (interquartile range). Normally distributed variables were compared using the Student t-test, and the Mann–Whitney U test was used to compare independent non-normally distributed variables. Differences between categorical variables were assessed using the chi-square test. Matched pair variables with normal distribution were compared using matched-pair t test, and otherwise, the Wilcoxon matched-pairs signed-ranks test was used to compare independent variables. Receiver operating characteristic (ROC) curves were plotted for NPT and IL-6 levels and LAEF, and the area under the curve (AUC) was calculated in order to determine values separating the patients with or without AF recurrence during the follow-up period. NPT and IL-6 levels 24 h before, 24 h after, and 7 days after CV were compared using repeated measures ANOVA test. Univariate and multiple logistic regression was used to assess the association between an early AF recurrence (between 1 week and 30 days after CV) and other variables (age, gender, AF history and AF episode duration before CV, medications, concomitant diseases, echocardiographic parameters, as well as plasma NPT and IL-6 levels). Cox proportional hazards analysis was performed to identify determinants associated with AF recurrence during 12 months of follow-up after CV. Variables with p≤ 0.2 by univariate analysis were then included in the stepwise multivariate analysis. A p value of <0.05 was considered statistically significant. Statistical analysis was performed using the STATA software (version 12.1, STATACorp, USA).
Results
Successful CV was performed in 60 patients (mean age 75±7 years) with persistent AF with a median duration of 51 days. There were no adverse events related to CV, anesthesia, or pacemaker malfunction after CV. The final analysis included 51 patients. Eight patients in whom AF recurred within the first week after CV and one patient whose baseline blood sample was lost were excluded from further analysis. The control group consisted of 17 healthy subjects, including nine men (mean age 74±6 years).
No difference was found in baseline serum CRP levels between the study group and the control subjects: 2.3±1.7 mg/L (range 0.8–8 mg/L) vs. 2.6±1.9 mg/L (range 0.8–8.2 mg/L), p=0.4. Baseline NPT levels were significantly higher in the study group than in control subjects (19±7 nmol/L vs. 11±5 nmol/L, p<0.001). Baseline IL-6 levels did not differ between the study group and the control group (Table 1).
Table 1.
Plasma levels of neopterin and interleukin-6 measured 24 h before, 24 h after, and 7 days after successful electrical cardioversion in patients with persistent atrial fibrillation and in subjects in the control group
| Control group | 24 h before CV | 24 h after CV | 7 days after CV | P Before vs. 24 h vs. 7 days after CV | |
|---|---|---|---|---|---|
| Neopterin, nmol/L | 11±5* (5.8–26) | 19±7* (6.3–38) | 17.8±7Δ (7.7–35) | 19.8±8Δ (6.4–37) | <0.05 |
| Interleukin-6, pg/mL | 3.4±5 (0.7–20) | 3.6±4 (0.3–19) | 4.8±5 (0.4–20) | 3±2.7 (0.2–12) | 0.5 |
CV - electrical cardioversion. Repeated-measures ANOVA test was used to compare cytokine levels 24 h before vs. 24 h after vs. 7 days after electrical cardioversion.
P<0.001 (Student t-test);
P=0.03 (matched-pair t-test)
Changes in NPT and IL-6 levels after CV are shown in Table 1. NPT levels increased significantly at 7 days after CV compared with values obtained at 24 h after CV (p=0.03). Plasma IL-6 levels did not change after CV compared with baseline values.
Atrial fibrillation recurred after successful CV in all but five patients. AF recurred between 1 week and 30 days after CV (Group 1) in 28 patients, between 31 days and 1 year after CV (Group 2) in 18 patients, and only in five (10%) patients no recurrent AF episodes were noted within 12 months after CV (Group 3). The patients who remained free from AF for 1 year after CV were all treated with class-III antiarrhythmic drugs, whereas those with early AF recurrence after CV were characterized by lower LAEF (Table 2).
Table 2.
Clinical characteristics of patients in relation to the timing of the first atrial fibrillation recurrence after electrical cardioversion
| Variable | Group 1 (n=28) | Group 2 (n=18) | Group 3 (n=5) | P |
|---|---|---|---|---|
| Age, years | 76±6 (67–85) | 75±7 (60–90) | 75±14 (54–88) | 0.7 |
| Male gender, % | 15 (54) | 9 (50) | 5 (100) | 0.1 |
| BMI, kg/m2 | 29±6 (24–42) | 28±3 (20–34) | 31±3 (28–35) | 0.2 |
| AF episode duration before CV, days | 52 [QI 6; QIII 104] | 47 [QI 35; QIII 83] | 58 [QI 3; QIII 151] | 0.9 |
| AF history, years | 5 [QI 2.8; QIII 7.3] | 4.2 [QI 2.9; QIII 9.2] | 1.7 [QI 0.6; QIII 4.4] | 0.18 |
| Pacing indications | ||||
| Sick sinus syndrome, % | 15 (54) | 8 (50) | 3 (60) | 1.0 |
| 2nd or 3rd degree atrio-ventricular block, % | 13 (46) | 8 (50) | 2 (40) | 1.0 |
| Percentage of atrial pacing, % | 66±31 | 68±249 | 67±35 | 0.5 |
| Percentage of ventricular pacing, % | 57±18 | 54±16 | 55±26 | 0.6 |
| Arterial hypertension, % | 23 (82) | 16 (89) | 5 (100) | 0.7 |
| Coronary artery disease, % | 9 (32) | 6 (33) | 2 (40) | 1.0 |
| Heart failure, % | 10 (36) | 10 (56) | 1 (20) | 0.3 |
| Diabetes, % | 9 (32) | 6 (33) | 3 (60) | 0.5 |
| Chronic obstructive pulmonary disease, % | 4 (14) | 3 (17) | 0 (0) | 1.0 |
| Dyslipidemia, % | 23 (82) | 13 (72) | 5 (100) | 0.5 |
| Chronic kidney disease, % | 13 (46) | 9 (50) | 1 (20) | 0.5 |
| CHA2DS2-VASc score | 2.4 | 2.2 | 2.3 | 0.7 |
| LVEF, % | 54±6 (45–65) | 56±5 (46–62) | 58±6 (52–64) | 0.2 |
| Left atrial diameter, mm | 47±3 (40–53) | 45±3 (38–52) | 48±5 (43–53) | 0.08 |
| Left atrial area, cm2 | 29±4 (23–38) | 28±5 (21–40) | 31±5 (28–41) | 0.3 |
| Right atrial area, cm2 | 25±4 (20–36) | 26±4 (18–32) | 27±4 (22–33) | 0.6 |
| LAEF, % | 34±4 (26–44) | 39±5 (31–49) | 39±6 (30–44) | <0.001 |
| RAEF, % | 35±5 (24–46) | 35±7 (23–45) | 37±2 (31–41) | 0.5 |
| Antiarrhythmic drugs | ||||
| Class I, % | 4 (14) | 4 (22) | 1 (20) | 0.9 |
| Class III, % | 10 (36) | 6 (33) | 5 (100) | 0.02 |
| ACEI or ARB, % | 18 (64) | 11 (61) | 4 (80) | 0.8 |
| Statin, % | 23 (82) | 14 (78) | 5 (100) | 0.7 |
| Vitamin K antagonists, % | 27 (96) | 17 (94) | 5 (100) | 1.0 |
| Acetylsalicylic acid, % | 3 (11) | 2 (11) | 0 (0) | 1.0 |
Group 1 - AF between 1 week and 30 day after CV; Group 2 - AF between 31 day and 1 year after CV; Group 3 - no AF within 1 year after CV; ACEI - angiotensin-converting enzyme inhibitor; AF - atrial fibrillation; ARB - angiotensin II receptor blocker; CHA2DS2-VASc - congestive heart failure, Hypertension, Age >75 years (doubled), Diabetes; LAEF - left atrial emptying fraction; LVEF - left ventricular ejection fraction, Stroke (doubled) - Vascular disease, Age 65–74 years; RAEF - right atrial emptying fraction, Sex category (calculates stroke risk in patients with non-valvular atrial fibrillation); CV - electrical cardioversion.
Kruskal–Wallis test or the one-way ANOVA was used to compare the multiple groups depending on whether data fit the assumptions of normality
Baseline plasma NPT levels of ≥14.6 nmol/L separated the patients with and without AF recurrence during the follow-up with a sensitivity and specificity of 73% and 57%, respectively. AUC for baseline NPT levels were 0.68 [95% confidence interval (CI) 0.49–0.87, Fig. 1a]. The strongest predictor of AF recurrence was NPT levels at 7 days after CV, with the cutoff value of ≥13.3 nmol/L; AF recurrence was predicted with a sensitivity and specificity of 81% and 75%, respectively. AUC for the NPT levels at 7 days after CV was 0.84 (95%CI 0.69–0.99, Fig. 1b).
Figure 1.
Receiver-operator characteristic curve analysis of (a) the plasma neopterin (NPT) levels before electrical cardioversion (CV) and (b) that at 7 days after CV for separation of patients with or without atrial fibrillation recurrence
When comparing NPT and IL-6 levels after CV in the three groups of patients (Table 3), the only difference was borderline lower NPT levels (p=0.09) at 24 h after CV in subjects with no AF recurrence during 12 months of follow-up (Group 3) than in patients with AF recurrence (Groups 1 and 2). We found no significant differences in IL-6 levels between Groups 1, 2, and 3. However, using a repeated measures ANOVA test, we found that changes in IL-6 levels were significant when consecutive IL-6 level measurements were compared (p=0.04), and a trend was noted near significant borderline (p=0.07) when comparing the three groups of patients (Fig. 2).
Table 3.
Plasma inflammatory marker levels and their changes in serial blood samples in the three groups of patients with different timing of the first atrial fibrillation recurrence after electrical cardioversion. Plasma blood samples were obtained 24 h before (I), 24 h after (II), and 7 days (III) after successful cardioversion
| Cytokine | Group 1 AF between 1 week and 30 day after CV (n=28) | Group 2 AF between 1 week day and 1 year after (n=18) | Group 3 AF between 31 1 year after CV CV (n=5) | P |
|---|---|---|---|---|
| CRP, mg/L | 2.1±1.6 (0.7–7) | 2.5±2 (0.7–8) | 2.6±1.6 (0.8–5) | 0.7 |
| NPT I, nmol/L | 18.5±7 (6–38) | 20.7±8 (9–34) | 14±1 (13–16) | 0.22 |
| NPT II, nmol/L | 18.4±7 (8–33) | 18.5±8 (10–35) | 11.8±2 (9–15) | 0.09 |
| NPT III, nmol/L | 20.4±9 (6–37) | 21±8 (8–35) | 13.8±2 (11–15) | 0.23 |
| IL-6 I, pg/mL | 4.2±5 (0.3–19) | 2.1±2 (0.4–8) | 7.3±7 (1.3–15) | 0.17 |
| IL-6 II, pg/mL | 5.1±5 (0.4–20) | 5.4±3 (0.4–10) | 1±0.5 (0.7–1.5) | 0.15 |
| IL-6 III, pg/mL | 2±1.8 (0.3–5) | 3.1±3 (0.2–12) | 1.6±1.5 (0.7–3.3) | 0.9 |
AF - atrial fibrillation; CRP-C - reactive protein (at baseline); CV - electrical cardioversion; IL-6 - interleukin 6; NPT - neopterin.
Kruskal–Wallis test or the one-way ANOVA was used to compare the multiple groups depending on whether data fit the assumptions of normality
Figure 2.

Plasma interleukin 6 (IL-6) levels measured 24 h before (I), 24 h after (II), and 7 days (III) after electrical cardioversion (CV) of persistent atrial fibrillation (AF) in three groups of patients with different timing of the first AF recurrence after CV (repeated measures ANOVA). Group 1–AF occurrence within 7–30 days after CV; Group 2–AF recurrence between 31 days and 1 year after CV; Group 3–no AF during 1 year follow-up after CV
P=0.04 for comparison between IL-6 levels
P=0.07 for comparison between Groups 1, 2, and 3
Analysis of echocardiographic parameters in the three groups (Table 2) showed that LAEF was significantly lower in patients with early AF recurrence (p<0.001). The cutoff value of LAEF of <38% could predict AF recurrence with a sensitivity and specificity of 71% and 70%, respectively. AUC for LAEF was 0.69 (95%CI 0.45–0.92, Fig. 3).
Figure 3.
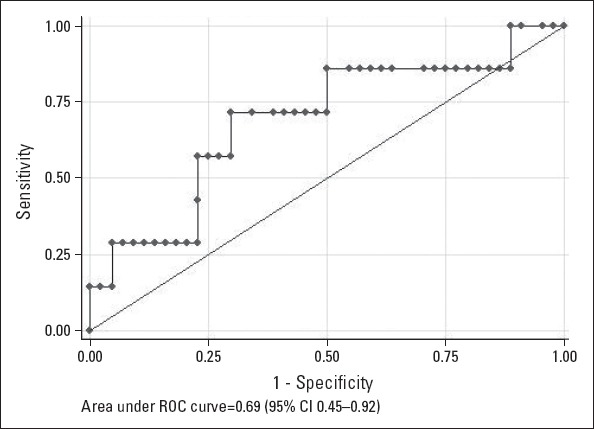
Receiver-operator characteristic curve analysis of left atrial emptying fraction by echocardiography for separation of patients with or without atrial fibrillation recurrence
We performed analyses using age, gender, AF history, AF episode duration before CV, comorbidities, echocardiographic parameters, medications, and plasma NPT and IL-6 levels to identify predictors of AF recurrence within 30 days after CV (Table 4). None of these variables was found to predict the risk of early AF recurrence. In the Cox proportional hazard analysis (with the endpoint of AF recurrence during 12 months after CV), univariate models revealed that LVEF and NPT levels at 7 days after ≥13.3 nmol/L were independent predictors of AF recurrence after CV (Table 5). In stepwise multivariate analysis, LAEF of <38% was the only independent predictor of AF recurrence, whereas LVEF (p=0.052) and NPT levels of ≥13.3 nmol/L at 7 days after CV (p=0.07) were of borderline statistical significance.
Table 4.
Univariate and multivariate predictors of atrial fibrillation recurrence between 1 week and 30 days after electrical cardioversion
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR [CI 95%] | P | OR [CI 95%] | P | |
| Age, years | 1.07 (0.96–1.19) | 0.24 | ||
| Gender, male=1 | 0.99 (0.19–4.94) | 0.9 | ||
| AF history, years | 1.15 (0.89–1.49) | 0.3 | ||
| AF episode duration before CV, days | 1.003 (0.99–1.02) | 0.6 | ||
| Coronary artery disease | 3.42 (0.38–31.08) | 0.3 | ||
| Diabetes | 1.43 (0.25–8.23) | 0.7 | ||
| Chronic obstructive pulmonary disease | 0.95 (0.09–9.31) | 0.9 | ||
| Chronic kidney disease | 2.28 (0.39–13.05) | 0.4 | ||
| Left ventricular ejection fraction, % | 0.86 (0.72–1.03) | 0.09 | 0.79 (0.54–1.15) | 0.2 |
| Left atrial diameter, mm | 1.06 (0.85–1.32) | 0.6 | ||
| Left atrial emptying fraction <38% | 3.18 (0.62–16.24) | 0.16 | 10.35(0.29–36.24) | 0.2 |
| NPT I, nmol/L | 1.11 (0.96–1.28) | 0.17 | 0.96 (0.65–1.41) | 0.8 |
| NPT II, nmol/L | 1.31 (0.99–1.72) | 0.05 | 1.79 (0.61–5.24) | 0.3 |
| NPT III, nmol/L | 1.26 (0.96–1.65) | 0.09 | 1.32 (0.66–2.61) | 0.4 |
| IL-6 I, pg/mL | 1.09 (0.82–1.45) | 0.5 | ||
| IL-6 II, pg/mL | 0.98 (0.81–1.17) | 0.8 | ||
| IL-6 III, pg/mL | 0.93 (0.62–1.41) | 0.7 | ||
| ACEI/ARB | 2.85 (0.56–14.52) | 0.21 | ||
| Statin | 0.75 (0.08–7.12) | 0.8 | ||
ACEI - angiotensin-converting enzyme inhibitor; AF - atrial fibrillation; ARB - angiotensin II receptor blocker; CI - confidence interval; CV - electrical cardioversion; IL-6 - interleukin-6; NPT - neopterin; OR - odds ratio
Table 5.
Predictors of atrial fibrillation recurrence during 12 months after electrical cardioversion
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR [CI 95%] | P | HR [CI 95%] | P | |
| Age, years | 1.003 (0.98–1.04) | 0.8 | ||
| Gender, male=1 | 0.92 (0.51–1.67) | 0.8 | ||
| AF history, years | 0.99 (0.93–1.07) | 0.9 | ||
| AF episode duration before CV, days | 1.001 (0.99–1.006) | 0.7 | ||
| Coronary artery disease | 0.90 (0.48–1.71) | 0.7 | ||
| Diabetes | 0.75 (0.39–1.41) | 0.4 | ||
| Chronic obstructive pulmonary disease | 1.18 (0.53–2.65) | 0.7 | ||
| Chronic kidney disease | 1.23 (0.68–2.24) | 0.5 | ||
| Left ventricular ejection fraction, % | 0.94 (0.89–0.99) | 0.03 | 0.93 (0.86–1.00) | 0.052 |
| Left atrial diameter, mm | 1.01 (0.94–1.09) | 0.7 | ||
| Left atrial emptying fraction <38% | 1.78 (0.97–3.27) | 0.06 | 2.36 (1.09–5.09) | 0.03 |
| NPT I, nmol/L | 1.02 (0.98–1.06) | 0.4 | ||
| NPT II, nmol/L | 1.03 (0.99–1.07) | 0.15 | 0.99 (0.93–1.06) | 0.8 |
| NPT III, nmol/L | 1.02 (0.98–1.07) | 0.3 | ||
| NPT III≥13.3 nmol/L | 2.81 (1.10–7.16) | 0.03 | 3.21(0.90–11.5) | 0.07 |
| IL-6 I, pg/mL | 0.98 (0.90–1.07) | 0.7 | ||
| IL-6 II, pg/mL | 1.05 (0.97–1.14) | 0.19 | 1.04 (0.95–1.13) | 0.4 |
| IL-6 III, pg/mL | 0.97 (0.84–1.12) | 0.7 | ||
| ACE/ARB | 0.82 (0.44–1.52) | 0.5 | ||
| Statin | 0.69 (0.33–1.44) | 0.3 | ||
ACEI - angiotensin-converting enzyme inhibitor; AF - atrial fibrillation; ARB - angiotensin II receptor blocker; CI - confidence interval; CV - electrical cardioversion; IL-6 - interleukin-6; NPT - neopterin; OR - odds ratio
Discussion
In our study, we focused on the possible relation between inflammation and AF, examining changes in NPT and IL-6 levels in patients with preserved LV systolic function who underwent CV of persistent AF. We found elevated plasma NPT levels in patients with persistent AF. We showed that NPT levels of ≥14.6 nmol/L before CV predicted AF recurrence during the follow-up with a sensitivity and specificity of 73% and 57%, respectively. More importantly, NPT levels of ≥13.3 nmol/L at 7 days after CV could predict arrhythmia recurrence with a sensitivity and specificity of 81% and 75%, respectively. Neither NPT and IL-6 levels at baseline nor their changes within 7 days after CV were predictive for AF recurrence during 12 months of follow-up, although NPT levels of ≥13.3 nmol/L at 7 days after CV showed borderline statistical significance in multivariate analysis (p=0.07). Among various clinical variables, only low LAEF (<38%) by echocardiography was an independent predictor of AF recurrence. Our findings are in agreement with the study by Ökçün et al. (23) who showed that LAEF <30% on transesophageal echocardiography was a predictor of AF recurrence during 6 months of follow-up after CV. It should be emphasized that we adopted a very strict criterion of AF recurrence in our study; this was AF episode lasting ≥30 min and detected by pacemaker data logs. Similarly strict criteria have been implemented in some studies which assessed the outcome of AF ablation. AF recurrence criterion, we adopted, may explain the high percentage of patients with AF relapse during 12-month observation in our study, in comparison with about 50% of patients with AF recurrence in epidemiological studies with different methodology.
Interleukin-6 is one of the most important factors that directly stimulate CRP synthesis (an acute phase reactant) in hepatocytes. Despite some inconsistent results, the majority of studies have indicated that high plasma CRP and IL-6 levels correlate with the presence and duration of AF (11, 24). It has been demonstrated that elevated CRP and IL-6 levels are associated with the risk of immediate CV failure and AF recurrence after CV (9, 25–27). Moreover, the prognostic value of these inflammatory markers was documented and both CRP and IL-6 levels could predict cardiovascular events, development of heart failure, and all-cause mortality (28–30) irrespective of the presence of cardiovascular disease (31–34).
Elevated plasma NPT levels were reported in conditions characterized by increased cellular immune response, such as inflammation, autoimmune and malignant diseases, and after organ transplantation (18, 35). NPT levels are considered an indirect indicator of oxidative stress induced by NPT in these conditions. Growing evidence indicates a role of NPT in cardiovascular risk stratification. NPT has been indicated as a predictor of LV dysfunction in patients with chronic stable coronary artery disease (36). It was associated with mortality among survivors of an acute myocardial infarction (15) and with an increased cardiac event rate in patients with heart failure (21). Data on NPT in patients with AF are very limited. Barani et al. (11) examined inflammatory factors in patients with critical limb ischemia and found a significantly higher serum NPT levels in those with AF than in patients in sinus rhythm. Rubaj et al. (37) reported a significant decrease in NPT levels at 1 week of biatrial pacing in comparison to right atrial appendage pacing.
In our study, we found significantly higher NPT levels in patients with persistent AF than in healthy subjects. In addition, NPT levels at 7 days after CV were significantly higher than in control subjects. This may suggest that an immune response with activation of monocytes/macrophages is involved in the inflammatory reactions during AF. However, the lack of changes in NPT levels after CV may suggest that in patients with persistent AF in whom sinus rhythm was successfully restored, normalization of NPT levels requires a longer time than normalization of CRP and IL-6 levels (3).
In our study, we found no significant differences in baseline IL-6 and CRP levels between patients with persistent AF and control subjects. This is not consistent with other studies that documented increased levels of these inflammatory factors in patients with persistent AF. However, our findings are plausible in the context of the inflammation pathway, because CRP is a downstream product and IL-6 is the most important stimulus for its release. In our study, we excluded patients with increased CRP levels and comorbidities that may cause inflammation. Moreover, most patients were treated with statins and this may have influenced the results, because it was demonstrated that statin administration results in significant decrease of CRP levels (38–40). On the other hand, the fact that NPT levels, but not IL-6 levels, were increased in our sample may indicate NPT as a promising marker of AF induced inflammation and oxidative stress, possibly identifying a patient phenotype vulnerable to AF recurrence.
Interleukin-6 is a pleiotropic proinflammatory cytokine that is produced not only by immune cells but also by endothelial cells, vascular smooth muscle cells, and ischemic cardiomyocytes (41). A recent meta-analysis (42) has demonstrated that IL-6 is not associated with AF recurrence after CV, as it was shown in our study. Our results are not consistent with the studies that reported increased plasma IL-6 levels in patients with AF (1, 3, 5, 10, 11, 28) but our study group was small and highly selected compared to large cohort studies that included as many as 47000 subjects (43) or epidemiological cross-sectional studies (1, 44, 45). However, similarly to Leftheriotis et al. (8), we found a rapid decrease in the IL-6 level following CV in patients who maintained sinus rhythm during the follow-up.
In our study, NPT and IL-6 levels and their changes within 7 days after CV were not useful for predicting AF recurrence during 12 months of follow-up. Among various clinical variables, only low LAEF by echocardiography was an independent predictor of AF recurrence after CV. Of note, our data on AF recurrence were obtained by means of dual-chamber pacemakers that enable a very accurate determination of AF recurrences.
Study limitations
Clearly, our study included a relatively small overall patient sample and a small number of patients in the study group with no AF recurrence during the follow-up after CV. The study group was heterogeneous in regard to concomitant diseases and AF history but in our opinion, this group was representative for most patients with AF referred for CV to our center. We cannot exclude a potential deleterious effect of permanent ventricular pacing on the development of AF in our patients. Furthermore, additional measurements of NPT and IL-6 levels performed at later intervals could provide additional data on the changes of these markers during a longer follow-up. It would also be interesting to obtain data on hs-CRP levels changes following CV but we were unable to measure this parameter in our laboratory.
Conclusions
Patients with persistent AF and preserved LV systolic function have significantly higher NPT levels. Restoration of sinus rhythm did not cause changes in NPT or IL-6 levels within 1 week after CV but NPT levels of ≥13.3 nmol/L at 7 days after CV predicted AF recurrence with high sensitivity and specificity. Further studies are needed to determine the potential significance of NPT measurements in clinical practice, and whether this inflammatory marker plays any role in the pathogenesis of AF.
Acknowledgements:
This study was supported by the Polish Committee for Scientific Research, grant KBN N N402 2383 33.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – E.L., J.D.G.; Design – E.L., J.D.G.; Supervision – E.L., G.R.; Funding – E.L.; Materials – L.D.S.; Data collection &/or processing – A.D.K., P.Z.; Analysis and/or interpretation – P.Z., G.R.; Literature search – L.D.S., G.R.; Writing – A.D.K., A.L.; Critical review – A.L.
References
- 1.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Benjamin EJ. Current perceptions of the epidemiology of atrial fibrillation. Cardiol Clin. 2009;27:13–24. doi: 10.1016/j.ccl.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sata N, Hamada N, Horinouchi T, Amitani S, Yamashita T, Moriyama Y, et al. C- reactive protein and atrial fibrillation. Is inflammation a consequence or a cause of atrial fibrillation? Jpn Heart J. 2004;45:441–5. doi: 10.1536/jhj.45.441. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T, Takeishi Y, Hirono O, Itoh M, Matsui M, Nakamura K, et al. C-reactive protein elevation predicts the occurrence of atrial structural remodeling in patients with paroxysmal atrial fibrillation. Heart Vessels. 2005;20:45–9. doi: 10.1007/s00380-004-0800-x. [DOI] [PubMed] [Google Scholar]
- 5.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, et al. Activation of the complement system during and after cardiopulmonary bypass surgery:postsurgery activation involves C- reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–8. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 6.Psychiari SN, Apostolou TS, Sinos L, Hamodraka E, Liakos G, Kremastinos DT. Relation of elevated C-reactive protein and interleukin -6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am Cardiol. 2005;95:764–7. doi: 10.1016/j.amjcard.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 7.Gómez Martínez E, Borrás Pallé S, Valls Grima F, Miralles Serrano LL, MoltóGuillamont L, Jarabo Bueno MM, et al. Inflammatory state in patients with atrial fibrillation before and after electrical cardioversion. Med Intensiva. 2007;31:126–32. doi: 10.1016/s0210-5691(07)74790-3. [DOI] [PubMed] [Google Scholar]
- 8.Leftheriotis DI, Fountoulaki KT, Flevari PG, Parissis JT, Panou FK, Andreadou IT, et al. The predictive value of inflammatory and oxidative markers following the successful cardioversion of persistent lone atrial fibrillation. Int J Cardiol. 2009;135:361–9. doi: 10.1016/j.ijcard.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Henningsen KM, Therkelsen SK, Bruunsgaard H, Krabbe KS, Pedersen BK, Svendsen JH. Prognostic impact of hs-CRP and IL-6 in patients with persistent atrial fibrillation treated with electrical cardioversion. Scand J Clin Lab Invest. 2009;69:425–32. doi: 10.1080/00365510802676848. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–44. doi: 10.1016/j.hrthm.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barani J, Mattiasson I, Lindblad B, Gottsater A. Cardiac function, inflammatory mediators and mortality in critical limb ischemia. Angiology. 2006;57:437–45. doi: 10.1177/0003319706290743. [DOI] [PubMed] [Google Scholar]
- 12.Liew R, Khairunnisa K, Gu Y, Tee N, Yin NO, Naylynn TM, et al. Role of tumor necrosis factor-αin the pathogenesis of atrial fibrosis and development of an arrhythmogenic substrate. Circ J. 2013;77:1171–9. doi: 10.1253/circj.cj-12-1155. [DOI] [PubMed] [Google Scholar]
- 13.Gramley F, Lorenzen J, Koellensperger E, Kettering K, Weiss C, Munzel T. Atrial fibrosis and atrial fibrillation:the role of the TGF-β1 signaling pathway. Int J Cardiol. 2010;143:405–13. doi: 10.1016/j.ijcard.2009.03.110. [DOI] [PubMed] [Google Scholar]
- 14.Avanzas P, Arroyo-Espliguero R, Quiles J, Roy D, Kaski JC. Elevated serum neopterin predicts future adverse cardiac events in patients with chronic stable angina pectoris. Eur Heart J. 2005;26:457–63. doi: 10.1093/eurheartj/ehi111. [DOI] [PubMed] [Google Scholar]
- 15.Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzales P. Usefulness of neopterin levels and left ventricular function for risk assessment in survivors of acute myocardial infarction. Int J Cardiol. 2006;111:318–20. doi: 10.1016/j.ijcard.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Kaski JC, Consuegra-Sanchez L, Fernandez-Berges DJ, Cruz-Fernandez JM, Garcia-Moll X, Marrugat J, et al. Elevated serum neopterin levels and adverse cardiac events at 6 months follow-up in Mediterranean patients with non-ST-segment elevation acute coronary syndrome. Atherosclerosis. 2008;201:176–83. doi: 10.1016/j.atherosclerosis.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Grammer TB, Fuchs D, Boehm BO, Winkelmann BR, Maerz W. Neopterin as a predictor of total and cardiovascular mortality in individuals undergoing angiography in the Ludwigshafen Risk and Cardiovascular Health study. Clin Chem. 2009;55:1135–46. doi: 10.1373/clinchem.2008.118844. [DOI] [PubMed] [Google Scholar]
- 18.Avanzas P, Arroyo-Espliguero R, Kaski JC. Neopterin and cardiovascular disease:growing evidence for a role in patient risk stratification. Clinical Chemistry. 2009;55:1056–7. doi: 10.1373/clinchem.2009.127084. [DOI] [PubMed] [Google Scholar]
- 19.Dominguez-Rodriguez A, Abreu-Gonzalez P, Avanzas P, Laynez-Cerdena I, Kaski JC. Neopterin predicts left ventricular remodeling in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Atherosclerosis. 2010;211:574–8. doi: 10.1016/j.atherosclerosis.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Caruso R, De Chiara B, Campolo J, Verde A, Musca F, Belli O, et al. Neopterin levels are independently associated with cardiac remodeling in patients with chronic heart failure. Clin Biochem. 2013;46:94–8. doi: 10.1016/j.clinbiochem.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki T, Takeishi Y, Suzuki S, Niizeki T, Kitahara T, Katoh S, et al. High serum level of neopterin is a risk factor of patients with heart failure. Int J Cardiol. 2010;145:318. doi: 10.1016/j.ijcard.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 22.Lang R, Bierig M, Devereux R, Flachskampf F, Foster E, Pellikka PA, et al. Recommendations for chamber quantification:a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Ökçün B, Yiğit Z, Küçükoğlu MS, Şansoy V, Güzelsoy D, Üner S. Predictors for maintance of sinus rhythm after cardioversion in patients with nonvalvular atrial fibrillation. Echocadiography. 2002;19:351–7. doi: 10.1046/j.1540-8175.2002.00351.x. [DOI] [PubMed] [Google Scholar]
- 24.Roldan V, Marin F, Blann AD, Garcia A, Marco P, Sogorb F, et al. Interleukin-6, endothelial activation and thrombogenesis in chronic atrial fibrillation. Eur Heart J. 2003;24:1373–80. doi: 10.1016/s0195-668x(03)00239-2. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe E, Arakawa T, Uchiyama T, Kodama I, Hishida H. High-sensitivity C-reactive protein is predictive of successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversion. Int J Cardiol. 2006;108:346–53. doi: 10.1016/j.ijcard.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 26.Zarauza J, Rodriguez Lera MJ, Farinas Alvarez C, Hernando JP, Ceballos B, Gutierres B, et al. Relationship between C-reactive protein level and early recurrence of atrial fibrillation after electrical cardioversion. Rev Esp Cardiol. 2006;59:125–9. [PubMed] [Google Scholar]
- 27.Smit MD, Maass AH, De Jong AM, Muller Kobold AC, Van Veldhuisen DJ, Van Gelder IC. Role of inflammation in early atrial fibrillation recurrence. Europace. 2012;14:810–7. doi: 10.1093/europace/eur402. [DOI] [PubMed] [Google Scholar]
- 28.Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin-6 and atrial fibrillation in patients with coronary artery disease;data from the Heart and Soul Study. Am Heart J. 2008;155:303–9. doi: 10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kardys I, Knetsch AM, Bleumink GS, Deckers JW, Hofman A, Stricker BH, et al. C-reactive protein and risk of heart failure. The Rotterdam Study. Am Heart J. 2006;152:514–20. doi: 10.1016/j.ahj.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Lip GY, Patel JV, Hughes E, Heart RG. High-sensitivity C-reactive protein and soluble CD40 ligand as indices of inflammation and platelet activation in 880 patients with nonvalvular atrial fibrillation:relationship to stroke risk factors, stroke risk stratification schema, and prognosis. Stroke. 2007;38:1229–37. doi: 10.1161/01.STR.0000260090.90508.3e. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 32.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and onset of cardiovascular events:results from the Health ABC study. Circulation. 2003;108:2317–22. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 33.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality:an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein and all-cause mortality-the Copenhagen City Heart Study. Eur Heart J. 2010;31:1624–32. doi: 10.1093/eurheartj/ehq103. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs D, Weiss G, Reibnegger G, Wachter H. The role of the neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious and malignant disease. Crit Rev Clin Lab Sci. 1992;29:307–41. doi: 10.3109/10408369209114604. [DOI] [PubMed] [Google Scholar]
- 36.Estévez-Loureiro R, Recio-Mayoral A, Sieira-Rodríguez-Moret JA, Trallero-Araguás E, Kaski JC. Neopterin levels and left ventricular dysfunction in patients with chronic stable angina pectoris. Atherosclerosis. 2009;207:514–8. doi: 10.1016/j.atherosclerosis.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 37.Rubaj A, Ruciński P, Kutarski A, Dąbrowska-Kugacka A, Oleszczak K, Zimon B, et al. Cardiac hemodynamics and proinflammatory cytokines during biatrial and right appendage pacing in patients with interatrial block. J Interv Card Electrophysiol. 2013;37:147–54. doi: 10.1007/s10840-013-9792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dernellis J, Panaretou M. Effect of C-reactive protein reduction on paroxysmal atrial fibrillation. Am Heart J. 2005;150:1064. doi: 10.1016/j.ahj.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 39.Kumagai K. Upstream therapy for atrial fibrillation. Circ J. 2007;(Suppl A):A75–81. doi: 10.1253/circj.71.a75. [DOI] [PubMed] [Google Scholar]
- 40.Loffredo L, Angelico F, Perri L, Violi F. Upstream therapy with statin and recurrence of atrial fibrillation after electrical cardioversion. Review of the literature and meta-analysis. BMC Cardiovasc Disord. 2012;12:107. doi: 10.1186/1471-2261-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–70. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 42.Wu N, Xu B, Xiang Y, Wu L, Zhang Y, Ma X, et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation:a meta-analysis. Int J Cardiol. 2013;169:62–72. doi: 10.1016/j.ijcard.2013.08.078. [DOI] [PubMed] [Google Scholar]
- 43.Marott SC, Nordestgaard BG, Zacho J. Does elevated C-reactive protein increase atrial fibrillation risk. J Am Coll Cardiol. 2010;56:789–95. doi: 10.1016/j.jacc.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 44.Anderson JL, Allen Maycock CA, Lappé DL, Crandall BG, Horne BD, Bair TL, et al. Intermountain Heart Collaborative Study group. Frequency of elevation of C-reactive protein in atrial fibrillation. Am J Cardiol. 2004;94:1255–9. doi: 10.1016/j.amjcard.2004.07.108. [DOI] [PubMed] [Google Scholar]
- 45.Asselbergs FW, van den Berg MP, Diercks GF, van Gilst WH, van Veldhuisen DJ. C-reactive protein and microalbuminuria are associated with atrial fibrillation. Int J Cardiol. 2005;98:73–7. doi: 10.1016/j.ijcard.2003.12.028. [DOI] [PubMed] [Google Scholar]



