Abstract
Objective:
This study aimed to evaluate the association between the history of stroke/transient ischemic attack (TIA) and inter- and intra-atrial electromechanical delay (EMD) in patients with paroxysmal atrial fibrillation (PAF).
Methods:
Patients diagnosed with PAF were included in this retrospective study. Patients who had a history of stroke or TIA were defined as the symptomatic group, whereas those who did not have such a history were defined as the asymptomatic group. On the basis of the transthoracic echocardiographic records, atrial electromechanical coupling (time interval from the onset of the P wave on the surface electrocardiogram to the beginning of the A’ wave interval with tissue Doppler echocardiography) and intra- and interatrial EMD were measured.
Results:
In this study, 160 patients were included, 52 of whom were symptomatic. While the intra-left atrial EMD was 68.2±6.1 ms in the symptomatic group, it was found to be 50.8±6.5 ms in the asymptomatic group (p<0.001). Interatrial EMD was 91.3±5.0 ms in the symptomatic group, whereas it was 71.5±7.0 ms in the asymptomatic group (p<0.001). In multiple logistic regression analysis, intra-left atrial [odds ratio (OR): 1.417, 95% confidence interval (CI): 1.193–1.684, p<0.001] and interatrial EMDs (OR:1.398, 95% CI: 1.177–1.661, p<0.001) were found to be independently associated with the presence of stroke/TIA.
Conclusion:
Prolonged inter- and intra-left atrial EMDs in patients with PAF is associated with stroke/TIA. Evaluating this parameter in addition to the CHA2DS2-VASc score in patients with PAF may be helpful in identifying patients who are at a high risk of stroke/TIA. (Anatol J Cardiol 2016; 16: 572-8)
Keywords: atrial fibrillation, stroke, electromechanical delay
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia and is responsible for 50% of cardioembolic strokes. One third of all stroke cases are cryptogenic, occurring particularly in younger patients. Paroxysmal atrial fibrillation (PAF) is considered to play a possible major role in these cases (1–3). Because of the asymptomatic nature of PAF attacks, it is difficult to identify patients having a rare and short-term PAF. The echocardiographic parameters identifying PAF in patients are not specific and can be influenced by concomitant diseases and the patient’s hemodynamic status.
Prolonged inter- and intra-atrial conduction times are indicators of atrial conduction heterogeneity, which is important in the development of AF. The prolongation of atrial electromechanical delay (EMD) is linked to various mechanisms, including atrial dilatation and remodeling, chronic inflammation within the atrial myocardium, and atrial fibrosis (4–6). In atrial EMD measurement, although the assessment made by electrophysiological studies is a gold standard, it is not possible for routine use because of its invasive nature. However, in recent studies, when compared with EMD values which measured by electrophysiologic study, also tissue Doppler measurements were reported to have acceptable accuracy (7, 8).
Prolonged atrial EMD time was reported to be helpful in identifying patients with PAF and in predicting the incidence of atrial fibrillation (9,10). However, data regarding the importance of atrial EMD measurement is limited to identifying patients with PAF who were at a high risk of stroke (11, 12). We designed our study to compare inter- and intra-atrial EMDs among patients having PAF with or without neurological symptoms. Moreover, we aimed to assess whether inter- and intra-atrial EMDs additionally contributed to the CHA2DS2-VASc score in identifying patients with PAF who were at a high risk of stroke/transient ischemic attack (TIA).
Methods
One hundred and sixty patients who were admitted to our clinic from 2011 to 2014 with non-rheumatic PAF were enrolled in this single-centered retrospective study. PAF diagnosis was made on the basis of the 12-lead electrocardiography (ECG) or Holter ECG recordings. AF attacks spontaneously terminating in a period less than 7 days were considered to be PAF. Patients with heart failure (36 patients), moderate to severe valvular disease (42 patients), and thyroid dysfunction (12 patients), which was not euthyroid, were excluded from this study. Patients with electrolyte imbalance, previous myocardial infarction, collagen tissue disease, cardiomyopathy, patent foramen ovale, and other congenital heart diseases and patients using antiarrhythmic drugs were also excluded from this study. Baseline demographic features were registered. The patients’ CHA2DS2-VASc scores were calculated (13). Stroke is defined as the brain, spinal cord, or retinal cell death that is attributable to ischemia based on the neuropathological, neuroimaging, and/or clinical evidence of permanent injury. TIAs are episodes of temporary and focal dysfunction of vascular origin, which are variable in duration, commonly lasting from 2 to 15 min but occasionally lasting for long as a day (24 h) (14). Patients who had a history of stroke or TIA were defined as the symptomatic group, whereas those who did not have such a history were defined as the asymptomatic group. This study was approved by the Local Ethics Board.
ECG with 12 derivations (Nihon Kohden, Tokyo, Japan) was performed for all patients. All patients were subjected to two-dimensional, M-mode, pulsed and color Doppler flow echocardiographic examinations (Philips EPIQ 7 Cardiac Ultrasound) by the same cardiologist who was incognizant regarding the stroke/TIA history of patients. During echocardiography, a single-lead (D2) electrocardiogram was simultaneously recorded. Data were recorded from the average of three cardiac cycles. M-mode and Doppler measurements were performed according to the American Society of Echocardiography guidelines by the same cardiologist (15). All measurements were recorded over an average of at least three cycles.
Tissue Doppler ECG was performed with a transducer at a frequency of 3.5–4.0 MHz by adjusting the spectral pulsed Doppler signal filters until a Nyquist limit of 15–20 cm/s was obtained and using the minimal optimal gain. The monitor sweep speed was set to 100 mm/s. In the apical four-chamber view, the pulsed Doppler sample volume was placed at the level of LV lateral mitral annulus, septal mitral annulus, and RV tricuspid annulus. Atrial electromechanical coupling (PA’), which is the time interval from the onset of the P wave on the surface electrocardiogram to the beginning of the late diastolic wave (A’), was obtained from the lateral mitral annulus (PA’lateral), septal mitral annulus (PA’septal), and tricuspid annulus (PA’tricuspid), respectively (Fig.1). The difference between PA’lateral and PA’tricuspid was defined as the interatrial EMD, whereas the time difference between PA’septal and PA’tricuspid was defined as the intra-right atrial EMD; the difference between PA’septal and PA’lateral was defined as the intra-left atrial EMD.
Figure 1.
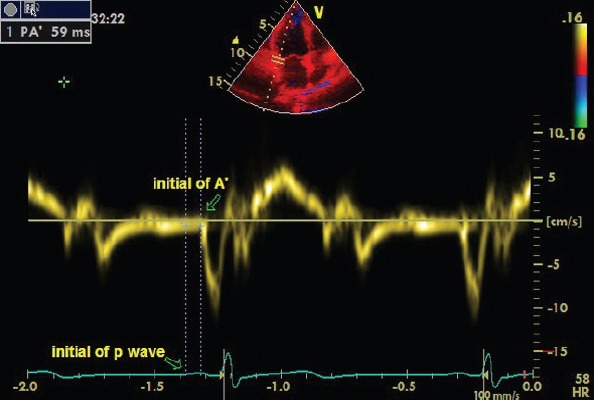
Atrial electromechanical coupling (PA’), the time interval from the onset of the P wave on the surface electrocardiogram to the beginning of the late diastolic wave A’.
Statistical analysis
Data were analyzed using the SPSS software version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). Categorical variables were presented as frequency and percentage. The c2 test was used to compare the categorical variables. The Kolmogorov–Smirnov test was used to assess the distribution of continuous variables. Student’s t-test was used for variables with normal distribution, and the values were presented as mean±SD. Continuous variables without normal distribution were analyzed using Mann–Whitney U test, and the values obtained were presented as median (50th) values and interquartile ranges (25th and 75th). Receiver operating characteristic (ROC) curve analysis was performed to identify the optimal cut-off values for stroke/TIA history. The effects of different variables on stroke/TIA history were calculated by univariate analysis for each variable. The variables for which the unadjusted p value was 0.10 in logistic regression analysis were identified as potential risk markers and were included in the full model. Model A was developed to evaluate the effect of intra-left atrial EMD in multiple logistic regression analysis, while Model B was developed to evaluate the effect of inter-atrial EMD. The odds ratios (OR) and 95% confidence intervals (CI) were calculated. A two-tailed p value of <0.05 was considered statistically significant.
Results
In this study, 160 patients were included, of which 52 were symptomatic. The mean age in the symptomatic group was 68±9 years, whereas it was 62±13 years in the asymptomatic group (p=0.028). There was no statistical difference between the symptomatic and asymptomatic groups with respect to gender; use of beta blocker; smoking; and presence of coronary artery disease, diabetes mellitus, hypertension, and hyperlipidemia. No significant difference was found between the groups in terms of the ratio of those patients who were within the target range for INR at the time of echocardiographic examination (59% vs. 62%, p=0.236) (Table 1).
Table 1.
Clinical characteristics of the study population
| Variables | Stroke/TIA (+)(n=52) | Stroke/TIA (-)(n=108) | P |
|---|---|---|---|
| Age, years | 68±9 | 62±13 | 0.028 |
| Male, n, % | 30 (58) | 46 (43) | 0.245 |
| Body mass index, kg/m2 | 27.5±4.1 | 28.1±3.8 | 0.544 |
| Hypertension, n, % | 46 (89) | 93 (86) | 1.000 |
| Diabetes mellitus, n, % | 18 (35) | 24 (22) | 0.287 |
| Coronary artery disease, n, % | 30 (58) | 38 (35) | 0.068 |
| Dyslipidemia, n, % | 28 (54) | 38 (35) | 0.148 |
| Smoking, n, % | 14 (27) | 24 (22) | 0.782 |
| Warfarine usage, n,% | 37 (71) | 85 (79) | 0.489 |
| Aspirin usage, n, % | 16 (31) | 39 (36) | 0.672 |
| Thyroid dysfunction, n, % | 8 (15) | 11 (10) | 0.491 |
| CHA2DS2-VASc score | 5.0±1.2 | 2.6±1.4 | <0.001 |
Data are expressed as mean±standard deviation for normally distributed data and percentage for categorical variables
The CHA2DS2-VASc score of the symptomatic group was 5.0±1.2, while that of the asymptomatic group was 2.6±1.4. However, no statistically significant difference was found between the CHA2DS2-VASc scores of the symptomatic and asymptomatic groups when two points given for stroke/TIA were subtracted from the CHA2DS2-VASc scores of the symptomatic group (3.0±1.2 vs. 2.6±1.4, p=0.07).
Regarding conventional parameters, such as left ventricular ejection fraction and left ventricular hypertrophy, no difference was ascertained between the two groups. Furthermore, no discrepancy was found between the two groups in terms of mild mitral regurgitation, mild aortic regurgitation, mild tricuspid regurgitation, systolic pulmonary arterial pressure, and E/A ratio. However, the diameter of the left atrium (parasternal long-axis) was found to be greater in the symptomatic group than in the asymptomatic group (p=0.002). In the symptomatic and asymptomatic groups, left ventricular end-systolic and -diastolic diameters were within normal limits; furthermore, the diameters were significantly higher in the symptomatic group than in the asymptomatic group (p=0.009, p=0.001, respectively) (Table 2).
Table 2.
Conventionally transthoracic echocardiographic parameters of the study population
| Variables (normal range) | Stroke/TIA (+)(n=52) | Stroke/TIA (-)(n=108) | P |
|---|---|---|---|
| LVEF,% (55%≤) | 60 (58–64) | 62 (60–65) | 0.321 |
| LVEDD, mm (39–59 mm) | 50.4±7.2 | 46.1±4.4 | 0.001 |
| LVESD, mm (21–40) | 34.3±9.0 | 29.7±6.4 | 0.009 |
| LAD, mm (27–40 mm) | 46±6.1 | 41±7.0 | 0.002 |
| LV hypertrophy presence, % | 58 | 59 | 1.000 |
| Mitral E/A ratio | 1.1 (0.6–1.5) | 1.4 (0.7–1.7) | 0.109 |
| Mitral EDT, ms | 225±70 | 213±59 | 0.444 |
| IVRT, ms | 112±16 | 108±22 | 0.417 |
| Aortic root, mm (22–36 mm) | 32.7±3.9 | 31.4±4.6 | 0.225 |
Data are expressed as mean±standard deviation for normally distributed data, median (50th) values and interquantile ranges (25th and 75th) for continuous variables without normal distribution, and percentage for categorical variables
EDT - E wave deceleration time; IVRT - isovolumetric relaxation time; LAD - left atrium diameter, parasternal long axis; LVEDD - left ventricular end-diastolic diameter; LVEF - left ventricular ejection fraction; LVESD - left ventricular end-systolic diameter
Intra-left atrial EMD was 68.2±6.1 ms in the symptomatic group, whereas it was 50.8±6.5 ms in the asymptomatic group (p<0.001). Moreover, interatrial EMD was 91.3±5 ms in the symptomatic group, whereas it was 71.5±7.0 ms in the asymptomatic group (p<0.001). There was no significant difference observed between the two groups with respect to intra-right atrial EMD (Table 3). The cut-off value of 60 ms, which was determined by the ROC curve for intra-left atrial EMD, had 80.6% sensitivity and 94.9% specificity to predict stroke/TIA (area under the curve, 0.895; p<0.001) (Fig. 2). The cut-off value of 81 ms, which was determined by the ROC curve for interatrial EMD, had 83.9% sensitivity and 98.3% specificity to predict stroke/TIA (area under the curve, 0.899; p<0.001) (Fig. 3).
Table 3.
Inter- and intra-atrial EMDs measured by tissue Doppler imaging
| Variables | Stroke/TIA (+)(n=52) | Stroke/TIA (-)(n=108) | P |
|---|---|---|---|
| P-A’septal, ms | 62.3±8.4 | 65.6±9.2 | 0.127 |
| P-A’lateral, ms | 131±9 | 116±10 | <0.001 |
| P-A’tricuspid, ms | 39.2±7.8 | 44.9±9.1 | 0.007 |
| Interatrial EMD, ms | 91.3±5.0 | 71.5±7.0 | <0.001 |
| Intra-left atrial EMD, ms | 68.2±6.1 | 50.8±6.5 | <0.001 |
| Intra-right atrial EMD, ms | 23.1±5.1 | 20.7±5.9 | 0.076 |
EMD - electromechanical delay; TIA - transient ischemic attack (variables with normal distribution were expressed as mean±SD)
Figure 2.
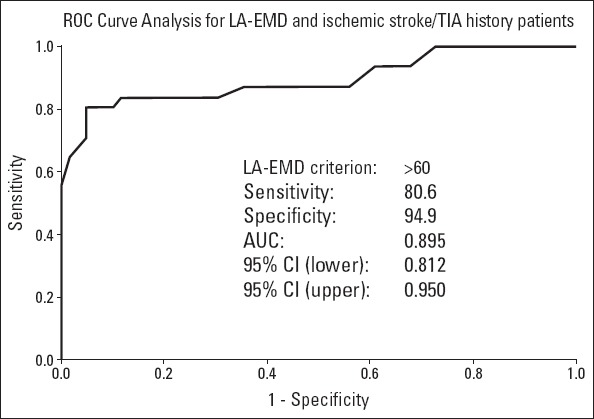
Receiver operating characteristics curve of intra-left atrial EMD for predicting stroke/TIA in patients with PAF
Figure 3.
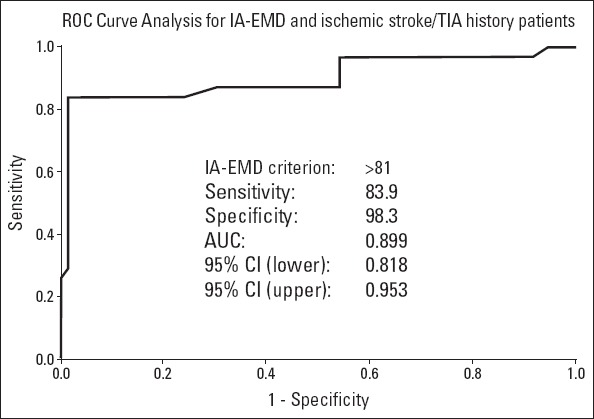
Receiver operating characteristics curve of interatrial EMD for predicting stroke/TIA in patients with PAF
Univariate and multiple logistic regression analyses were performed for the factors (LA-EMD, IA-EMD, age, left atrial diameter, DM, HT, CAD, smoking, and hyperlipidemia) that were associated with the history of stroke/TIA. Univariate logistic regression analysis revealed that there was an independent association between stroke/TIA and left atrial diameter, intra-left atrial EMD, and interatrial EMD. Multiple logistic regression analysis demonstrated that there was an independent association between the intra-left atrial EMD and stroke/TIA (OR: 1.417, 95% CI: 1.193–1.684, p<0.001). Multiple logistic regression analysis also demonstrated that there was an independent association between the inter-atrial EMD and stroke/TIA (OR: 1.398, 95% CI: 1.177–1.661, p<0.001) (Table 4).
Table 4.
Predictors of stroke/TIA in univariate and multivariate logistic regression analyses
| Model A (LA-EMD) | Model B (IA-EMD) | |||||
|---|---|---|---|---|---|---|
| Univariate | Multiple | Multiple | ||||
| Variable | OR (95% CI) | P | Adjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
| LA-EMD | 1.280 (1.156–1.418) | <0.001 | 1.417 (1.193–1.684) | <0.001 | ||
| IA-EMD | 1.271 (1.157–1.396) | <0.001 | 1.398 (1.177–1.661) | <0.001 | ||
| Age | 1.045 (1.003–1.089) | 0.034 | 1.008 (0.931–1091) | 0.840 | 1.004 (0.926–1.088) | 0.930 |
| LA | 1.245 (1.083–1.430) | 0.002 | 1.878 (0.948–3.718) | 0.071 | 1.574 (0.807–3.070) | 0.183 |
| DM | 1.531 (0.584–4.008) | 0.386 | 2.038 (0.233–17.844) | 0.520 | 0.284 (0.022–3.665) | 0.335 |
| HT | 0.936 (0.284–3.081) | 0.913 | 50.380 (1.349–188.644) | 0.034 | 84.635 (2.679–267.235) | 0.012 |
| CAD | 3.087 (1.253–7.606) | 0.014 | 0.039 (0.003–0.448) | 0.009 | 0.032 (0.002–0.423) | 0.009 |
| Smoking | 1.315 (0.493–3.505) | 0.584 | 0.978 (0.153–6.259) | 0.981 | 1.729 (0.189–15.824) | 0.628 |
| HL | 1.696 (0.701–4.103) | 0.241 | 9.731 (1.010–93.718) | 0.049 | 10.433 (0.898–121.139) | 0.061 |
CAD - coronary artery disease; CI - confidence interval; DM - diabetes mellitus; HL - hyperlipidemia; HT - hypertension; IA-EMD - interatrial electromechanical delay; LA - left atrium; LA-EMD - intra-left atrial electromechanical delay; OR - odds ratio
Discussion
Interatrial and intra-left atrial EMD were found to be associated with patients who had PAF and a history of stroke/TIA in our trial in this study. The cut-off value, which was identified by ROC curve to predict stroke/TIA, was found to be 81 ms for interatrial-EMD (83.9% sensitivity and 98.3% specificity) and 60 ms for intra-left atrial EMD (80.6% sensitivity and 94.9% specificity). The CHA2DS2-VASc scores were measured and found to be similar in the symptomatic and asymptomatic groups when two points given for stroke/TIA were subtracted from the CHA2DS2-VASc score of the symptomatic group, whereas interatrial and intra-left atrial EMDs remained significantly higher in the symptomatic group. In multiple logistic regression analysis, the prolongation of intra-left atrial and interatrial EMDs was found to be independent predictors for the presence of stroke/TIA for this patient group. This result was considered particularly important to identify patients with PAF who were at a higher risk of stroke/TIA if they had elevated means of interatrial and intra-left atrial EMDs.
Prolonged inter- and intra-atrial conduction times are indicators of atrial conduction heterogeneity. The gold standard method for atrial EMD measurement is electrophysiological studies. However, routine use is not possible because of the lack of easily accessible electrophysiological studies, which are invasive procedures. Therefore, in recent studies, the measurability of atrial EMD by tissue Doppler echocardiography, which is a noninvasive method, was evaluated. In a study conducted by Deniz et al. (8), in the measurement of atrial conduction time in patients with PAF, the results of electrophysiological studies and tissue Doppler examination were compared, and intra-left atrial EMD measured by ECG and left intra-left atrial conduction time were found to be correlated. Moreover, in this study, the measurement of intra-left atrial EMD by ECG was reported to be useful in identifying patients with PAF. In contrast, in a study conducted by Evranos et al. (7), the predictors of AF recurrence after ablation were investigated. Intra-left atrial EMD was reported to be independent predictors of AF recurrence. Furthermore, in this study, moderate correlation between intra-left atrial EMD and intra-left atrial conduction time measured by electrophysiological studies was found. In our study population, comprising patients with PAF, tissue Doppler ECG records were used to investigate the association between atrial EMD measurements and presence of stroke/TIA history. We believe that this technique can contribute more to the clinical practice because it is easily accessible and non-invasive.
AF is responsible for 10% of all strokes and 50% of cardioembolic strokes (1). AF-related strokes have poor prognosis, cause severe deficits, and persist in >50% of living patients. The recurrence rate of AF-related stroke is up to 12% per year (16). Recent trials have demonstrated that the risk of stroke in patients with PAF is similar to the risk in patients with chronic AF (17). Thus, it may be essential to identify patients with PAF who are at a high risk of stroke at an early stage. In previous trials, inter- and intra-atrial EMDs were demonstrated to be elevated in patients with lone AF compared with the values obtained from a normal population. Moreover, the measurement of inter- and intra-atrial EMDs may help in identifying patients with lone AF (9, 10). However, in real life, most patients with PAF have accompanying diseases. We enrolled patients with PAF and accompanying diseases. The importance of measuring inter- and intra-atrial EMDs to identify patients with PAF and with an increased risk of stroke/TIA was evaluated in our study. Intra- and inter-atrial EMDs were prolonged in patients with a history of stroke/TIA despite similar CHA2DS2-VASc scores when two points given for stroke/TIA were subtracted.
AF is characterized by uncoordinated atrial activation with consequent deterioration of atrial mechanical function, and the underlying cause of AF is a recurrent condition (18, 19). Intra-atrial EMD and marked fragmentation of atrial activity have been recently suggested to be the characteristic of PAF (20). Several trials demonstrated that the measurement of inter- and intra-atrial EMDs identified patients with PAF and patients with an increased risk of AF after cardiac surgery (9, 10, 21). Moreover, interatrial and intra-left atrial EMDs were reported to be useful in predicting the progression from PAF to chronic AF (22). Deftereos et al. (23) evaluated interatrial EMD (high right atrium- distal coronary sinus) by means of an electrophysiological study in 612 patients without a history of atrial fibrillation or valvular heart disease, and interatrial EMD was demonstrated to be associated with the risk of AF development during a follow-up of 43 months. Interatrial EMD was found to increase in patients who developed AF compared with that in patients without AF. Furthermore, measuring interatrial EMD was reported to be beneficial to ascertain patients with an increased risk of AF (23). Another study, which included patients with paroxysmal AF, demonstrated that increased intra-left atrial and interatrial EMDs were associated with an increased number of annual AF attacks even in patients with normal LA diameter. Furthermore, it was asserted that these parameters might predict patients with PAF even in early stage (24). In our study, left atrial diameter was found to be greater in the symptomatic group than in the asymptomatic group. In addition, in accordance with an increase in the diameter of the left atrium, intra-left atrial and interatrial conduction times have been found to be longer in this group.
The inception and continuation of AF require electrical and structural changes in the atria. In a trial comprising 213 patients, total atrial conduction time was reported to be important to predict AF recurrence after radiofrequency catheter ablation (25). In another trial comprising 279 patients, the PA-PDI interval (time interval between the P wave on ECG and peak A’ of mitral inflow obtained with pulse wave Doppler examination) was reported to be useful in identifying patients with an increased risk of stroke after successful AF ablation. Patients were followed up for 46.5±17.2 months after ablation. Increased CHA2DS2-VASc scores and prolonged PA-PDI interval were detected in patients who developed a stroke during the follow up. A cut-off value of 150 ms for the PA-PDI interval to predict a stroke had a positive predictive value of 86.7% and negative predictive value of 100%. A cut-off value of 150 ms, which was identified by the ROC curve, had 100% sensitivity and 85.7% specificity in predicting stroke following ablation. According to these results, the continuation of warfarin treatment was recommended following AF ablation in patients with the PA-PDI interval equal to or >150 ms (26).
Hoshi et al. (27) stated that patients with PAF had a larger left atrial volume index and longer interatrial EMD compared with the normal population. When patients with PAF were reassessed according to their history of cardioembolic stroke, age and interatrial EMD were found to be independently related to cardioembolic stroke; however, no association was demonstrated between the left atrial volume index and history of cardioembolic stroke. Moreover, an increased incidence of cardioembolic stroke was found in patients with PAF and interatrial EMD of 82 ms or longer. The measurements of EMD to investigate the association with cardioembolic stroke were only obtained from lateral mitral tissue Doppler in this study. Similar to the abovementioned trial, interatrial and intra-left atrial EMDs were prolonged in patients with a history of stroke/TIA in our study. Recently Vatan et al. (11) found a significant association between the left atrial mechanical functions and CHA2DS2-VASc score in patients with PAF, whereas they did not find an association between the atrial EMD and CHA2DS2-VASc score. However, the CHA2DS2-VASc scores of patients were lower, and there were a relatively low number of patients in this trial compared with that in our study.
Anticoagulant treatment that was initiated following the diagnosis of AF decreases the risk of stroke by 40% as opposed to antiplatelet therapy alone (28). Therefore, diagnosing AF and identifying patients having an increased risk of stroke is vital to initiate maximal stroke preventive therapy. ECG on admission and 24 h Holter ECG are usually performed for patients with TIA/stroke in our daily practice. However, short-term PAF attacks are not easily ascertained with these methods. The measurement of interatrial and intra-left atrial EMDs is a fast, easy, and highly reproducible technique and can be performed through almost all echocardiography devices. There have been some patients with PAF who have had stroke, although they have had low CHA2DS2-VASc scores in our clinical practice. Calculating interatrial and intra-left atrial EMDs might be helpful in predicting the risk of stroke, particularly in these patients.
The value of EMD to identify patients with PAF was reported in previous studies (9, 10). However, a significant correlation was found in our study between the presence of stroke/TIA in patients with PAF and EMD. More prolonged EMD was considered to reflect the increased risk of stroke/TIA in patients with PAF, probably because of the increased burden of arrhythmia. Therefore, we consider that the measurement of EMD, which is a non-invasive and easily accessible technique, may contribute not only to identify patients with PAF but also to define patients with an increased risk of recurrent stroke/TIA during the evaluation of patients with stroke/TIA. However, there is a requirement for prospective and large trials for this matter.
Study limitations
Our study is a retrospective, non-randomized, and small sample size trial. The carotid artery Doppler studies and Holter ECG recordings could not be performed in all patients. Echocardiographic examinations were performed in the period when the patients were in sinus rhythm; however, it could not be determined with certainty how long the patient was in sinus rhythm. Therefore, this may have influenced the atrial conduction time in patients with atrial stunning. Left atrial diameter was merely used to evaluate the left atrial size, and the measurement of the left atrial volume index was not possible in all patients because of the retrospective nature of our study. Most patients were suffering from palpitations for at least 1 month according to the patients’ medical records. However, the exact duration of PAF was not established because of the fact that PAF attacks might be asymptomatic. As TEE was not performed in most patients having PAF without a history of stroke in our study, no analysis was conducted for the association between EMD and the presence of LAA thrombus. Moreover, electrophysiological study, which is the gold standard method to calculate inter- and intra-atrial EMDs, was not performed in patients enrolled in this study. In accordance with the relatively advanced average age in groups, our results may not accurately reflect the findings in younger patients because of the frequency of comorbidities. The prolongation of atrial EMD effects on stroke/TIA recurrence could not be assessed because our study plan did not include long-term follow-up.
Conclusion
Intra-left atrial EMD of >60 ms and interatrial EMD of >81 ms are associated with the history of stroke/TIA, and they may be determinants in predicting the stroke risk. The measurement of interatrial and intra-left atrial EMDs may provide additional benefit to the CHA2DS2-VASc score for predicting the stroke risk in patients with PAF. There is a requirement for prospective and large trials to explore the association between EMD and stroke in patients with PAF.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – N.B.; Design – N.B., Ş.A.; Supervision – G.Ç., Z.E.; Funding – Z.E., Ş.A., Ç.M.Ü.; Materials – S.K., Z.E.; Data collection &/or processing – S.K., S.Ç.; Analysis and/or interpretation – G.Ç., Ş.A., S.Ç.; Literature search- S.K.; Writing – N.B., Z.E.; Critical review – Ş.A., N.B., Ç.M.Ü.
References
- 1.Hart RG. Stroke prevention in atrial fibrillation. Curr Cardiol Rep. 2000;2:51–5. doi: 10.1007/s11886-000-0025-2. [DOI] [PubMed] [Google Scholar]
- 2.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria:incidence, recurrence, and long-term survival in ischemic stroke subtypes:a population-based study. Stroke. 2001;32:2735–40. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 3.Seet RC, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation. 2011;124:477–86. doi: 10.1161/CIRCULATIONAHA.111.029801. [DOI] [PubMed] [Google Scholar]
- 4.Pham TD, Fenoglio JJ., Jr Right atrial ultrastructural in chronic rheumatic heart disease. Int J Cardiol. 1982;1:289–304. doi: 10.1016/0167-5273(82)90091-2. [DOI] [PubMed] [Google Scholar]
- 5.Perzanowski C, Ho AT, Jacobson AK. Increased P–wave dispersion predicts recurrent atrial fibrillation after cardioversion. J Electrocardiol. 2005;38:43–6. doi: 10.1016/j.jelectrocard.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Çağdaş M, Velibey Y, Güvenç TS, Güngör B, Güzelburç O, Çalık N, et al. Evaluation of atrial electromechanical conduction delay in case of hemodynamically insignificant rheumatic heart disease:A tissue Doppler study. Cardiol J. 2015 Jul 23; doi: 10.5603/CJ.a2015.0043. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Evranos B, Aytemir K, Oto A, Okutucu S, Karakulak U, Şahiner L, et al. Predictors of atrial fibrillation recurrence after atrial fibrillation ablation with cryoballoon. Cardiol J. 2013;20:294–303. doi: 10.5603/CJ.2013.0075. [DOI] [PubMed] [Google Scholar]
- 8.Deniz A, Şahiner L, Aytemir K, Kaya B, Kabakçı G, Tokgözoğlu L, et al. Tissue Doppler echocardiography can be a useful technique to evaluate atrial conduction time. Cardiol J. 2012;19:487–93. doi: 10.5603/cj.2012.0089. [DOI] [PubMed] [Google Scholar]
- 9.Platonov PG, Yuan S, Hertervig E, Kongstad O, Roijer A, Vygovsky AB, et al. Further evidence of localized posterior interatrial conduction delay in lone paroxysmal atrial fibrillation. Europace. 2001;3:100–7. doi: 10.1053/eupc.2001.0150. [DOI] [PubMed] [Google Scholar]
- 10.Deniz A, Yavuz B, Aytemir K, Hayran M, Köse S, Okutucu S, et al. Intra-left atrial mechanical delay detected by tissue Doppler echocardiography can be a useful marker for paroxysmal atrial fibrillation. Echocardiography. 2009;26:779–84. doi: 10.1111/j.1540-8175.2008.00881.x. [DOI] [PubMed] [Google Scholar]
- 11.Vatan MB, Yılmaz S, Ağaç MT, Çakar MA, Erkan H, Aksoy M, et al. Relationship between CHA2DS2-VASc score and atrial electromechanical function in patients with paroxysmal atrial fibrillation:A pilot study. J Cardiol. 2015 Mar 24; doi: 10.1016/j.jjcc.2015.02.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Nar G, İnci S, Aksan G, Soylu K, Demirelli S, Nar R. The relationships between atrial electromechanical delay and CHA2DS2-VASc score in patients diagnosed with paroxysmal AF. Echocardiography. 2015;32:1359–66. doi: 10.1111/echo.12855. [DOI] [PubMed] [Google Scholar]
- 13.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation:the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 14.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21stcentury:a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–89. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group;American Society of Echocardiography’s Guidelines and Standards Committee;European Association of Echocardiography. Recommendations for chamber quantification:a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Mattle HP. Long-term outcome after stroke due to atrial fibrillation. Cerebrovasc Dis. 2003;16(Suppl 1):3–8. doi: 10.1159/000069934. [DOI] [PubMed] [Google Scholar]
- 17.Inoue H, Atarashi H, Okumura K, Yamashita T, Kumagai N, Origasa H. Thromboembolic events in paroxysmal vs. permenant non-valvular atrial fibrillation. Subanalysis of the J-RHYTHM Registry. Circ J. 2014;78:2388–93. doi: 10.1253/circj.cj-14-0507. [DOI] [PubMed] [Google Scholar]
- 18.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 19.Allessie MA. Reentrant mechanisma underlying atrial fibrillation. In: Zipes DP, Jalife J, editors. From Cell to Bedside. Philadelphia: Saunders; 1995. pp. 562–6. [Google Scholar]
- 20.Shimizu A, Fukatani M, Tanigawa M, Mori M, Hashiba K. Intra-atrial conduction delay and fragmented atrial activity in patients with paroxysmal atrial fibrillation. Jpn Circ J. 1989;53:1023–30. doi: 10.1253/jcj.53.1023. [DOI] [PubMed] [Google Scholar]
- 21.Karaca M, Demirbaş MI, Biçeroğlu S, Çevik A, Çetin Y, Arpaz M, et al. Prediction of early postoperative atrial fibrillation after cardiac surgery:is it possible? Cardiovasc J Afr. 2012;23:34–6. doi: 10.5830/CVJA-2011-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakabe K, Fukuda N, Fukuda Y, Morishita S, Shinohara H, Tamura Y. Interatrial dyssynchrony on tissue Doppler imaging predicts progression to chronic atrial fibrillation in patients with non-valvular paroxysmal atrial fibrillation. Heart. 2009;95:988–93. doi: 10.1136/hrt.2008.152561. [DOI] [PubMed] [Google Scholar]
- 23.Deftereos S, Kossyvakis C, Efremidis M, Bouras G, Panagopoulou V, Papadimitriou C, et al. Interatrial conduction time and incident atrial fibrillation:A prospective cohort study. Heart Rhythm. 2014;11:1095–101. doi: 10.1016/j.hrthm.2014.03.053. [DOI] [PubMed] [Google Scholar]
- 24.Çalık AN, Özcan KS, Çağdaş M, Güngör B, Karaca G, Gürkan U, et al. Electromechanical delay detected by tissue Doppler echocardiography is associated with the frequency of attacks in patients with lone atrial fibrillation. Cardiol J. 2014;21:138–43. doi: 10.5603/CJ.a2013.0106. [DOI] [PubMed] [Google Scholar]
- 25.den Uijl DW, Gawrysiak M, Tops LF, Trines SA, Zeppenfeld K, Schalij MJ, et al. Prognostic value of total atrial conduction time estimated with tissue Doppler imaging to predict the recurrence of atrial fibrillation after radiofrequency catheter ablation. Europace. 2011;13:1533–40. doi: 10.1093/europace/eur186. [DOI] [PubMed] [Google Scholar]
- 26.Chao TF, Lin YJ, Tsao HM, Chang SL, Lo LW, Hu YF, et al. Prolonged atrium electromechanical interval is associated with stroke in patients with atrial fibrillation after catheter ablation. J Cardiovasc Electrophysiol. 2013;24:375–80. doi: 10.1111/jce.12054. [DOI] [PubMed] [Google Scholar]
- 27.Hoshi Y, Nozawa Y, Ogasawara M, Yuda S, Sato S, Sakasai T, et al. Atrial electromechanical interval may predict cardioembolic stroke in apparently low risk elderly patients with paroxysmal atrial fibrillation. Echocardiography. 2014;31:140–8. doi: 10.1111/echo.12329. [DOI] [PubMed] [Google Scholar]
- 28.Hart RG, Pearce LA, Aguilar MI. Meta-analysis:anti-thrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]


