Abstract
Rosemary (Rosmarinus officinalis L.) is a popular herb in cooking, traditional healing, and aromatherapy. The essential oils of R. officinalis were obtained from plants growing in Victoria (Australia), Alabama (USA), Western Cape (South Africa), Kenya, Nepal, and Yemen. Chemical compositions of the rosemary oils were analyzed by gas chromatography-mass spectrometry as well as chiral gas chromatography. The oils were dominated by (+)-α-pinene (13.5%–37.7%), 1,8-cineole (16.1%–29.3%), (+)-verbenone (0.8%–16.9%), (−)-borneol (2.1%–6.9%), (−)-camphor (0.7%–7.0%), and racemic limonene (1.6%–4.4%). Hierarchical cluster analysis, based on the compositions of these essential oils in addition to 72 compositions reported in the literature, revealed at least five different chemotypes of rosemary oil. Antifungal, cytotoxicity, xanthine oxidase inhibitory, and tyrosinase inhibitory activity screenings were carried out, but showed only marginal activities.
Keywords: essential oil, chemical composition, chiral gas chromatography, enantiomeric distribution, mass spectrometry, hierarchical cluster analysis, antifungal, cytotoxic, enzyme inhibition
1. Introduction
Rosmarinus officinalis L., “rosemary” (Lamiaceae) is an evergreen shrub with aromatic needle-like leaves. It is native to the Mediterranean and Asia, but is now cultivated in temperate locations around the world as a decorative garden plant and culinary herb. Rosemary leaves have been used to flavor foods such as lamb, pork, chicken, fish, and stuffings, and to prepare herbal oils, butters, and vinegars.
Rosemary has long been used in traditional medicine for a variety of conditions [1]. In the Mediterranean region, an infusion of the aerial parts is taken internally to treat colds and cough [2,3] as an antispasmodic, antihypertensive, and antiepileptic [4,5,6,7,8,9,10], to treat diabetes [6,9], and intestinal parasites [11]. A maceration of R. officinalis in alcohol or olive oil is used externally to treat contusions, rheumatism, and muscular and joint pains [3,12,13]. In Mexico, native peoples inhale the smoke from the burning plant to treat cough [14] or drink an infusion to treat vomiting, stomachache, or intestinal parasites [15]. Rosemary extracts contain a number of phytochemicals, including carnosic acid, carnosol, 12-O-methylcarnosic acid, rosmarinic acid, and genkwanin [16]. Rosemary essential oils contain 1,8-cineole [17], α-pinene [18], verbenone [19], camphor, and borneol [20], but the compositions can vary widely. There are several varieties of R. officinalis. The Missouri Botanical Garden currently lists 16 sub-taxa [21] and numerous cultivars have been developed as well.
Rosemary essential oil is used in aromatherapy as a nerve stimulant for purposes including memory loss and lethargy [22]. Rosemary oil has shown psychostimulant activity. In a mouse model, a dose of 100 μg/kg caused a significant increase in locomotor activity [23]. In humans, massaged [24] or inhaled [25] rosemary oil has caused significant increased blood pressure, heart rate, and respiratory rate; oral administration of rosemary oil significantly increased blood pressure in hypotensive patients [26]. Inhaled rosemary oil has been shown to increase memory and concentration abilities [27]. In addition to psychostimulatory effects, rosemary essential oil has shown anticholinesterase [28], acaricidal [29,30], antibacterial [31,32], antifungal [33,34], and antinociceptive [35] activities.
The biological activities of R. officinalis essential oils doubtless depend on the chemical compositions, and at least 13 different rosemary oil chemotypes have been previously identified, based on the relative percentages of α-pinene, 1,8-cineole, camphor, borneol, verbenone, and bornyl acetate [31,36,37,38,39,40,41]. In this work, we have characterized the essential oils of R. officinalis collected from Alabama (USA), Western Cape (South Africa), Victoria (Australia), Kenya, Nepal, and Yemen, and screened these essential oils for antifungal activity. In addition, a hierarchical cluster analysis has been carried out based on the compositions of an additional 72 rosemary essential oils reported in the literature.
2. Materials and Methods
2.1. Plant Material
Leaves of R. officinalis from flowering plants in Huntsville, Alabama (34°38′27.2″ N, 86°33′44.4″ W, elevation 183 m above sea level (asl)) were identified by W.N. Setzer, and collected on 4 August 2016. The fresh plant material (55.44 g) was hydrodistilled for 4 h using a Likens-Nickerson apparatus with continuous extraction with dichloromethane to give 950 mg (1.7% yield) colorless essential oil, which was stored at −20 °C until analysis.
The leaves of R. officinalis were collected during the flowering stage in May 2013, from Dhamar province, Yemen. The plant was identified by Dr. Hassan M. Ibrahim of the Botany Department, Faculty of Sciences, Sana’a University. A voucher specimen of the plant material (YMP-lam-31) has been deposited at the Pharmacognosy Department, Sana’a University, Yemen. The dried leaves were hydrodistilled for 3 h in a Clevenger type apparatus according to the European Pharmacopoeia [42]. The obtained oil was subsequently dried over anhydrous Na2SO4 and kept at 4 °C until analysis. After filtration, the yield of the oil was 1.1% w/w.
R. officinalis, in the flowering stage, from Jumla, Nepal (29°16′28.99″ N, 82°11′1.79″ E, elevation 2500 m asl), was identified by Prasun Satyal, and collected on 2 July 2016. The fresh plant material (100 g) was hydrodistilled for 4 h using a Clevenger-type apparatus and collected to give 500 mg (0.5% yield) colorless essential oil after drying with Na2SO4.
R. officinalis from Ribeeck Kasteel, Western Cape, South Africa (33°23′7.21″ S, 18°53′54.65″ E, elevation 300 m asl), was identified by Prabodh Satyal, and collected on 20 August 2016. The fresh plant material (500 g) was subjected to steam distillation for 4 h using a Clevenger-type apparatus and collected to give 4 g (0.8% yield) colorless essential oil after drying with Na2SO4.
Flowering R. officinalis from Lancefield, Victoria, Australia (37°16′ S, 144°43′ E, elevation 495 m asl), was identified by Chris Burder, and collected on 20 December 2015. The fresh plant material (1 kg) was subjected to steam distillation for 3 h using a Clevenger-type apparatus and collected to give 9 g (0.9% yield) colorless essential oil after drying with Na2SO4.
R. officinalis from Thika, Kenya (1°1′ S, 37°5′ E, elevation 1500 m asl), was identified by Aaron Sorensen, and collected on 8 July 2016. The fresh plant material (1 kg) was subjected to steam distillation for 3.5 h using a Clevenger-type apparatus and collected to give 10 g (1.0% yield) pale yellow essential oil after drying with Na2SO4.
2.2. Gas Chromatography-Mass Spectrometry (GC-MS)
The essential oils of R. officinalis were analyzed by GC-MS using a Shimadzu GCMS-QP2010 Ultra operated in the electron impact (EI) mode (electron energy = 70 eV), scan range = 40–400 atomic mass units, scan rate = 3.0 scans/s, and GC-MS solution software. The GC column was a ZB-5 fused silica capillary column with a (5% phenyl)-polymethylsiloxane stationary phase and a film thickness of 0.25 μm. The carrier gas was helium with a column head pressure of 552 kPa and flow rate of 1.37 mL/min. Injector temperature was 250 °C and the ion source temperature was 200 °C. The GC oven temperature program was programmed for 50 °C initial temperature, temperature increased at a rate of 2 °C/min to 260 °C. A 5% w/v solution of the sample in CH2Cl2 was prepared and 0.1 μL was injected with a splitting mode (30:1). Identification of the oil components was based on their retention indices determined by reference to a homologous series of n-alkanes, and by comparison of their mass spectral fragmentation patterns with those reported in the literature [43], and stored in our in-house MS library.
2.3. Chiral Gas Chromatography-Mass Spectrometry
Chiral analysis of the essential oils was performed on a Shimadzu GCMS-QP2010S operated in the EI mode (electron energy = 70 eV), scan range = 40–400 amu, scan rate = 3.0 scans/s. GC was equipped with a Restek B-Dex 325 capillary column (30 mL × 0.25 mm ID × 0.25 μm film). Oven temperature was started at 50 °C, and then gradually raised to 120 °C at 1.5 °C/min. The oven was then raised to 200 °C at 2 °C/min and held for 5 min. Helium was the carrier gas and the flow rate was maintained at 1.8 mL/min. Samples were diluted 3% w/v with CH2Cl2 and then a 0.1 μL sample was injected in a split mode with a split ratio of 1:45.
2.4. Hierarchical Cluster Analysis
A total of 72 R. officinalis essential oil compositions from the published literature [17,18,19,20,28,29,30,31,32,33,34,35,36,37,39,41,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81] in addition to the six samples from this study were treated as operational taxonomic units (OTUs). The percentage composition of 23 major essential oil components (1,8-cineole, α-pinene, camphor, verbenone, borneol, camphene, myrcene, bornyl acetate, β-pinene, limonene, α-terpineol, linalool, β-caryophyllene, p-cymene, terpinen-4-ol, γ-terpinene, geraniol, α-phellandrene, terpinolene, α-terpinene, α-humulene, sabinene, and caryophyllene oxide) was used to determine the chemical relationship between the various R. officinalis essential oil samples by agglomerative hierarchical cluster (AHC) analysis using the XLSTAT software, version 2015.4.01 (Addinsoft™, New York, NY, USA). Pearson correlation was selected as a measure of similarity, and the unweighted pair-group method with arithmetic average (UPGMA) was used for cluster definition.
2.5. Antifungal Screening
Antifungal activity was carried out as previously described [82]. Briefly, minimum inhibitory concentrations were determined using microdilution methods for Candida albicans (American Type Culture Collection, ATCC #18804), Cryptococcus neoformans (serotype D or var. neoformans) (ATCC #24067), and Aspergillus niger (ATCC #16888). Single colonies from potato dextrose agar plates were grown in potato dextrose broth for three days. Cells were diluted to 2 × 103 cells/mL using MOPS (3-(N-morpholino)propanesulfonic acid) buffered RPMI (Roswell Park Memorial Institute) medium and aliquoted into sterile 12 × 75 mm tubes (900 μL). Essential oil (100 μL) was added to each tube followed by incubation at 37 °C for three days in a shaking incubator (175 rpm). Minimum inhibitory concentrations (MICs) were calculated from microdilution in 96-well plates, performed in triplicate. Serial dilution was performed by adding 50 μL of RPMI to each well, then an equal volume of essential oil to be tested to the first row. After mixing, 50 μL was removed and added to the next row. The procedure was repeated for each row, discarding the final 50 μL removed. To the mixture in each well, 50 μL of cells were added. The plates were incubated for two days at 37 °C before growth was quantitated from turbidity.
2.6. Xanthine Oxidase Inhibition Assay
Using xanthine as substrate, the xanthine oxidase (XO) activity of R. officinalis oil from Yemen was assayed spectrophotometrically according to Apaya and Hernandez [83]. A mixture containing 1 mL of 100 μg/mL of R. officinalis oil or allopurinol, 1.9 mL of 50 mM potassium phosphate buffer, and 1 mL of xanthine substrate (0.6 mM) was preincubated for 10 min at 25 °C, and the reaction was started by the addition of 0.1 mL of XO enzyme (0.1 U/mL in phosphate buffer). The reaction was incubated at 25 °C for 30 min, and the absorbance was measured against phosphate buffer as blank at 295 nm using quartz cuvettes. Allopurinol was used as standard enzyme inhibitors. The percent xanthine oxidase inhibition was calculated according to the following formula:
| % inhibition = 100 − (A1 − B) × 100/(Ao − B) | (1) |
where A1 is the activity of the enzyme in presence of the oil, B is the absorbance in absence of the enzyme, and Ao is the absorbance in absence of the oil or inhibitor.
2.7. Tyrosinase Inhibition Assay
In vitro mushroom tyrosinase inhibitory activity of R. officinalis oil from Yemen was determined by a spectrophotometric approach using l-tyrosine as the substrate [84]. In a total volume of 200 µL, the enzyme activity was measured in buffer containing 50 mM phosphate buffer, pH 6.5, 50 U/mL mushroom tyrosinase, and 50 µg/mL l-tyrosine. The reaction (conversion of l-tyrosine to DOPAchrome) was conducted at 37 °C for 30 min, and absorbance was then measured at the wavelength of 490 nm using a microplate reader. Blanks were run for the same concentration of essential oil in the absence of the enzyme. The inhibition assays were carried out in the presence of 100 µg/mL of the essential oil. Kojic acid and arbutin were used as positive control inhibitors in these assays. The absorbance of the same mixture without the essential oil was used as the negative control. The percent inhibition of tyrosinase activity was calculated as follows:
| % inhibition = ((A − B)/A) × 100 | (2) |
where A is the absorbance difference at 490 nm of the negative control (enzyme activity without oil)—the absorbance of the blank (no enzyme, no oil); B is the absorbance of test sample (with enzyme)—the absorbance of test sample blank (no enzyme).
2.8. Cytotoxicity Screening
Human colorectal cancer cell lines (SW480 and HCT116) were generously provided by Dr. Rick F. Thorne (University of Newcastle, Australia) and were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal calf serum (Bio Whittaker, Verviers, Belgium). The rosemary oil from Yemen was tested for acute cytotoxic effect; the essential oil was dissolved in dimethylsulfoxide (DMSO) and different dilutions in culture buffer were prepared. The acute cytotoxic effect of the essential oil on colorectal cancer cells was determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay [85]. Briefly, cells were seeded at 5000 cells per well onto flat-bottomed 96-well culture plates and allowed to grow for 24 h before the desired treatment. Cells were then incubated with 200 µL of essential oil concentrations (0–200 μg/mL) for 72 h. Cells were then labeled with MTT from the Vybrant MTT Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR, USA) according to the manufacturer’s instruction and resulting formazan was solubilized with DMSO. Absorbance was read in a microplate reader at 540 nm.
3. Results and Discussion
3.1. Essential Oil Compositions
The chemical compositions of six Rosmarinus officinalis essential oils are compiled in Table 1. α-Pinene and 1,8-cineole dominated the essential oils of all six samples (13.5%–38.1% and 16.3%–29.4%, respectively). Verbenone was also relatively abundant in the samples from Alabama (USA), Kenya, and Yemen. The sample from Yemen, with its relatively high verbenone content (18.6%) and relatively low α-pinene content (13.5%), along with 1,8-cineole (20.6%) and camphor (7.0%), firmly places it in the verbenoniferum chemotype, type IIIA, according to Napoli et al. [39]. The other five samples in this study have higher concentrations of α-pinene than 1,8-cineole or verbenone, and can be characterized, based on Napoli et al., as cineoliferum type VIA [39].
Table 1.
Chemical compositions of essential oils of Rosmarinus officinalis from six different geographical locations.
| RI | Compound | Percent Composition | |||||
|---|---|---|---|---|---|---|---|
| Alabama | Western Cape | Kenya | Victoria | Nepal | Yemen | ||
| 800 | n-Octane | --- a | --- | --- | tr b | --- | --- |
| 801 | Hexanal | tr | --- | --- | --- | --- | --- |
| 849 | (2E)-Hexenal | tr | --- | --- | --- | --- | --- |
| 850 | (3Z)-Hexenol | tr | --- | 0.1 | --- | --- | --- |
| 920 | Artemisia triene | --- | --- | --- | tr | --- | --- |
| 922 | Tricyclene | 0.1 | 0.4 | 0.1 | 0.2 | 0.2 | 0.1 |
| 925 | α-Thujene | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 |
| 934 | α-Pinene | 25.4 | 33.6 | 31.7 | 37.9 | 38.1 | 13.5 |
| 941 | Thujadiene | --- | --- | --- | --- | --- | tr |
| 947 | α-Fenchene | tr | 0.2 | tr | tr | 0.1 | --- |
| 950 | Camphene | 2.5 | 7.6 | 2.6 | 4.6 | 4.6 | 1.5 |
| 953 | Thuja-2,4(10)-diene | 0.5 | 1.2 | 0.4 | 0.8 | 0.8 | 0.3 |
| 972 | Sabinene | tr | --- | 0.1 | tr | 0.1 | tr |
| 978 | β-Pinene | 1.4 | 2.3 | 2.1 | 3.0 | 2.8 | 1.0 |
| 890 | 1-Octen-3-ol | --- | 0.6 | --- | --- | --- | 0.1 |
| 984 | 6-Methyl-5-Hepten-2-one | --- | --- | tr | tr | tr | --- |
| 984 | 3-Octanone | --- | 0.2 | --- | --- | --- | tr |
| 989 | Myrcene | 1.3 | 1.6 | 1.2 | 1.5 | 1.4 | 0.7 |
| 989 | Dehydro-1,8-cineole | --- | --- | 0.1 | --- | --- | --- |
| 1004 | p-Mentha-1(7),8-diene | --- | --- | --- | tr | tr | --- |
| 1005 | α-Phellandrene | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | --- |
| 1009 | δ-3-Carene | --- | --- | --- | --- | 0.1 | 0.2 |
| 1017 | α-Terpinene | 0.3 | 0.3 | 0.4 | 0.5 | 0.6 | tr |
| 1019 | p-Cymene | 0.9 | 1.6 | 0.4 | 1.6 | 1.5 | 1.1 |
| 1029 | Limonene | 2.7 | 4.4 | 2.3 | 3.5 | 3.5 | 1.6 |
| 1031 | 1,8-cineole | 18.8 | 16.3 | 20.9 | 29.4 | 23.0 | 20.6 |
| 1035 | (Z)-β-Ocimene | --- | 0.6 | tr | tr | tr | --- |
| 1044 | (E)-β-Ocimene | --- | 0.2 | --- | --- | --- | --- |
| 1048 | Thujol | --- | --- | --- | --- | --- | 0.1 |
| 1058 | γ-Terpinene | 1.0 | 0.4 | 1.0 | 0.8 | 1.2 | 0.1 |
| 1069 | cis-Sabinene hydrate | 0.1 | tr | 0.3 | tr | --- | 0.4 |
| 1070 | cis-4-Thujanol | --- | --- | --- | --- | tr | --- |
| 1085 | Terpinolene | 0.7 | 0.3 | 0.8 | 0.5 | 0.7 | 0.2 |
| 1086 | trans-Linalool oxide (furanoid) | --- | --- | --- | --- | --- | 0.1 |
| 1090 | p-Cymenene | tr | 0.1 | tr | 0.1 | 0.1 | --- |
| 1091 | Rosefuran | --- | --- | --- | tr | --- | --- |
| 1098 | Linalool | 2.7 | 3.1 | 2.8 | 2.3 | 2.2 | 3.8 |
| 1098 | cis-Decahydronaphthalene + Perillene | --- | --- | --- | --- | --- | 0.1 |
| 1100 | trans-Sabinene hydrate | --- | --- | 0.2 | --- | --- | --- |
| 1103 | Hotrienol | --- | --- | --- | tr | --- | --- |
| 1105 | (2E)-Hexenyl propanoate | --- | --- | --- | tr | --- | --- |
| 1106 | Isochrysanthenone | tr | 0.1 | tr | tr | tr | --- |
| 1117 | endo-Fenchol | tr | 0.1 | tr | tr | tr | tr |
| 1117 | 2,4-Dimethyl-2,4 heptadienal | --- | --- | --- | --- | --- | 0.1 |
| 1122 | Chrysanthenone | 0.2 | 0.5 | 0.5 | 0.5 | 0.3 | 0.5 |
| 1123 | cis-p-Menth-2-en-1-ol | tr | --- | tr | tr | --- | --- |
| 1126 | α-Campholenal | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1139 | trans-Pinocarveol | 0.1 | 0.5 | --- | 0.1 | 0.1 | 0.1 |
| 1141 | cis-Verbenol | 0.2 | 0.1 | 0.3 | 0.1 | tr | 0.2 |
| 1144 | trans-Verbenol | 0.4 | 0.2 | 0.5 | 0.1 | 0.1 | --- |
| 1146 | Camphor | 2.4 | 0.7 | 2.1 | 1.7 | 1.7 | 7.0 |
| 1154 | trans-β-Terpineol | tr | 0.1 | tr | --- | tr | tr |
| 1155 | Camphene hydrate | --- | --- | --- | tr | --- | --- |
| 1161 | trans-Pinocamphone | 0.2 | 0.4 | 0.1 | 0.1 | 0.1 | 0.2 |
| 1162 | Pinocarvone | 0.3 | 0.1 | 0.3 | 0.2 | 0.1 | 0.2 |
| 1165 | Isofenchol | 0.2 | tr | 0.2 | tr | 0.1 | --- |
| 1169 | δ-Terpineol | 0.3 | --- | 0.3 | 0.1 | 0.2 | --- |
| 1171 | Borneol | 4.0 | 7.0 | 2.8 | 2.1 | 2.5 | 5.5 |
| 1175 | cis-Pinocamphone | 0.7 | 1.1 | 0.6 | 0.4 | 0.4 | 0.9 |
| 1177 | p-1,8-Menthadien-4-ol | tr | tr | tr | tr | --- | tr |
| 1179 | Terpinen-4-ol | 1.1 | 0.7 | 1.0 | 0.5 | 2.2 | 1.0 |
| 1185 | p-Cymen-8-ol | 0.1 | tr | 0.1 | tr | tr | 0.3 |
| 1194 | α-Terpineol | 2.9 | 1.0 | 2.6 | 0.9 | 1.5 | 3.2 |
| 1197 | Methyl chavicol | tr | --- | tr | tr | tr | --- |
| 1206 | Verbenone | 17.1 | 0.8 | 11.9 | 2.5 | 2.7 | 18.6 |
| 1213 | 3-Oxo-1,8-cineole | 0.1 | --- | 0.1 | tr | --- | tr |
| 1218 | (4-Methylpentyl)-cyclohexane | 0.4 | --- | 0.2 | --- | tr | 0.2 |
| 1218 | trans-Carveol | --- | --- | --- | tr | --- | --- |
| 1225 | Citronellol | 0.3 | --- | 0.3 | tr | 0.2 | 0.3 |
| 1228 | Bornyl formate. | --- | --- | --- | tr | --- | --- |
| 1238 | Neral | 0.1 | --- | 0.2 | tr | 0.1 | 0.1 |
| 1239 | cis-Shisool | 0.3 | 0.3 | 0.1 | tr | 0.1 | 0.3 |
| 1242 | Carvone | 0.1 | --- | tr | tr | tr | tr |
| 1242 | Hexyl isovalerate | --- | --- | --- | --- | tr | --- |
| 1246 | trans-Shisool | 0.4 | 0.3 | 0.2 | tr | 0.1 | 0.5 |
| 1247 | Carvotanacetone | --- | --- | --- | --- | --- | 3.0 |
| 1250 | Geraniol | 4.8 | --- | 4.6 | 0.7 | 1.6 | 3.8 |
| 1252 | cis-Myrtanol | 0.1 | --- | tr | --- | --- | 0.1 |
| 1257 | Methyl citronellate | --- | --- | --- | --- | tr | --- |
| 1267 | Geranial | 0.1 | --- | 0.2 | 0.1 | 0.1 | --- |
| 1268 | Isopiperitenone | 0.1 | 0.1 | 0.2 | --- | 0.1 | 0.3 |
| 1280 | cis-Verbenyl acetate | --- | tr | --- | tr | --- | 0.1 |
| 1284 | Bornyl acetate | 1.7 | 2.0 | 1.0 | 0.9 | 1.0 | 1.8 |
| 1290 | Thymol | --- | --- | --- | --- | --- | 0.5 |
| 1296 | Carvacrol | tr | --- | --- | --- | --- | --- |
| 1297 | Perilla alcohol | tr | --- | --- | --- | --- | --- |
| 1297 | Geranyl formate + Carvacrol | --- | --- | --- | --- | --- | 0.1 |
| 1321 | Myrtenyl acetate | tr | --- | tr | --- | --- | --- |
| 1321 | Methyl geranate | --- | --- | --- | --- | tr | --- |
| 1332 | δ-Elemene | 0.1 | --- | --- | tr | --- | --- |
| 1332 | cis-Piperitol acetate | --- | --- | tr | --- | tr | tr |
| 1337 | Piperitenone. | tr | --- | 0.1 | --- | tr | 0.1 |
| 1348 | Citronellyl acetate | tr | --- | --- | --- | --- | --- |
| 1349 | Eugenol | 0.1 | --- | --- | --- | --- | --- |
| 1361 | Neoisodihydrocarvyl acetate | --- | --- | --- | --- | --- | tr |
| 1367 | Linalyl isobutanoate | 0.1 | --- | --- | tr | --- | |
| 1368 | α-Ylangene | --- | 0.2 | --- | --- | --- | --- |
| 1174 | α-Copaene | --- | 0.8 | --- | --- | --- | 0.1 |
| 1378 | Geranyl acetate | 0.2 | 0.2 | tr | 0.1 | 0.3 | |
| 1392 | 2-Ethylidene-6-methyl-3,5-heptadienal | --- | 0.1 | 0.2 | 0.1 | --- | --- |
| 1400 | Methyleugenol | 0.4 | 0.2 | 0.2 | tr | 0.1 | 0.2 |
| 1419 | β-Caryophyllene | 0.6 | 2.5 | 0.7 | 1.3 | 1.4 | 1.2 |
| 1427 | β-Gurjunene | --- | --- | --- | --- | --- | 0.1 |
| 1431 | α-Maaliene | --- | --- | --- | --- | --- | tr |
| 1438 | Aromadendrene | --- | 0.2 | --- | --- | --- | --- |
| 1448 | Geranyl acetone | tr | 0.1 | tr | --- | --- | 0.1 |
| 1454 | α-Humulene | 0.1 | 0.5 | 0.1 | 0.1 | 0.2 | 0.2 |
| 1472 | cis-Cadina-1(6),4-diene | --- | 0.1 | --- | --- | --- | --- |
| 1475 | trans-Cadina-1(6),4-diene | --- | 0.7 | --- | --- | tr | 0.1 |
| 1481 | ar-Curcumene | --- | tr | --- | --- | --- | --- |
| 1488 | β-Selinene | --- | 0.1 | --- | --- | --- | --- |
| 1492 | γ-Amorphene | --- | 0.1 | --- | --- | --- | --- |
| 1496 | α-Selinene | --- | 0.2 | --- | --- | --- | --- |
| 1499 | α-Muurolene | --- | 0.3 | --- | --- | 0.1 | --- |
| 1503 | Valencene. | --- | 0.1 | --- | --- | --- | --- |
| 1509 | β-Bisabolene | --- | 0.1 | --- | --- | --- | --- |
| 1513 | δ-Amorphene | --- | 0.6 | --- | --- | 0.1 | tr |
| 1516 | δ-Cadinene | --- | 1.0 | --- | --- | 0.3 | 0.1 |
| 1522 | trans-Calamenene | --- | 0.2 | --- | --- | --- | --- |
| 1533 | trans-Cadine-1,4-diene | --- | 0.1 | --- | --- | --- | --- |
| 1537 | α-Cadinene | --- | 0.1 | --- | --- | --- | --- |
| 1541 | α-Calacorene | --- | 0.1 | --- | --- | --- | --- |
| 1548 | Elemol | --- | --- | --- | --- | 0.5 | --- |
| 1566 | Maaliol | --- | --- | --- | --- | --- | 1.6 |
| 1573 | Spathulenol | --- | --- | --- | --- | --- | 0.1 |
| 1582 | Caryophyllene oxide | 0.2 | 0.3 | 0.2 | tr | 0.1 | 0.5 |
| 1582 | Gleenol | --- | --- | --- | --- | --- | tr |
| 1608 | Humulene epoxide II | tr | 0.1 | --- | --- | --- | tr |
| 1631 | γ-Eudesmol | --- | --- | --- | --- | 0.1 | --- |
| 1635 | Caryophylla-4(12),8(13)-dien-5-ol | tr | --- | --- | --- | --- | --- |
| 1654 | 14-Hydroxy-9-epi-(Z)-Caryophyllene | 0.1 | 0.1 | --- | --- | --- | --- |
| 1655 | α-Eudesmol | --- | --- | --- | --- | 0.2 | --- |
| 1683 | α-Bisabolol | --- | --- | --- | --- | --- | tr |
| 1764 | Benzyl benzoate | tr | --- | --- | --- | --- | --- |
| 1777 | 8α-Acetoxyelemol | --- | --- | --- | --- | tr | --- |
| Total Compounds Identified | 53 | 66 | 52 | 36 | 52 | 59 | |
| Percent Composition Identified | 99.1 | 99.7 | 99.5 | 99.9 | 99.7 | 98.8 | |
a --- = not detected. b tr = trace (<0.05%).
The enantiomeric distributions of monoterpenoids of the rosemary oils, determined by chiral GC-MS, are summarized in Table 2. (+)-α-Pinene was the predominant enantiomer (97%–99%) in the five samples analyzed in this study. β-Pinene, on the other hand, showed 29%–33% (+)-enantiomer, in general agreement with that reported by Presti and co-workers, 36%–40% (+)-β-pinene [86]. The enantiomeric distributions of limonene (40%–54% (+)-limonene), linalool (95%–97% (−)-linalool), terpinen-4-ol (69%–70% (+)-terpinen-4-ol), and α-terpineol (66%–75% (+)-α-terpineol), are also in qualitative agreement with those reported by Presti et al., 48%–53% (+)-limonene, 99% (−)-linalool, 73%–76% (+)-terpinen-4-ol, and 66%–67% (+)-α-terpineol [86]. Presti and co-workers found (+)-sabinene to be dominant in rosemary oil (96%–98%) [86], but sabinene concentrations were too low to determine the enantiomeric distribution in the samples in this study. König and co-workers found the enantiomeric distribution of borneol in rosemary oils to show significant differences; the (−)-enantiomer generally dominated, but was much larger in rosemary oil originating in Spain [87]. In this current study, (−)-borneol also dominated (95%–98%). Furthermore, bornyl acetate in this study was enantiomerically pure with 100% (−)-bornyl acetate. Verbenone was also enantiomerically pure, 100% (+)-verbenone, in all rosemary oils examined. Ravid and co-workers also found high enantiomeric purity (96%–100% (+)-verbenone) in their R. officinalis essential oil samples [88]. Thus, we can conclude that the (+)/(−) ratios of each of the monoterpenoids remains relatively constant in rosemary oils, regardless of geographical location of the oil source.
Table 2.
Enantiomeric distribution of monoterpenoids in Rosmarinus officinalis essential oils from six different geographical locations.
| Compound | Enantiomeric Distribution, (+)/(−) | ||||
|---|---|---|---|---|---|
| Alabama | Western Cape | Kenya | Victoria | Nepal | |
| α-Pinene | 99/1 | 99/1 | 97/3 | 99/1 | 98/2 |
| Camphene | 75/25 | 78/22 | 75/25 | 75/25 | 75/25 |
| β-Pinene | 33/67 | 29/71 | 31/69 | 30/70 | 30/70 |
| Limonene | 48/52 | 40/60 | 53/47 | 54/46 | 54/46 |
| Linalool | 5/95 | 3/97 | 5/95 | 3/97 | 5/95 |
| Camphor | 15/85 | 16/84 | 15/85 | 15/85 | 15/85 |
| Borneol | 2/98 | 2/98 | 5/95 | 5/95 | 4/96 |
| Terpinen-4-ol | 69/31 | 70/30 | 70/30 | 70/30 | 70/30 |
| α-Terpineol | 74/26 | 75/25 | 71/29 | 68/32 | 66/34 |
| Verbenone | 100/0 | 100/0 | 100/0 | 100/0 | 100/0 |
| Bornyl acetate | 0/100 | 0/100 | 0/100 | 0/100 | 0/100 |
3.2. Chemotypes of Rosemary
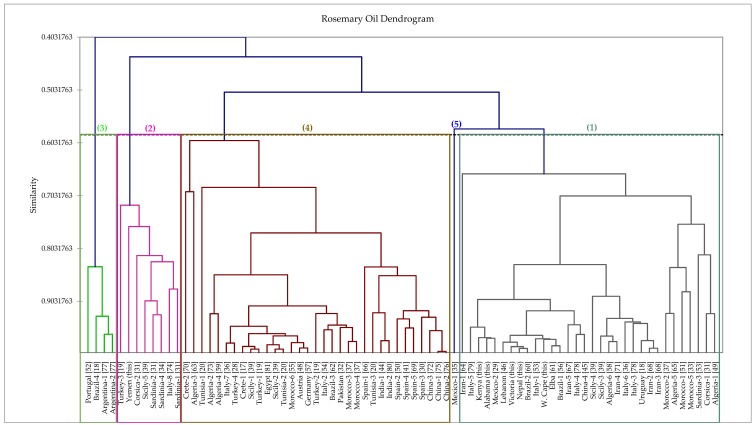
In order to provide additional insight into the chemotypes of rosemary essential oils, we have carried out a hierarchical cluster analysis based on the chemical compositions of the six oils in this study along with 72 additional rosemary oil chemical compositions from the literature. The dendrogram of the analysis is shown in Figure 1. Based on this analysis, there are five different chemotypes: (1) α-pinene/1,8-cineole, (2) verbenone/α-pinene/camphor/1,8-cineole, (3) myrcene/1,8-cineole/camphor, (4) 1,8-cineole/camphor/α-pinene, and (5) α-pinene/β-pinene/camphene. Chemotype 1, dominated by α-pinene [36,37,89,90,91,92], is a cluster made up of 32 samples, including those from Victoria (Australia), Nepal, Kenya, Western Cape (South Africa), and Alabama (USA). Chemotype 2, dominated by verbenone (i.e., the verbenoniferum chemotype) [39,74], has eight samples, including the sample from Yemen. Chemotype 3, dominated by myrcene [89], has four samples in this analysis. Chemotype 4 is dominated by 1,8-cineole [36,38,93,94,95] and is comprised of 33 samples, and can be considered comparable to the cineoliferum chemotype described by Napoli and co-workers [39]. The fifth chemotype, represented by only one sample from Mexico [35], has nearly equal quantities of α-pinene, β-pinene, and camphene; the relatively high β-pinene concentration (12%) separates this sample from chemotype 1.
Figure 1.
Dendrogram obtained from the agglomerative hierarchical cluster analysis of 78 Rosmarinus officinalis essential oil compositions.
3.3. Antifungal Activity
Antifungal activity was tested against three common opportunistic fungal pathogens. Yeast-like C. albicans and mold-like A. niger are Ascomycota while C. neoformans is a Basidiomycota. Overall, no inhibitory activity at or below 2500 ppm for the R. officinalis essential oil chemotypes was overserved against either ascomycete. Inhibitory activity was observed against C. neoformans for the majority of R. officinalis chemotypes tested. Though not exceedingly promising, R. officinalis from Australia did demonstrate an MIC of 625 ppm. Previous investigations of antifungal activity rosemary oil against A. niger [76,96,97] and C. albicans [19,98,99] have also found rosemary oil to be inactive, while against C. neoformans it had been found to be moderately active (MIC = 156 μg/mL) [100]. Thus, rosemary oil cannot be considered a worthwhile antifungal agent.
3.4. Other Biological Assays
The rosemary oil from Yemen was screened for xanthine oxidase inhibitory, tyrosinase inhibitory, and cytotoxic (SW480 and HCT116 human colorectal carcinoma cells) activity, but showed no activity in any of these assays. Yemeni rosemary oil showed only 8.6% ± 4.7% inhibition of xanthine oxidase. Against tyrosinase, Yemeni rosemary oil showed only 3.3% ± 1.5% inhibition at 100 μg/mL. In contrast, rosemary oil from cultivated plants from Mauritius did show tyrosinase inhibitory activity (median inhibitory concentration, IC50 = 97 μg/mL) [101]. Rosemary oil was found to be relatively non-toxic to SK-OV-3, HO-8910, Bel-7402 (IC50 ≥250 μg/mL) [102], MCF-7, LNCaP, and NIH-3T3 cells (IC50 >180 μg/mL) [32], but active against A549 cells (IC50 = 80 μg/mL) [103]. In summary, the enzyme inhibitory potential of rosemary oil is fairly low. In high concentrations, however, rosemary oil may be harmful to some mammalian tissues.
4. Conclusions
Rosemary (Rosmarinus officinalis) essential oil has been extensively studied for its chemical composition and its biological activities. α-Pinene and 1,8-cineole generally dominate the essential oil compositions, but camphor, verbenone, camphene, and myrcene may also appear in high concentrations. The enantiomeric distributions of monoterpenoids in rosemary oils seem to remain relatively constant regardless of environmental factors. Based on the relative concentrations of the major components in rosemary oils, we can define at least five separate chemotypes. The different chemotypes are likely to present different biological activities, but rosemary oil, in general, is relatively inactive in terms of antifungal, enzyme inhibitory, or antitumor potential.
Acknowledgments
We thank Joshua Thomerson, Chris Burder, Aaron Sorensen, and Prasun Satyal for assistance with plant collection and distillation, and Badri P. Mainali for help with library research. T.H.J. was supported, in part, by a grant to R.L.M. from the Herman Frasch Foundation for Chemical Research in conjunction with the American Chemical Society.
Author Contributions
P.S., R.L.M., N.A.A.A., and W.N.S. conceived and designed the experiments; T.H.J., E.M.L., I.M., and A.G.A. performed the experiments; P.S., R.L.M., N.A.A.A., and W.N.S. analyzed the data; R.L.M. contributed reagents/materials/analysis tools; R.L.M., N.A.A.A., and W.N.S. wrote the paper.
Conflicts of Interest
The authors declare no conflicts of interest. The funding agency had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Heinrich M., Kufer J., Leonti M., Pardo-de-Santayana M. Ethnobotany and ethnopharmacology—Interdisciplinary links with the historical sciences. J. Ethnopharmacol. 2006;107:157–160. doi: 10.1016/j.jep.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Savo V., Giulia C., Maria G.P., David R. Folk phytotherapy of the Amalfi Coast (Campania, Southern Italy) J. Ethnopharmacol. 2011;135:376–392. doi: 10.1016/j.jep.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Calvo M.I., Akerreta S., Cavero R.Y. Pharmaceutical ethnobotany in the Riverside of Navarra (Iberian Peninsula) J. Ethnopharmacol. 2011;135:22–33. doi: 10.1016/j.jep.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Novais M.H., Santos I., Mendes S., Pinto-Gomes C. Studies on pharmaceutical ethnobotany in Arrabida Natural Park (Portugal) J. Ethnopharmacol. 2004;93:183–195. doi: 10.1016/j.jep.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Boudjelal A., Henchiri C., Sari M., Sarri D., Hendel N., Benkhaled A., Ruberto G. Herbalists and wild medicinal plants in M’Sila (North Algeria): An ethnopharmacology survey. J. Ethnopharmacol. 2013;148:395–402. doi: 10.1016/j.jep.2013.03.082. [DOI] [PubMed] [Google Scholar]
- 6.Eddouks M., Maghrani M., Lemhadri A., Ouahidi M.-L., Jouad H. Ethnopharmacological survey of medicinal plants esed for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet) J. Ethnopharmacol. 2002;82:97–103. doi: 10.1016/S0378-8741(02)00164-2. [DOI] [PubMed] [Google Scholar]
- 7.Eddouks M., Ajebli M., Hebi M. Ethnopharmacological survey of medicinal plants used in Daraa-Tafilalet Region (Province of Errachidia), Morocco. J. Ethnopharmacol. 2016 doi: 10.1016/j.jep.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Al-Qura’n S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009;123:45–50. doi: 10.1016/j.jep.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Jouad H., Haloui M., Rhiouani H., El Hilaly J., Eddouks M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the north centre region of Morocco (Fez-Boulemane) J. Ethnopharmacol. 2001;77:175–182. doi: 10.1016/S0378-8741(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 10.Ivancheva S., Stantcheva B. Ethnobotanical inventory of medicinal plants in Bulgaria. J. Ethnopharmacol. 2000;69:165–172. doi: 10.1016/S0378-8741(99)00129-4. [DOI] [PubMed] [Google Scholar]
- 11.El-Hilaly J., Hmammouchi M., Lyoussi B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate Province (Northern Morocco) J. Ethnopharmacol. 2003;86:149–158. doi: 10.1016/S0378-8741(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 12.González J.A., García-Barriuso M., Amich F. Ethnobotanical study of medicinal plants traditionally used in the Arribes Del Duero, western Spain. J. Ethnopharmacol. 2010;131:343–355. doi: 10.1016/j.jep.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Cavero R.Y., Akerreta S., Calvo M.I. Pharmaceutical ethnobotany in the Middle Navarra (Iberian Peninsula) J. Ethnopharmacol. 2011;137:844–855. doi: 10.1016/j.jep.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Juárez-Vázquez M.D. C., Carranza-Álvarez C., Alonso-Castro A.J., González-Alcaraz V.F., Bravo-Acevedo E., Chamarro-Tinajero F.J., Solano E. Ethnobotany of medicinal plants used in Xalpatlahuac, Guerrero, México. J. Ethnopharmacol. 2013;148:521–527. doi: 10.1016/j.jep.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 15.Josabad Alonso-Castro A., Jose Maldonado-Miranda J., Zarate-Martinez A., Jacobo-Salcedo M.D.R., Fernández-Galicia C., Alejandro Figueroa-Zuñiga L., Abel Rios-Reyes N., Angel De León-Rubio M., Andrés Medellín-Castillo N., Reyes-Munguia A., et al. Medicinal plants used in the Huasteca Potosina, México. J. Ethnopharmacol. 2012;143:292–298. doi: 10.1016/j.jep.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 16.Del Baño M.J., Lorente J., Castillo J., Benavente-García O., Del Río J.A., Ortuño A., Quirin K.W., Gerard D. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxidant activity. J. Agric. Food Chem. 2003;51:4247–4253. doi: 10.1021/jf0300745. [DOI] [PubMed] [Google Scholar]
- 17.Daferera D.J., Ziogas B.N., Polissiou M.G. GC-MS Analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem. 2000;48:2576–2581. doi: 10.1021/jf990835x. [DOI] [PubMed] [Google Scholar]
- 18.Dellacassa E., Lorenzo D., Moyna P., Frizzo C.D., Serafini L.A., Dugo P. Rosmarinus officinalis L. (Labiatae) essential oils from the south of Brazil and Uruguay. J. Essent. Oil Res. 1999;11:27–30. doi: 10.1080/10412905.1999.9701061. [DOI] [Google Scholar]
- 19.Celiktas O.Y., Kocabas E.E. H., Bedir E., Sukan F.V., Ozek T., Baser K.H. C. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2007;100:553–559. doi: 10.1016/j.foodchem.2005.10.011. [DOI] [Google Scholar]
- 20.Zaouali Y., Messaoud C., Salah A. Ben; Boussaïd, M. Oil composition variability among populations in relationship with their ecological areas in Tunisian Rosmarinus officinalis L. Flavour Fragr. J. 2005;20:512–520. doi: 10.1002/ffj.1428. [DOI] [Google Scholar]
- 21.Missouri Botanical Garden. [(accessed on 26 January 2017)]. Available online: Tropicos.org www.tropicos.org.
- 22.Lawless J. Aromatherapy and the Mind. Thorsons; London, UK: 1994. [Google Scholar]
- 23.Alnamer R., Alaoui K., Bouidida E.H., Benjouad A. Psychostimulant activity of Rosmarinus officinalis essential oils. J. Nat. Prod. (India) 2012;5:83–92. [Google Scholar]
- 24.Hongratanaworakit T. Simultaneous aromatherapy massage with rosemary oil on humans. Sci. Pharm. 2009;77:375–387. doi: 10.3797/scipharm.0903-12. [DOI] [Google Scholar]
- 25.Sayorwan W., Ruangrungsi N., Piriyapunyporn T., Hongratanaworakit T., Kotchabhakdi N., Siripornpanich V. Effects of inhaled rosemary oil on subjective feelings and activities of the nervous system. Sci. Pharm. 2013;81:531–542. doi: 10.3797/scipharm.1209-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernández L.F., Palomino O.M., Frutos G. Effectiveness of Rosmarinus officinalis essential oil as antihypotensive agent in primary hypotensive patients and its influence on health-related quality of life. J. Ethnopharmacol. 2014;151:509–516. doi: 10.1016/j.jep.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Dobetsberger C., Buchbauer G. Actions of essential oils on the central nervous system: An updated review. Flavour Fragr. J. 2011;26:300–316. doi: 10.1002/ffj.2045. [DOI] [Google Scholar]
- 28.Orhan I., Aslan S., Kartal M., Şener B., Hüsnü Can Başer K. Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem. 2008;108:663–668. doi: 10.1016/j.foodchem.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Velazquez M., Rosario-Cruz R., Castillo-Herrera G., Flores-Fernandez J.M., Alvarez A.H., Lugo-Cervantes E. Acaricidal effect of essential oils from Lippia graveolens (Lamiales: Verbenaceae), Rosmarinus officinalis (Lamiales: Lamiaceae), and Allium sativum (Liliales: Liliaceae) against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) J. Med. Entomol. 2011;48:822–827. doi: 10.1603/ME10140. [DOI] [PubMed] [Google Scholar]
- 30.Laborda R., Manzano I., Gamón M., Gavidia I., Pérez-Bermúdez P., Boluda R. Effects of Rosmarinus officinalis and Salvia officinalis essential oils on Tetranychus urticae Koch (Acari: Tetranychidae) Ind. Crops Prod. 2013;48:106–110. doi: 10.1016/j.indcrop.2013.04.011. [DOI] [Google Scholar]
- 31.Pintore G., Usai M., Bradesi P., Juliano C., Boatto G., Tomi F., Chessa M., Cerri R., Casanova J. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica. Flavour Fragr. J. 2002;17:15–19. doi: 10.1002/ffj.1022. [DOI] [Google Scholar]
- 32.Hussain A.I., Anwar F., Chatha S.A.S., Jabbar A., Mahboob S., Nigam P.S. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and antibacterial activities. Braz. J. Microbiol. 2010;41:1070–1078. doi: 10.1590/S1517-83822010000400027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouchra C., Achouri M., Hassani L.M.I., Hmamouchi M. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J. Ethnopharmacol. 2003;89:165–169. doi: 10.1016/S0378-8741(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 34.Sacchetti G., Maietti S., Muzzoli M., Scaglianti M., Manfredini S., Radice M., Bruni R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005;91:621–632. doi: 10.1016/j.foodchem.2004.06.031. [DOI] [Google Scholar]
- 35.Martinez A.L., Eva Gonzalez-Trujano M., Pellicer F., Lopez-Munoz F.J., Navarrete A. Antinociceptive effect and GC/MS analysis of Rosmarinus officinalis L. essential oil from its aerial parts. Planta Med. 2009;75:508–511. doi: 10.1055/s-0029-1185319. [DOI] [PubMed] [Google Scholar]
- 36.Flamini G., Cioni P.L., Morelli I., Macchia M., Ceccarini L. Main agronomic-productive characteristics of two ecotypes of Rosmarinus officinalis L. and chemical composition of their essential oils. J. Agric. Food Chem. 2002;50:3512–3517. doi: 10.1021/jf011138j. [DOI] [PubMed] [Google Scholar]
- 37.Lahlou M., Berrada R. Composition and niticidal activity of essential oils of three chemotypes of Rosmarinus officinalis L. acclimatized in Morroco. Flavour Fragr. J. 2003;18:124–127. doi: 10.1002/ffj.1160. [DOI] [Google Scholar]
- 38.Zaouali Y., Bouzaine T., Boussaid M. Essential oils composition in two Rosmarinus officinalis L. varieties and incidence for antimicrobial and antioxidant activities. Food Chem Toxicol. 2010;48:3144–3152. doi: 10.1016/j.fct.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Napoli E.M., Curcuruto G., Ruberto G. Screening of the essential oil composition of wild Sicilian rosemary. Biochem. Systemat. Ecol. 2010;38:659–670. doi: 10.1016/j.bse.2010.04.001. [DOI] [Google Scholar]
- 40.Matsuzaki Y., Tsujisawa T., Nishihara T., Nakamura M., Kakinoki Y. Antifungal activity of chemotype essential oils from rosemary against Candida albicans. J. Stomatol. 2013;3:176–182. doi: 10.4236/ojst.2013.32031. [DOI] [Google Scholar]
- 41.Jordán M.J., Lax V., Rota M.C., Lorán S., Sotomayor J.A. Effect of bioclimatic area on the essential oil composition and antibacterial activity of Rosmarinus officinalis L. Food Cont. 2013;30:463–468. doi: 10.1016/j.foodcont.2012.07.029. [DOI] [Google Scholar]
- 42.Council of Europe . European Pharmacopoeia. 3rd ed. Council of Europe Press; Strasbourg, France: 1997. [Google Scholar]
- 43.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing; Carol Stream, IL, USA: 2007. [Google Scholar]
- 44.Mallavarapu R.G., Singh M., Chandra Shekhar R., Ramesh S., Kumar S. Rosemary oil: Prospects of its production in India. Med. Aromat. Plant Sci. 2000;22:298–301. [Google Scholar]
- 45.Chen Z.-F., Yang J.-L., Wang C., Cui S.-Y. Study on chemical constituents of essential oil of Rosmarinus officinalis. Zhongeaoyao. 2001;32:1085–1086. [Google Scholar]
- 46.Diab Y., Auezova L., Chebib H., Chalchat J.-C., Figueredo G. Chemical composition of Lebanese rosemary (Rosmarinus officinalis L.) essential oil as a function of the geographical region and the harvest time. J. Essent. Oil Res. 2002;14:449–452. doi: 10.1080/10412905.2002.9699918. [DOI] [Google Scholar]
- 47.Angelini L.G., Carpanese G., Cioni P.L., Morelli I., Macchia M., Flamini G. Essential oils from Mediterranean Lamiaceae as weed germination inhibitors. J. Agric. Food Chem. 2003;51:6158–6164. doi: 10.1021/jf0210728. [DOI] [PubMed] [Google Scholar]
- 48.Jirovetz L., Buchbauer G., Denkova Z., Stoyanova A., Murgov I., Schmidt E., Geissler M. Antimicrobial testing and gas chromatographic analysis of pure oxygenated monoterpenes 1,8-cineole, α-terpineol, terpinen-4-ol, and camphor, as well as target compounds in essential oils of pine (Pinus pinaster), rosemary (Rosmarinus officinalis), tea tree (Melaleuca alternifolia) Sci. Pharm. 2005;73:27–38. [Google Scholar]
- 49.Boutekedjiret C., Buatois B., Bessiere J.M. Characterization of rosemary essential oil of different areas of Algeria. J. Essent. Oil Bear. Plants. 2005;8:65–70. doi: 10.1080/0972060X.2005.10643423. [DOI] [Google Scholar]
- 50.Kubeczka K.H., Formáček V. In: Essential Oils Analysis by Capillary Gas Chromatography and Carbon-13 NMR Spectroscopy. 2nd ed. Kubeczka K.H., Formáček V., editors. John Wiley & Sons Ltd.; Chichester, UK: 2002. pp. 285–300. [Google Scholar]
- 51.Lahlou M., Berrada R., Agoumi A., Hmamouchi M. The potential effectiveness of essential oils in the control of human head lice in Morocco. Int. J. Aromather. 2001;10:108–128. doi: 10.1016/S0962-4562(01)80005-9. [DOI] [Google Scholar]
- 52.Serrano E., Palma J., Tinoco T., Venancio F., Martins A. Evaluation of the essential oils of rosemary (Rosmarinus officinalis L.) from different zones of “Alentejo” (Portugal) J. Essent. Oil Res. 2002;14:87–92. doi: 10.1080/10412905.2002.9699779. [DOI] [Google Scholar]
- 53.Angioni A., Barra A., Cereti E., Barile D., Coïsson J.D., Arlorio M., Dessi S., Coroneo V., Cabras P. Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J. Agric. Food Chem. 2004;52:3530–3535. doi: 10.1021/jf049913t. [DOI] [PubMed] [Google Scholar]
- 54.Kartnig T., Fischer U., Bucar F. Vergleichende gaschromatographische Untersuchungen an ätherischen Wacholderölen, Fenchelölen und Rosmarinölen. Sci. Pharm. 1998;66:237–252. [Google Scholar]
- 55.Ait-Ouazzou A., Lorán S., Bakkali M., Laglaoui A., Rota C., Herrera A., Pagán R., Conchello P. Chemical composition and antimicrobial activity of essential oils of Thymus algeriensis, Eucalyptus globulus and Rosmarinus officinalis from Morocco. J. Sci. Food Agric. 2011;91:2643–2651. doi: 10.1002/jsfa.4505. [DOI] [PubMed] [Google Scholar]
- 56.Atti-Santos A.C., Rossato M., Pauletti G.F., Rota L.D., Rech J.C., Pansera M.R., Agostini F., Serafini L.A., Moyna P. Physico-chemical evaluation of Rosmarinus officinalis L. essential oils. Braz. Arch. Biol. Technol. 2005;48:1035–1039. doi: 10.1590/S1516-89132005000800020. [DOI] [Google Scholar]
- 57.Baratta M.T., Dorman H.J. D., Figueiredo A.C., Barroso J.G., Ruberto G. Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Fragr. J. 1998;13:235–244. doi: 10.1002/(SICI)1099-1026(1998070)13:4<235::AID-FFJ733>3.0.CO;2-T. [DOI] [Google Scholar]
- 58.Bousbia N., Abert Vian M., Ferhat M.A., Petitcolas E., Meklati B.Y., Chemat F. Comparison of two isolation methods for essential oil from rosemary leaves: Hydrodistillation and microwave hydrodiffusion and gravity. Food Chem. 2009;114:355–362. doi: 10.1016/j.foodchem.2008.09.106. [DOI] [Google Scholar]
- 59.Boutekedjiret C., Bentahar F., Belabbes R., Bessiere J.M. Extraction of rosemary essential oil by steam distillation and hydrodistillation. Flavour Fragr. J. 2003;18:481–484. doi: 10.1002/ffj.1226. [DOI] [Google Scholar]
- 60.Cassel E., Vargas R.M. F., Martinez N., Lorenzo D., Dellacassa E. Steam distillation modeling for essential oil extraction process. Ind. Crops Prod. 2009;29:171–176. doi: 10.1016/j.indcrop.2008.04.017. [DOI] [Google Scholar]
- 61.Conti B., Canale A., Bertoli A., Gozzini F., Pistelli L. Essential oil composition and larvicidal activity of xix Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae) Parasitol. Res. 2010;107:1455–1461. doi: 10.1007/s00436-010-2018-4. [DOI] [PubMed] [Google Scholar]
- 62.De Azeredo G.A., Stamford T.L. M., Nunes P.C., Gomes Neto N.J., De Oliveira M.E. G., De Souza E.L. Combined application of essential oils from Origanum vulgare L. and Rosmarinus officinalis L. to inhibit bacteria and autochthonous microflora associated with minimally processed vegetables. Food Res. Int. 2011;44:1541–1548. doi: 10.1016/j.foodres.2011.04.012. [DOI] [Google Scholar]
- 63.Djeddi S., Bouchenah N., Settar I., Skaltsa H.D. Composition and antimicrobial activity of the essential oil of Rosmarinus officinalis from Algeria. Chem. Nat. Comp. 2007;43:487–490. doi: 10.1007/s10600-007-0172-4. [DOI] [Google Scholar]
- 64.Gachkar L., Yadegari D., Rezaei M.B., Taghizadeh M., Astaneh S.A., Rasooli I. Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem. 2007;102:898–904. doi: 10.1016/j.foodchem.2006.06.035. [DOI] [Google Scholar]
- 65.Giordani R., Hadef Y., Kaloustian J. Compositions and antifungal activities of essential oils of some Algerian aromatic plants. Fitoterapia. 2008;79:199–203. doi: 10.1016/j.fitote.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Guillen M.D., Cabo N., Burillo J. Characterisation of the essential oils of some cultivated aromatic plants of industrial interest. J. Sci. Food Agric. 1996;70:359–363. doi: 10.1002/(SICI)1097-0010(199603)70:3<359::AID-JSFA512>3.0.CO;2-0. [DOI] [Google Scholar]
- 67.Jalali-Heravi M., Moazeni R.S., Sereshti H. Analysis of Iranian rosemary essential oil: Application of gas chromatography-mass spectrometry combined with chemometrics. J. Chromatogr. A. 2011;1218:2569–2576. doi: 10.1016/j.chroma.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 68.Jamshidi R., Afzali Z., Afzali D. Chemical composition of hydrodistillation essential oil of rosemary in different origins in Iran and comparison with other countries. Am. Eurasian J. Agric. Environ. Sci. 2009;5:78–81. [Google Scholar]
- 69.Jordán M.J., Lax V., Rota M.C., Lorán S., Sotomayor J.A. Effect of the phenological stage on the chemical composition, and antimicrobial and antioxidant properties of Rosmarinus officinalis L. essential oil and its polyphenolic extract. Ind. Crops Prod. 2013;48:144–152. doi: 10.1016/j.indcrop.2013.04.031. [DOI] [Google Scholar]
- 70.Katerinopoulos H.E., Pagona G., Afratis A., Stratigakis N., Roditakis N. Composition and insect attracting activity of the essential oil of Rosmarinus officinalis. J. Chem. Ecol. 2005;31:111–122. doi: 10.1007/s10886-005-0978-0. [DOI] [PubMed] [Google Scholar]
- 71.Minaiyan M., Ghannadi A.R., Afsharipour M., Mahzouni P. Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res. Pharmaceut. Sci. 2011;6:13–21. [PMC free article] [PubMed] [Google Scholar]
- 72.Sui X., Liu T., Ma C., Yang L., Zu Y., Zhang L., Wang H. Microwave irradiation to pretreat rosemary (Rosmarinus officinalis L.) for maintaining antioxidant content during storage and to extract essential oil simultaneously. Food Chem. 2012;131:1399–1405. doi: 10.1016/j.foodchem.2011.10.007. [DOI] [Google Scholar]
- 73.Touafek O., Nacer A., Kabouche A., Kabouche Z., Bruneau C. Chemical composition of the essential oil of Rosmarinus officinalis cultivated in the Algerian Sahara. Chem. Nat. Comp. 2004;40:28–29. doi: 10.1023/B:CONC.0000025460.78222.69. [DOI] [Google Scholar]
- 74.Usai M., Marchetti M., Foddai M., Del Caro A., Desogus R., Sanna I., Piga A. Influence of different stabilizing operations and storage time on the composition of essential oil of thyme (Thymus officinalis L.) and rosemary (Rosmarinus officinalis L.) LWT-Food Sci. Technol. 2011;44:244–249. doi: 10.1016/j.lwt.2010.05.024. [DOI] [Google Scholar]
- 75.Wang W., Wu N., Zu Y.G., Fu Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008;108:1019–1022. doi: 10.1016/j.foodchem.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 76.Jiang Y., Wu N., Fu Y.-J., Wang W., Luo M., Zhao C.-J., Zu Y.-G., Liu X.-L. Chemical composition and antimicrobial activity of the essential oil of rosemary. Environ. Toxicol. Pharmacol. 2011;32:63–68. doi: 10.1016/j.etap.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 77.Mizrahi I., Juarez M.A., Bandoni A.L. The essential oil of Rosmarinus officinalis growing in Argentina. J. Essent. Oil Res. 1991;3:11–15. doi: 10.1080/10412905.1991.9697900. [DOI] [Google Scholar]
- 78.Moretti M.D.L., Peana A.T., Passino G.S., Bazzoni A., Solinas V. Effects of iron on yield and composition of Rosmarinus officinalis L. essential oil. J. Essent. Oil Res. 1998;10:43–49. doi: 10.1080/10412905.1998.9700836. [DOI] [Google Scholar]
- 79.Moretti M.D.L., Peana A.T., Passino G.S., Solinas V. Effects of soil properties on yield and composition of Rosmarinus officinalis essential oil. J. Essent. Oil Res. 1998;10:261–267. doi: 10.1080/10412905.1998.9700898. [DOI] [Google Scholar]
- 80.Rao L.J., Singh M., Raghavan B., Abraham K.O. Rosemary (Rosmarinus officinalis L.): Impact of drying on its flavor quality. J. Food Qual. 1998;21:107–115. doi: 10.1111/j.1745-4557.1998.tb00508.x. [DOI] [Google Scholar]
- 81.El-Ghorab A.H. Supercritical fluid extraction of the Egyptian rosemary (Rosmarinus officinalis) leaves and Nigella sativa L. seeds volatile oils and their antioxidant activities. J. Essent. Oil Bear. Plants. 2003;6:67–77. doi: 10.1080/0972-060X.2003.10643331. [DOI] [Google Scholar]
- 82.Satyal P., Murray B.L., McFeeters R.L., Setzer W.N. Essential oil characterization of Thymus vulgaris from various geographical locations. Foods. 2016;5:70. doi: 10.3390/foods5040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Apaya K.L., Chichioco-Hernandez C.L. Xanthine oxidase inhibition of selected Philippine medicinal plants. J. Med. Plants Res. 2011;5:289–292. [Google Scholar]
- 84.Ai N., Welsh W.J., Santhanam U., Hu H., Lyga J. Novel virtual screening approach for the discovery of human tyrosinase inhibitors. PLoS ONE. 2014;9:1–11. doi: 10.1371/journal.pone.0112788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mhaidat N.M., Al-Smadi M., Al-Momani F., Alzoubi K.H., Mansi I., Al-Balas Q. Synthesis, antimicrobial and in vitro antitumor activities of a series of 1,2,3-thiadiazole and 1,2,3-selenadiazole derivatives. Drug Des. Devel. Ther. 2015;9:3645–3652. doi: 10.2147/DDDT.S86054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Presti M.L., Ragusa S., Trozzi A., Dugo P., Visinoni F., Fazio A., Dugo G., Mondello L. A comparison between different techniques for the isolation of rosemary essential oil. J. Sep. Sci. 2005;28:273–280. doi: 10.1002/jssc.200400037. [DOI] [PubMed] [Google Scholar]
- 87.König W.A., Fricke C., Saritas Y., Momeni B., Hohenfeld G. Adulteration or natural variability? Enantioselective gas chromatography in purity control of essential oils. J. High Res. Chromatogr. 1997;20:55–61. doi: 10.1002/jhrc.1240200202. [DOI] [Google Scholar]
- 88.Ravid U., Putievsky E., Katzir I., Lewinsohn E., Dudai N. Identification of (1R)(+)-verbenone in essential oils of Rosmarinus officinalis L. Flavour Fragr. J. 1997;12:109–112. doi: 10.1002/(SICI)1099-1026(199703)12:2<109::AID-FFJ618>3.0.CO;2-A. [DOI] [Google Scholar]
- 89.Moreno S., María A., Sana O., Gaya M., Barni M.V., Castro O.A., van Baren C. Rosemary compounds as nutraceutical health products. In: El-Shamragy Y., editor. Food Additive. InTech; Rijeka, Croatia: 2012. pp. 157–174. Chapter 9. [Google Scholar]
- 90.Beretta G., Artali R., Facino R.M., Gelmini F. An analytical and theoretical approach for the profiling of the antioxidant activity of essential oils: The case of Rosmarinus officinalis L. J. Pharmaceut. Biomed. Anal. 2011;55:1255–1264. doi: 10.1016/j.jpba.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 91.Meziane-Assami D., Tomao V., Ruiz K., Meklati B.Y., Chemat F. Geographical differentiation of rosemary based on GC/MS and fast HPLC analyses. Food Anal. Meth. 2013;6:282–288. doi: 10.1007/s12161-012-9430-6. [DOI] [Google Scholar]
- 92.Li G., Cervelli C., Ruffoni B., Shachter A., Dudai N. Volatile diversity in wild populations of rosemary (Rosmarinus officinalis L.) from the Tyrrhenian Sea vicinity cultivated under homogeneous environmental conditions. Ind. Crops Prod. 2016;84:381–390. doi: 10.1016/j.indcrop.2016.02.029. [DOI] [Google Scholar]
- 93.Elamrani A., Zrira S., Benjilali B., Berrada M. A study of Moroccan rosemary oils. J. Essent. Oil Res. 2000;12:487–495. doi: 10.1080/10412905.2000.9699572. [DOI] [Google Scholar]
- 94.Nowak A., Kalemba D., Krala L., Piotrowska M., Czyzowska A. The effects of thyme (Thymus vulgaris) and rosemary (Rosmarinus officinalis) essential oils on Brochothrix thermosphacta and on the shelf life of beef packaged in high-oxygen modified atmosphere. Food Microbiol. 2012;32:212–216. doi: 10.1016/j.fm.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 95.Lakušić D.V., Ristić M.S., Slavkovska V.N., Šinžar-Sekulić J.B., Lakušić B.S. Environment-related variations of the composition of the essential oils of rosemary (Rosmarinus officinalis L.) in the Balkan Penninsula. Chem. Biodivers. 2012;9:1286–1302. doi: 10.1002/cbdv.201100427. [DOI] [PubMed] [Google Scholar]
- 96.Shin S. Anti-Aspergillus activities of plant essential oils and their combination effects with ketoconazole or amphotericin B. Arch. Pharm. Res. 2003;26:389–393. doi: 10.1007/BF02976696. [DOI] [PubMed] [Google Scholar]
- 97.Fu Y.-J., Zu Y.-G., Chen L.-Y., Shi X.-G., Wang Z., Sun S., Efferth T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother. Res. 2007;21:989–994. doi: 10.1002/ptr.2179. [DOI] [PubMed] [Google Scholar]
- 98.Giordani R., Regli P., Kaloustian J., Mikaïl C., Abou L., Portugal H. Antifungal effect of various essential oils against Candida albicans. Potentiation of antifungal action of amphotericin B by essential oil from Thymus vulgaris. Phytother. Res. 2004;18:990–995. doi: 10.1002/ptr.1594. [DOI] [PubMed] [Google Scholar]
- 99.Tampieri M.P., Galuppi R., MacChioni F., Carelle M.S., Falcioni L., Cioni P.L., Morelli I. The inhibition of Candida albicans by selected essential oils and their major components. Mycopathologia. 2005;159:339–345. doi: 10.1007/s11046-003-4790-5. [DOI] [PubMed] [Google Scholar]
- 100.Braga F.G., Bouzada M.L.M., Fabri R.L., de O. Matos M., Moreira F.O., Scio E., Coimbra E.S. Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J. Ethnopharmacol. 2007;111:396–402. doi: 10.1016/j.jep.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 101.Aumeeruddy-Elalfi Z., Gurib-Fakim A., Mahomoodally M.F. Kinetic studies of tyrosinase inhibitory activity of 19 essential oils extracted from endemic and exotic medicinal plants. S. Afr. J. Bot. 2016;103:89–94. doi: 10.1016/j.sajb.2015.09.010. [DOI] [Google Scholar]
- 102.Wang W., Li N., Luo M., Zu Y., Efferth T. Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules. 2012;17:2704–2713. doi: 10.3390/molecules17032704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miladi H., Slama R.B., Mili D., Zouari S., Bakhrouf A., Ammar E. Essential oil of Thymus vulgaris L. and Rosmarinus officinalis L.: Gas chromatography-mass spectrometry analysis, cytotoxicity and antioxidant properties and antibacterial activities against foodborne pathogens. Nat. Sci. 2013;5:729–739. [Google Scholar]