Abstract
Objective:
Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a transcriptional coactivator that has been proposed to play a protective role in mouse models of cardiac ischemia and heart failure, suggesting that PGC-1α could be relevant as a prognostic marker. Our previous studies showed that the estimation of peripheral mRNA PGC-1α expression was feasible and that its induction correlated with the extent of myocardial necrosis and left ventricular remodeling in patients with myocardial infarction. In this study, we sought to determine if the myocardial and peripheral expressions of PGC-1α are well correlated and to analyze the variability of PGC-1α expression depending on the prevalence of some metabolic disorders.
Methods:
This was a cohort of 35 consecutive stable heart failure patients with severe aortic stenosis who underwent an elective aortic valve replacement surgery. mRNA PGC-1α expression was simultaneously determined from myocardial biopsy specimens and blood samples obtained during surgery by quantitative PCR, and a correlation between samples was made using the Kappa index. Patients were divided into two groups according to the detection of baseline expression levels of PGC-1α in blood samples, and comparisons between both groups were made by chi-square test or unpaired Student’s t-test as appropriate.
Results:
Based on myocardial biopsies, we found that mRNA PGC-1α expression in blood samples showed a statistically significant correlation with myocardial expression (Kappa index 0.66, p<0.001). The presence of higher systemic PGC-1α expression was associated with a greater expression of some target genes such as silent information regulator 2 homolog-1 (x-fold expression in blood samples: 4.43±5.22 vs. 1.09±0.14, p=0.044) and better antioxidant status in these patients (concentration of Trolox: 0.40±0.05 vs. 0.34±0.65, p=0.006).
Conclusions:
Most patients with higher peripheral expression also had increased myocardial expression, so we conclude that the non-invasive estimation of mRNA PGC-1α expression from blood samples provides a good approach of the constitutive status of the mitochondrial protection system regulated by PGC-1α and that this could be used as prognostic indicator in cardiovascular disease. (Anatol J Cardiol 2016; 16: 622-9)
Keywords: peroxisome proliferator-activated receptor-γ coactivator-1α, mRNA expression, cardiac surgery, prognosis markerIntroduction
Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a transcriptional coactivator and master regulator of genes involved in oxidative metabolism and mitochondrial biogenesis that controls cellular responses to metabolic demands inducing genes that coordinately increase the oxidative capacity of the cell, including mitochondrial biogenesis and energy metabolism genes (1). Enhanced oxidative phosphorylation is normally associated with increased mitochondrial reactive oxygen species (ROS) production, and this negative effect is amply compensated by PGC-1α induction of antioxidant systems, resulting in a net reduction of cellular ROS levels when PGC-1α is active (2). Thereby, PGC-1α has been shown to play a fundamental role in metabolic control as well as in the terminal differentiation of high-energy metabolism tissues including the myocardium (3, 4).
Constitutive PGC-1α expression levels are likely to reflect the general metabolic status of patients, with high levels presumably associated with a higher level of oxidative metabolism, high oxygen consumption rates, and reduced general oxidative stress, while low basal PGC-1α levels are expected to be associated with enhanced reliance on glycolytic metabolism, low oxygen consumption, and elevated ROS levels. This last condition tended to be more frequent in diabetic patients, as previously reported (5). Importantly, low PGC-1α levels may also be the cause of the high levels of ROS and poor tolerance to ischemic injury generally associated with those patients (6, 7).
In this regard, PGC-1α has been proposed to play a protective role in mouse models of cardiac ischemia and heart failure (8, 9), so it is suggested that PGC-1α levels are relevant as prognostic marker in patients with cardiovascular disease. Because the direct analysis of PGC-1α expression in the heart is not possible in clinical practice, we previously performed testing in acute myocardial infarction patients to determine whether PGC-1α activity in peripheral blood samples could be analyzed, and we found that the estimation of peripheral PGC-1α expression was feasible (10) and that its induction correlated with the extent of myocardial necrosis and left ventricular remodeling in ST-segment elevation myocardial infarction patients (11).
Nonetheless, if PGC-1α expression in blood samples is correlated to myocardial expression has not been previously evaluated, it would be imperative to provide value to these previous clinical studies. Therefore, in this study, we simultaneously analyzed the expression of PGC-1α in myocardial biopsies and peripheral blood in patients undergoing cardiac surgery and correlated it with the expression of superoxide dismutase 2 (SOD2), a target gene within the mitochondrial system of oxidative stress protection. The myocardial and peripheral mRNA expressions of both AMP-activated protein kinase (AMPK) and silent information regulator 2 homolog 1 (SIRT-1) were also analyzed as key regulators of PGC-1α activity. AMPK and SIRT-1 are two metabolic sensors that could directly affect PGC-1α activity through post-transcriptional modifications (12). Thus, phosphorylation by AMPK results in an increase in PGC-1α protein levels as a consequence of increased protein stability, and deacetylation via SIRT-1 keeps PGC-1α in an active state. Taken together, these players constitute an energy-sensing axis that controls metabolic homeostasis, and the abnormal function of this network could be essential in the pathophysiology of type 2 diabetes and other metabolic disorders (13).
In addition, total antioxidant status and autophagy markers were analyzed as indicators of comorbidity and advanced disease. Autophagy is the process for degrading and recycling long-lived proteins and cytoplasmic organelles through the lysosome, which plays an important role in cardiac remodeling, aging, and inflammation to maintain cellular homeostasis in the heart. In this regard, some stressors such as hypoxia could induce autophagy that protects against ischemia-reperfusion injury (14, 15); it is suggested that autophagy plays a key role in mediating the regression of cardiac hypertrophy during mechanical unloading (16, 17).
Therefore, the aim of the study was to determine if the myocardial and peripheral expressions of PGC-1α and its target genes are well correlated and to analyze the variability of PGC-1α expression depending on the pro-inflammatory status of patients and prevalence of some metabolic diseases such as diabetes.
Methods
Study population
This is a cohort of 35 consecutive patients electively referred to our Department of Cardiac Surgery (Cardiovascular Institute from General University Hospital, Valencia, Spain) to undergo aortic valve replacement surgery between June and December 2014. Inclusion criteria were as follows: severe aortic valve disease indicating replacement surgery, with or without concomitant coronary heart disease; no episodes of acute decompensated heart failure or acute coronary syndrome in the previous 3 months; and age of >18 years with a capacity to sign the informed consent form. Were selected stable patients with aortic valve disease to homogenize both the study population with respect to clinical and surgical characteristics as the protocol to obtain the cardiac biopsies?
Clinical and biochemical data
Clinical data collected included medical history with cardiovascular risk factors and medications and baseline biochemical values such as glucose, hemoglobin, and creatinine levels. The parameters analyzed to characterize immune response were as follows: leukocyte count prior to surgery and 48 h later (including total neutrophil, lymphocyte, and monocyte counts and their relative percentages); baseline, 24 h, and 48 h C-reactive protein (CRP) levels; and baseline, 8 h, 24 h, and 48 h lactate levels. As indirect marker of myocardial necrosis was used the maximum value of troponin I during admission.
Echocardiography
Transthoracic echocardiogram was performed in all patients as local protocol. Left ventricular ejection fraction, left ventricular end-diastolic and end-systolic diameters, tricuspid annular plane systolic excursion, and systolic pulmonary artery pressure through a tricuspid regurgitate jet were measured before surgery.
Variable of cardiac surgery
All patients underwent aortic valve replacement through medial sternotomy. Those patients with concomitant coronary artery disease underwent coronary bypass surgery in the same intervention. The total time of extracorporeal circulation and time of myocardial ischemia were recorded, as well as the number of coronary artery bypass grafts, where appropriate.
Acquisition of myocardial biopsies
Endomyocardial biopsies were obtained during aortic valve replacement. Briefly, after on-pump cannulation and aortic cross-clamping, the heart was arrested using a cold cardioplegia solution. Endoventricular access was performed through a 10–15-mm oblique aortotomy above the sinotubular junction, and endomyocardial specimens were excised from the left ventricular basal septum using a cold scalpel No. 11.
Preparation of mononuclear cells from blood samples
Immediately before starting extracorporeal circulation, 6–8 mL of peripheral blood collected in EDTA vacutainers (BD) were used to isolate mononuclear cells by Ficoll density gradient centrifugation using Ficoll-Paque™ (Miltenyi Biotec, Surrey, United Kingdom), following the manufacturer’s instructions. Isolated cells were analyzed using Cytospin and Fast Panoptic Staining (Panreac, Barcelona, Spain). Only preparations containing ≥90% mononuclear cells were used for the analysis.
Real-time quantitative PCR
Total RNA was isolated using Trizol™ (Invitrogen), following the manufacturer’s instructions. The quality of RNA was evaluated in a bioanalyzer and quantitated in a nanodrop. RNA used had a ratio of absorbance at 260 nm and 280 nm of ≥1.8 and an RNA integrity number of ≥8. Relative mRNA expression levels of PGC-1α, SOD2, AMPK, and SIRT-1 were determined by quantitative PCR of retrotranscribed cDNA with specific primers as previously described (18), both for peripheral blood samples and cardiac biopsies. Results were expressed as x-fold expression over the minimum value detected among all patients.
Antioxidant assay kit
The total antioxidant status of the patients was estimated using a Trolox™ Equivalent Antioxidant Capacity assay (Sigma-Aldrich, St. Louis, USA) in plasma samples based on the scavenging of the 2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical (ABTS•), a soluble chromogen that is converted into a colorless product when it reacts with the ferryl myoglobin radical (a product from the reaction between metmyoglobin and hydrogen peroxide). The concentration of Trolox, a water-soluble vitamin E analog, was used in analyses as the antioxidant control.
Ethics
The experimental protocol was approved by the Local Ethical Committees: Research Foundation and Cardiovascular Institute from University General Hospital of Valencia. All patients signed a written informed consent form, and all procedures conformed to the Declaration of Helsinki.
Statistics
Categorical values were expressed as absolute numbers and percentages and continuous variables as mean±standard deviation. The Kolmogorov–Smirnov test was used to test the normality of the distribution. Patients were divided in two groups according to the detection of baseline expression levels of PGC-1α in blood samples, assuming a two-fold expression over the minimum value detected as the cut-off value. Comparisons between both groups were made by the chi-square test or unpaired Student’s t-test as appropriate. The correlation between peripheral and cardiac samples was made using the Kappa index. All p-values refer to two-tailed tests of significance and p-values of <0.05 were considered to be significant. Statistical analyses were performed using SPSS for Windows, release 19.0 (SPSS Inc., Chicago, IL).
Results
Baseline patient characteristics
The general characteristics of the patients are presented in Table 1. The patients were divided in two groups according to the detection of the baseline expression levels of PGC-1α in blood samples. Both groups were comparable with respect to cardiovascular risk factors, medication, laboratory and echocardiographic parameters, and heart failure functional class, with a trend toward a higher prevalence of diabetic patients in the group with a lower baseline expression of PGC-1α.
Table 1.
Baseline patient characteristics according to the expression of PGC-1α in blood samples
| All patients (n=35) | Patients with peripheral PGC-1α expression >2 -fold (n=11) | Patients with peripheral PGC-1α expression <2 -fold (n=24) | P | |
|---|---|---|---|---|
| Age, years | 69.8±11.9 | 63.3±15.7 | 72.6±8.8 | 0.10 |
| Male, n (%) | 19 (54) | 7 (64) | 12 (50) | 0.22 |
| Cardiovascular risk factors, n (%) | ||||
| Hypertension | 29 (83) | 9 (82) | 20 (83) | 0.91 |
| Diabetes | 9 (26) | 1 (9) | 8 (33) | 0.13 |
| Dyslipidemia | 22 (63) | 6 (55) | 16 (67) | 0.50 |
| Smoking history | 18 (51) | 7 (64) | 11 (46) | 0.34 |
| Medication, n (%) | ||||
| ACEI or ARB | 23 (66) | 7 (64) | 16 (67) | 0.52 |
| Diuretics | 24 (69) | 6 (55) | 18 (75) | 0.24 |
| Beta-blockers | 14 (40) | 7 (64) | 7 (29) | 0.07 |
| Estatins | 20 (57) | 6 (55) | 14 (58) | 0.84 |
| Metformin | 6 (17) | 1 (9) | 5 (21) | 0.14 |
| ODM | 3 (9) | 1 (9) | 2 (9) | 0.92 |
| Insulin | 4 (12) | 0 (0) | 4 (18) | 0.14 |
| Laboratory | ||||
| Hemoglobin, g/dL | 13.1±1.1 | 13.4±1.2 | 12.9±1.1 | 0.15 |
| Creatinine, mg/dL | 0.8±0.2 | 0.9±0.2 | 0.8±0.3 | 0.62 |
| Hb1Ac (%) | 6.2±0.6 | 6.0±0.9 | 6.6±0.5 | 0.36 |
| Echocardiography | ||||
| LVEF (%) | 60.4±10.3 | 59.4±14.2 | 60.9±8.2 | 0.72 |
| LVEDD, mm | 48.8±9.4 | 51.6±10.9 | 47.5±8.5 | 0.20 |
| LVESD, mm | 30.7±9.0 | 33.9±10.5 | 29.1±8.0 | 0.23 |
| TAPSE, mm | 22.7±6.0 | 24.0±4.7 | 22.1±6.6 | 0.40 |
| sPAP, mm Hg | 39.1±9.9 | 35.5±8.2 | 40.8±10.3 | 0.11 |
| Coronarography | ||||
| CAD, n (%) | 8 (23) | 5 (45) | 3 (13) | 0.03 |
| VSS | 0.2±0.6 | 0.4±0.9 | 0.2±0.5 | 0.41 |
| History of heart failure | ||||
| NYHA functional class | 2.7±0.5 | 2.6±0.5 | 2.7±0.5 | 0.87 |
ACEI - angiotensin-converting enzyme inhibitor; ARB - angiotensin II receptor blocker; CAD - coronary artery disease; LVEF - left ventricular ejection fraction; LVEDD - left ventricular end-diastolic diameter; LVESD - left ventricular end-diastolic end-systolic diameter; ODM - other oral an diabetic medication; sPAP - systolic pulmonary artery pressure; TAPSE - tricuspid annular plane systolic excursion; VSS - No of vessels with significant stenosis. Comparisons between both groups were made by chi-square and unpaired Student’s t-tests. Quantitative data expressed as mean±standard deviation. Significant if p<0.05
When coronarography results were analyzed, a significantly higher prevalence of coronary artery disease was found in patients with a higher constitutive expression of PGC-1α. Nonetheless, if we compare cardiac surgery variables, such as time of ischemia or peak of troponin monitored after surgery, depending on this condition, no significant differences were detected (Table 2).
Table 2.
Cardiac surgery variables according to the expression of PGC-1α in blood samples
| All patients (n=35) | Patients with peripheral PGC-1α expression >2-fold (n=11) | Patients with peripheral PGC-1α expression <2-fold (n=24) | P | |
|---|---|---|---|---|
| Echocardiographic presurgical gradients, mm Hg | ||||
| Peak aortic gradient | 81.3±24.0 | 77.0±31.6 | 82.9±21.6 | 0.61 |
| Mean aortic gradient | 44.6±16.5 | 41.6±21.3 | 45.8±14.8 | 0.57 |
| Extracorporeal circulation | ||||
| Ischemia times, min | 104.5±35.9 | 111.7±41.2 | 100.9±33.6 | 0.47 |
| Coronary artery bypass grafting | ||||
| Concomitant BS | 5 (14) | 3 (27) | 2 (8) | 0.65 |
| Total coronary bypass | 9 | 5 | 4 | 0.83 |
| Myocardial necrosis related to surgery | ||||
| Peak of troponin, ng/mL | 1.4±2.6 | 1.7±2.7 | 1.2±2.6 | 0.59 |
Comparisons between both groups were made by chi-square and unpaired Student’s t-tests. Quantitative data expressed as mean±standard deviation. Significant if p<0.05.
BS - bypass surgery
Correlation between mononuclear cell and myocardial expressions
When a scatter plot with both variables was generated, we found a large dispersion of values, consistent with the variability of PGC-1α expression in clinical studies (Fig. 1). Therefore, to establish if peripheral and myocardial expressions of PGC-1α were indeed correlated, a cross table with both expressions was constructed classifying patients according to the cut-off value of 2-fold expression level, as described previously. With this, we observed a good correlation between both expressions with a kappa index of 0.66 (p<0.001); 8 of the 11 patients with higher peripheral expression also had increased myocardial expression.
Figure 1.
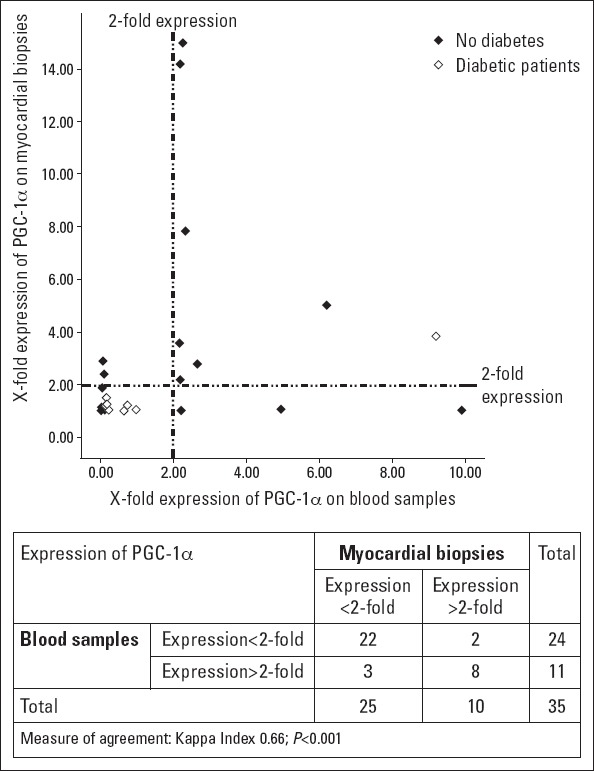
Comparative mRNA expression of PGC-1α in blood samples and myocardial biopsy specimens
Patients were divided according to the cut-off value of 2-fold PGC-1α expression in blood samples (i.e., patients with a significant higher expression: >2-fold expression and patients without detectable PGC-1α expression: <2-fold expression). Kappa index was used as measure of agreement between both determinations
PGC-1α target genes and regulators
To evaluate if peripheral PGC-1α expression correlated with the expression of target genes, SOD2 expression was determined both in blood samples and myocardial biopsy specimens. We found that SOD2 mRNA levels were slightly increased in patients with higher peripheral PGC-1α expression, although this was only in myocardial biopsy specimens, and differences were not statistically significant (x-fold: 2.01±3.21 vs. 1.03±0.03, p=0.14) (Fig. 2).
Figure 2.
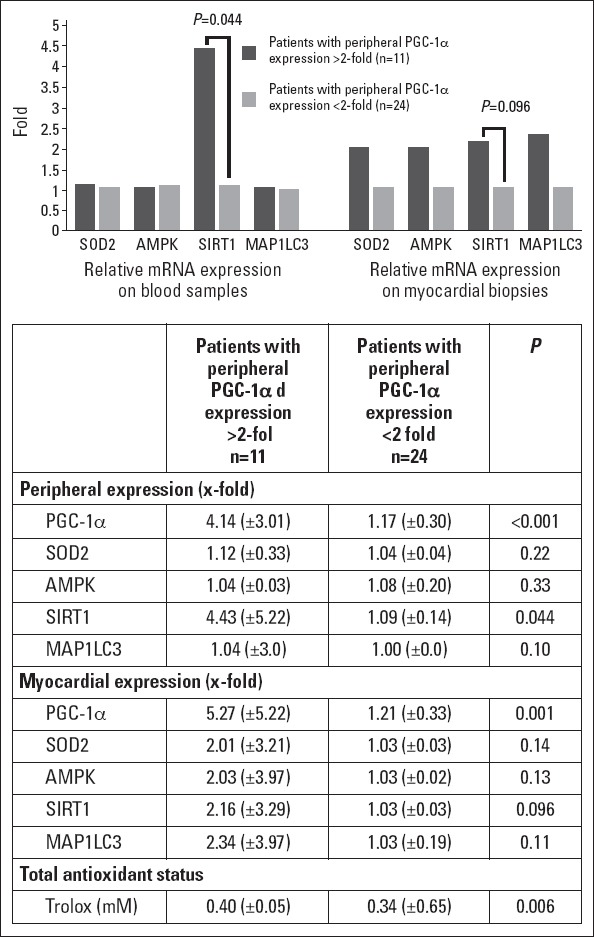
Relative mRNA expression of target genes and total antioxidant status according to PGC-1α expression in blood samples.
mRNA expression is represented as x -fold expression over the minimum expression detected among all patients. SOD2-superoxide dismutase 2. AMPK-AMP-activated protein kinase. SIRT-1-silent information regulator 2 homolog 1. MAP1LC3-microtubule-associated protein-1 light chain-3. Trolox: 6-hidroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
To assess if post-transcriptional regulators that have been shown to increase the activity of PGC-1α were differently expressed in both groups according to peripheral PGC-1α expression, we determined AMPK and SIRT-1 mRNA levels both in blood samples and myocardial biopsy specimens. We found that in patients with a higher peripheral PGC-1α expression, SIRT-1 mRNA expression was increased both in blood samples and myocardial biopsy specimens, although only in the first reached significance (x-fold: 4.43±5.22 vs. 1.09±0.14, p=0.044 in blood samples; and 2.16±3.29 vs. 1.03±0.03, p=0.096 in myocardial biopsy specimens) (Fig. 2). No differences were found in AMPK expression.
Autophagy and total antioxidant status
As a marker of autophagy, we analyzed the expression of microtubule-associated protein-1 light chain-3 (MAP1LC3), which is involved in the elongation of the membrane of autophagosomes, in both groups. We found that the expression of MAP1LC3 transcripts was mildly increased in the myocardial biopsy specimens of patients with a higher peripheral PGC-1α expression, although differences were not significant (x-fold: 2.34±3.97 vs. 1.03±0.19, p=0.11) (Fig. 2).
When we determine the total antioxidant status as an indirect marker of pro-inflammatory condition of the patients in both groups, we observed that those with a greater peripheral PGC-1α expression had a higher concentration of Trolox (0.40±0.05 vs. 0.34±0.65, p=0.006), suggesting higher oxidative metabolism levels, high oxygen consumption rates, and reduced general oxidative stress.
Immune response
To characterize the immune response triggered by surgery, absolute and relative leukocyte counts and CRP and lactate levels were monitored after cardiac intervention (Table 3A). We found that patients with a greater baseline PGC-1α expression in blood samples had a higher absolute monocyte count before surgery (0.68±0.16 vs. 0.55±0.11, p=0.01) and a trend toward a high absolute neutrophil count, without other significant differences in absolute and relative leukocyte counts. With respect to lactate levels, which are increased with anaerobic glycolysis, we found that patients with a higher expression of PGC-1α showed lower levels at 8 h after surgery, suggesting more reliance on oxidative metabolism. We found similar differences at 24 h, although these were not significant. Finally, regarding CRP levels, an inflammatory unspecific marker, we observed that patients with a high PGC-1α expression had significant lower levels before surgery (0.21±0.11 vs. 0.50±0.42, p=0.01), suggesting a less inflammatory burden in these patients.
Table 3.
Immune response triggered by cardiac surgery: leukocyte counts and C-reactive protein and lactate levels according to peripheral PGC-1α expression
| Patients with peripheral PGC-1α expression >2 fold (n=11) | Patients with peripheral PGC-1α expression <2 fold (n=24) | P | |
|---|---|---|---|
| A | |||
| Leukocytes | |||
| Absolute counts (·109/L) | |||
| Neutrophils at baseline | 7.41 (±2.30) | 5.51 (±2.78) | 0.06 |
| Neutrophils 48 h | 14.34 (±3.96) | 13.45 (±4.14) | 0.55 |
| Lymphocytes at baseline | 1.94 (±0.66) | 1.78 (±0.67) | 0.52 |
| Lymphocytes 48 h | 1.01 (±0.53) | 0.81 (±0.44) | 0.26 |
| Monocytes at baseline | 0.68 (±0.16) | 0.55 (±0.11) | 0.01 |
| Monocytes 48 h | 1.09 (±0.53) | 0.94 (±0.33) | 0.33 |
| Relative counts, (%) | |||
| Neutrophils at baseline | 67.1 (±9.5) | 65.7 (±10.7) | 0.71 |
| Neutrophils 48 h | 85.7 (±5.4) | 88.1 (±3.1) | 0.10 |
| Lymphocytes at baseline | 22.5 (±8.3) | 23.7 (±9.6) | 0.72 |
| Lymphocytes 48 h | 6.9 (±4.4) | 5.4 (±2.2) | 0.19 |
| Monocytes at baseline | 7.5 (±1.3) | 7.1 (±1.9) | 0.53 |
| Monocytes 48 h | 6.7 (±3.0) | 6.3 (±1.7) | 0.61 |
| Lactate, mmol/L | |||
| 8 h after surgery | 1.45 (±0.91) | 2.81 (±1.53) | 0.002 |
| 24 h after surgery | 2.11 (±0.92) | 2.74 (±1.11) | 0.09 |
| 48 h after surgery | 2.02 (±1.12) | 1.92 (±0.86) | 0.80 |
| C-reactive protein, mg/dL | |||
| Before surgery | 0.21 (±0.11) | 0.50 (±0.42) | 0.037 |
| 24 h after surgery | 9.06 (±4.93) | 7.59 (±3.36) | 0.31 |
| 48 h after surgery | 1.24 (±4.17) | 1.41 (±6.11) | 0.39 |
| B | |||
| Leukocytes | |||
| Percentage changes (%) | |||
| Neutrophil 48 h - 0 h/0 h | 104.3 (±63.3) | 167.7 (±93.0) | 0.026 |
| Lymphocyte 48 h - 0 h/0 h | -41.9 (±35.7) | -38.8 (±85.8) | 0.88 |
| Monocyte 48 h - 0 h/0 h | 65.1 (±78.6) | 78.5 (±82.5) | 0.65 |
Comparisons between both groups were made by unpaired Student’s t-test. Quantitative data expressed as mean±standard deviation. Percentage changes indicate the percentage difference between the value at baseline and the value at 48 h. Significant if p<0.05
To better compare the individual response to surgery, we analyzed percentage changes between the groups and found that patients with a lower PGC-1α expression showed a greater percentage increase in the absolute neutrophil count at 48 h, with no other significant differences (Table 3B).
PGC-1α expression in metabolic disorders
As shown in Figure 1, only 1 of the 9 diabetic patients in our population showed elevated expression of PGC-1α both in blood samples and myocardial biopsy specimens. When we also considered those patients with glycated hemoglobin of ≥6.5%, which is cut-off value recommended for diagnosing diabetes according current guidelines (19), we found similar significant distributions (Table 4). This downregulation in PGC-1α mRNA expression observed in patients with metabolic disorders, assuming both with previous diagnosis of diabetes and those with glycated hemoglobin of ≥6.5%, correlated with lower transcripts of SIRT-1 and SOD2, although the latter was not significant (Fig. 3). No significant differences were reached in the total antioxidant status and autophagy markers.
Table 4.
PGC-1α expression in blood samples and myocardial biopsy specimens according to the presence of metabolic disorders
| PGC-1 α expression in blood samples | PGC-1 α expression in cardiac samples | |||||
|---|---|---|---|---|---|---|
| >2-fold P | <2-fold | P | >2-fold | <2-fold | P | |
| Prior diagnosis of diabetes | ||||||
| Yes | 1 | 8 | 0.12 | 1 | 8 | 0.19 |
| No | 10 | 16 | 9 | 17 | ||
| Prior diagnosis of diabetes and/or glycated hemoglobin of ≥6.5% | ||||||
| Yes | 2 | 12 | 0.07 | 1 | 13 | 0.02 |
| No | 9 | 12 | 9 | 12 | ||
Comparisons between groups were made by the chi-square test. Significant if p<0.05
Figure 3.
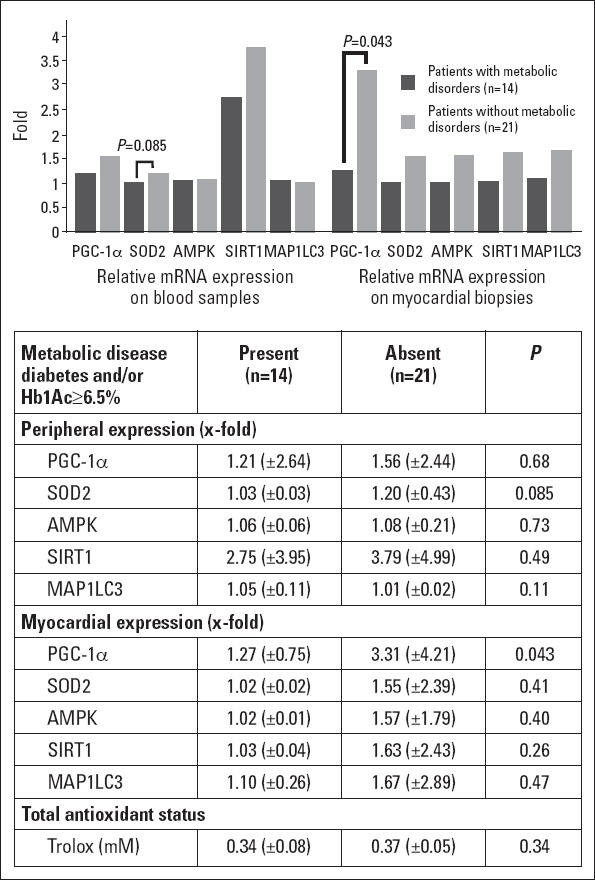
Relative mRNA expression of target genes and total antioxidant status according to the prevalence of metabolic disorders.
Were considered both known diabetic patients as those with Hb1Ac of ≥6.5%. mRNA expression is represented as x -fold expression over the minimum expression detected among all patients. SOD2 - superoxide dismutase 2. AMPK-AMP-activated protein kinase. SIRT-1 - silent information regulator 2 homolog 1. MAP1LC3 - microtubule-associated protein-1 light chain-3. Trolox- 6-hidroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
Discussion
This study shows that PGC-1α mRNA levels in blood samples are well correlated with myocardial expression for the first time and could thus be reliable to estimate the constitutive expression of PGC-1α and monitor changes in different clinical settings.
Our previous studies (10, 11) performed in acute myocardial infarction patients showed that the peripheral estimation of PGC-1α expression was feasible and that patients could be classified in two alternative ways based on PGC-1α levels: those with significant detectable levels of PGC-1α mRNA and those without detectable constitutive expression. The first study showed that infarct size positively correlated with the induction of PGC-1α expression after acute coronary syndrome. The second study supported those notions and found that both low basal PGC-1α levels and induction correlated with larger necrotic areas after myocardial infarction. To validate these results, we conducted the present study in cardiac surgery patients and suggested a good correlation between both expression levels.
Because the expression of PGC-1α considerably varies in clinical studies, unlike experimental studies where the control of certain variables is ensured, we divided patients into two groups according to the detectable expression, establishing a cut-off value of 2-fold expression over the minimum detected value among patients. This method of analysis of PGC-1α mRNA expression facilitates the classification of patients and easily allows the assessment of the clinical significance of results. To our knowledge, all previous clinical studies used biopsy specimens to determine PGC-1α mRNA expression both with myocardial (20) and skeletal muscle biopsy specimens (21, 22), the most commonly used. Our protocol estimating peripheral PGC-1α expression could provide a rough overview of the systemic activation of this coactivator and its target genes without using invasive or overly sophisticated techniques.
The constitutive higher expression of PGC-1α has been shown to be related to the upregulation of mitochondrial biogenesis and antioxidant defenses and an increased reliance on oxidative phosphorylation, especially in organs with a high metabolic rate (i.e., brain, muscle, liver, and heart). For example, PGC-1α plays a key role in regulating the expression of mitochondrial antioxidants such as SOD2 in protecting the heart against myocardial oxidative stress (23). Furthermore, it is assumed that post-transcriptional regulators that enhance PGC-1α activity would be upregulated under this condition. In our study, both SOD2 and SIRT-1 transcripts tended to be increased in patients with a higher PGC-1α expression, although differences were not significant in all comparisons. Nonetheless, these results together suggest a coordinated activation of the mitochondrial protection system regulated by PGC-1α.
In this regard, when we analyzed the potential link of PGC-1α mRNA expression with autophagy mechanisms and redox status, because these processes have been suggested to be related in some metabolic stress models (24, 25), we found that the upregulation of MAP1LC3 as a marker of autophagy was slight and not significant in the group of patients with a higher PGC-1α expression. However, the estimated antioxidant capacity by indirectly Trolox concentration was significantly increased, reinforcing the hypothesis of a better redox balance in these patients. In this respect, several studies have focused on redox homeostasis regulated by PGC-1α and cardioprotection, showing that the induction of PGC-1α expression could reduce mitochondrial damage (Flameng mitochondrial function score: 0.44±0.13 in the group with higher expression of PGC-1α vs. 1.70±0.03 in the control group) and prevent cellular apoptosis in the remote area of the left ventricular myocardium (Connexin 43 expression: 1.03±0.29 in the group with a higher expression of PGC-1α vs. 0.53±0.14 in the control group) in acute myocardial infarction models (26, 27). Therefore, the clinical use of our protocol to monitor PGC-1α expression may be useful in this context.
With regard to immune response analysis, the close relationship between metabolism and immunity and how the aberrant expression of PGC-1α could be linked to persistent systemic inflammation and a higher risk for some chronic diseases are well known (28). Our results indicate a better immune profile in patients with a higher PGC-1α expression with less baseline CRP levels before surgery and less lactate production at 8 h. Furthermore, acute unspecific response represented by neutrophil relative change was more tempered in these patients. These findings contribute to provide more evidence in recent years pointing to a potential link between low-grade inflammation in the heart and metabolic dysregulation.
In this respect, novel insights highlight the crosstalk between inflammatory processes and metabolic alterations in the failing heart in diabetic patients (29). Notably, PGC-1α activation might prevent metabolic disturbances occurring during diabetic cardiomyopathy, while inhibiting inflammatory processes in the heart, in particular, modulating nuclear factor-kB. Our previous results already indicated that diabetic patients show low PGC-1α mRNA levels in blood samples, compared with those without metabolic disorders. In this study, we proved that the vast majority of known diabetic patients showed lower PGC-1α expression both in blood samples and myocardial biopsy specimens, which was also observed in patients still not diagnosed with metabolic disease but with Hb1Ac of ≥6.5% determined during admission. This downregulation of PGC-1α expression was associated by a trend to the lower expression of different gene targets analyzed and the antioxidant status of patients.
Study limitations
There are some limitations such as the small sample size of patients, single-center experience, and unique blood samples and myocardial biopsy specimens for each patient. Nonetheless, although some results did not reach statistical significance, they are consistent with those found in our previous studies.
Conclusions
In this study performed in patients undergoing cardiac surgery, mRNA PGC-1α expression in blood samples showed a good correlation with the expression in myocardial biopsy specimens. The presence of a higher systemic PGC-1α expression was associated with higher peripheral SIRT-1 levels and Trolox concentration, suggesting a better antioxidant status in these patients. Conversely, lower PGC-1α levels were related to a significant inflammation profile, and this condition was more frequent in patients with metabolic disorders.
Acknowledgements:
Grant from Sociedad Valenciana de Cardiología, 2013 to Óscar Fabregat-Andrés.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – O.F.A., F.P., M.M., J.C.; Design – O.F.A., F.P., M.M., J.M., F.R.S., J.C.; Supervision – F.R.S., L.F., S.M., J.M.L., J.C.; Funding – J.C., J.M., O.F.A.; Materials – J.C., J.M., O.F.A.; Data collection &/or processing – J.C., S.G.H.; Analysis and/or interpretation – J.C., S.G.H.; Literature search – O.F.A., L.F., F.H., S.M., J.M.L., J.C.; Writing – O.F.A., L.F., F.H., J.C., J.M.; Critical review – F.R.S., S.M., J.M.L., J.C., L.F.
References
- 1.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis:the central role of PGC-1 alpha. Cardiovasc Res. 2008;79:208–17. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 2.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-1αlpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res. 2005;66:562–73. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Ding L, Liang X, Zhu D, Lou Y. Peroxisome proliferator-activated receptor alpha is involved in cardiomyocyte differentiation of murine embryonic stem cells in vitro. Cell Biol Int. 2007;31:1002–9. doi: 10.1016/j.cellbi.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Duncan JG, Finck BN. The PPARa- PGC-1α axis controls cardiac energy metabolism in healthy and diseased myocardium. PPAR Res. 2008;2008:253817. doi: 10.1155/2008/253817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1αlpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 6.Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:446–52. doi: 10.1097/MED.0b013e32833c3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu I, Minamino T, Toko H, Okada S, Ikeda H, Yasuda N, et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J Clin Invest. 2010;120:1506–14. doi: 10.1172/JCI40096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci USA. 2006;103:10086–91. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun CK, Chang LT, Sheu JJ, Wang CY, Youssef AA, Wu CJ, et al. Losartan preserves integrity of cardiac gap junctions and PGC-1 alpha gene expression and prevents cellular apoptosis in remote area of left ventricular myocardium following acute myocardial infarction. Int Heart J. 2007;48:533–46. doi: 10.1536/ihj.48.533. [DOI] [PubMed] [Google Scholar]
- 10.Fabregat-Andres O, Tierrez A, Mata M, Estornell-Erill J, Ridocci-Soriano F, Monsalve M. Induction of PGC-1αlpha expression can be detected in blood samples of patients with ST-segment elevation acute myocardial infarction. PLoS One. 2011;6:e26913. doi: 10.1371/journal.pone.0026913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabregat-Andrés O, Ridocci-Soriano F, Estornell-Erill J, Corbi-Pascual M, Valle-Muñoz A, Berenguer-Jofresa A, et al. Blood PGC-1α concentration predicts myocardial salvage and ventricular remodeling after ST-segment elevation acute myocardial infarction. Rev Esp Cardiol. 2015;68:408–16. doi: 10.1016/j.rec.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Uguccioni G, D’souza D, Hood DA. Regulation of PPARg coactivator-1α function and expression in muscle:effect of exercise. PPAR Res. 2010 Aug 19; doi: 10.1155/2010/937123. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantó C, Auwerx J. PGC-1αlpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–87. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Shen J, Xiong X, Xu Y, Zhang H, Huang C, et al. Remote ischemic preconditioning protects against liver ischemia-reperfusion injury via heme oxygenase-1-induced autophagy. PLoS One. 2014;9:e98834. doi: 10.1371/journal.pone.0098834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassiotis C, Ballal K, Wellnitz K, Vela D, Gong M, Salazar R, et al. Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation. 2009;120:S191–7. doi: 10.1161/CIRCULATIONAHA.108.842252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hariharan N, Ikeda Y, Hong C, Alcendor RR, Usui S, Gao S, et al. Autophagy plays an essential role in mediating regression of hypertrophy during unloading of the heart. PLoS One. 2013;8:e51632. doi: 10.1371/journal.pone.0051632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-1αlpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res. 2005;66:562–73. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care. 2014;37:S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 20.Karamanlidis G, Bautista-Hernandez V, Fynn-Thompson F, Del Nido P, Tian R. Impaired mitocondrial biogénesis precedes heart failure in right ventricular hypertrophy in congenital heart disease. Circ Heart Fail. 2011;4:707–13. doi: 10.1161/CIRCHEARTFAILURE.111.961474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez-Alvarez MI, Thabit H, Burns N, Shah S, Brema I, Hatunic M, et al. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1αlpha/Mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care. 2010;33:645–51. doi: 10.2337/dc09-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Mascher H, Psilander N, Blomstrand E, Sahlin K. Resistance exercise enhances the molecular signaling of mitochondrial biogenesis induced by endurance exercise in human skeletal muscle. J Appl Physiol (1985) 2011;111:1335–44. doi: 10.1152/japplphysiol.00086.2011. [DOI] [PubMed] [Google Scholar]
- 23.Lu Z, Xu X, Hu S, Fassett J, Zhu G, Tao Y, et al. PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal. 2010;13:1011–22. doi: 10.1089/ars.2009.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannavino J, Brocca L, Sandri M, Bottinelli R, Pellegrino MA. PGC-1α over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J Physiol. 2014;592:4575–89. doi: 10.1113/jphysiol.2014.275545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferraro E, Giammarioli AM, Chiandotto S, Spoletini I, Rosano G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: redox signaling and role of autophagy. Antioxid Redox Signal. 2014;21:154–76. doi: 10.1089/ars.2013.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun CK, Chang LT, Sheu JJ, Wang CY, Youssef AA, Wu CJ, et al. Losartan preserves integrity of cardiac gap junctions and PGC-1 alpha gene expression and prevents cellular apoptosis in remote area of left ventricular myocardium following acute myocardial infarction. Int Heart J. 2007;48:533–46. doi: 10.1536/ihj.48.533. [DOI] [PubMed] [Google Scholar]
- 27.Han JS, Wang HS, Yan DM, Wang ZW, Han HG, Zhu HY, et al. Myocardial ischaemic and diazoxide preconditioning both increase PGC-1 alpha and reduce mitochondrial damage. Acta Cardiol. 2010;65:639–44. doi: 10.1080/ac.65.6.2059860. [DOI] [PubMed] [Google Scholar]
- 28.Handschin C. Peroxisome proliferator-activated receptor-gamma coactivator-1 alpha in muscle links metabolism to inflammation. Clin Exp Pharmacol Physiol. 2009;36:1139–43. doi: 10.1111/j.1440-1681.2009.05275.x. [DOI] [PubMed] [Google Scholar]
- 29.Palomer X, Salvadó L, Barroso E, Vazquez-Carrera M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol. 2013;168:3160–72. doi: 10.1016/j.ijcard.2013.07.150. [DOI] [PubMed] [Google Scholar]


