Abstract
Background: Arginine is considered to be an essential amino acid in various (patho)physiologic conditions of high demand. However, dietary arginine supplementation suffers from various drawbacks, including extensive first-pass extraction. Citrulline supplementation may be a better alternative than arginine, because its only fate in vivo is conversion into arginine.
Objective: The goal of the present research was to determine the relative efficiency of arginine and citrulline supplementation to improve arginine availability.
Methods: Six-week-old C57BL/6J male mice fitted with gastric catheters were adapted to 1 of 7 experimental diets for 2 wk. The basal diet contained 2.5 g l-arginine/kg, whereas the supplemented diets contained an additional 2.5, 7.5, and 12.5 g/kg diet of either l-arginine or l-citrulline. On the final day, after a 3-h food deprivation, mice were continuously infused intragastrically with an elemental diet similar to the dietary treatment, along with l-[13C6]arginine, to determine the splanchnic first-pass metabolism (FPM) of arginine. In addition, tracers were continuously infused intravenously to determine the fluxes and interconversions between citrulline and arginine. Linear regression slopes were compared to determine the relative efficiency of each supplement.
Results: Whereas all the supplemented citrulline (105% ± 7% SEM) appeared in plasma and resulted in a marginal increase of 86% in arginine flux, supplemental arginine underwent an ∼70% FPM, indicating that only 30% of the supplemental arginine entered the peripheral circulation. However, supplemental arginine did not increase arginine flux. Both supplements linearly increased (P < 0.01) plasma arginine concentration from 109 μmol/L for the basal diet to 159 and 214 μmol/L for the highest arginine and citrulline supplementation levels, respectively. However, supplemental citrulline increased arginine concentrations to a greater extent (35%, P < 0.01).
Conclusions: Citrulline supplementation is more efficient at increasing arginine availability than is arginine supplementation itself in mice.
Keywords: amino acid, arginine, citrulline, first-pass metabolism, requirements
Introduction
Arginine is considered to be a dispensable amino acid in many species, including humans. However, certain physiologic (rapid growth, pregnancy) or pathophysiologic conditions with a high demand for arginine [e.g., preterm birth (1), sepsis (2, 3), and diabetes (4)] may lead to arginine becoming essential. Typically, such conditions may benefit from exogenous arginine supplementation, because this amino acid is required for NO (5), creatine (6), polyamine (7), and protein synthesis. However, oral supplementation with arginine may suffer from various drawbacks. First, arginine undergoes first-pass metabolism (FPM)6 via the gastrointestinal tract and liver. Earlier FPM determinations vary from 38% in humans (8) to 75% in mice (9). It is unclear whether this wide range is due to species differences or to different arginine intake amounts with respect to their requirements. Second, chronic dietary supplementation with arginine may cause gastrointestinal distress and diarrhea (10). Lastly, arginine supplementation may cause a sudden drop in blood pressure, and some reports have found arginine supplementation to cause adverse outcomes in critically ill patients (11).
Given these drawbacks of arginine supplementation, citrulline has been suggested as a potential alternative to increase arginine availability. The main support for citrulline supplementation comes from the premise that citrulline does not undergo FPM in the gut or liver (12) [although this has been questioned (13)] and that the only known fate of citrulline in vivo is its conversion to arginine (12). Furthermore, some studies have demonstrated that citrulline supplementation is as efficient as, if not more efficient than, arginine supplementation itself in increasing NO production (14, 15); however, there is a lack of quantitative data on the metabolic fate of these 2 supplements, and it is unclear which one is more efficient at increasing arginine availability. Thus, the purpose of this study was to quantify the metabolic fate of dietary arginine and citrulline supplementation under varying supplementation levels. Our hypothesis was that dietary supplementation with citrulline is more efficient than that with arginine to increase systemic arginine availability over the long term, because arginine undergoes increasing FPM.
Methods
Mice and housing.
Six-week-old male C57BL/6J mice were obtained from the Jackson Laboratory. Mice were housed in a pathogen-free temperature (22°C)- and humidity (55%)-controlled facility under a 12-h (0600–1800) light cycle. Ad libitum access to LabDiet 5V5R unpurified diet (59 g fat/kg, 24 g fiber/kg, 570 g carbohydrates/kg, 180 g crude protein/kg, 7.5 g arginine/kg, and 50 g ash/kg) and water was provided. Upon 2 d of acclimatization, mice were surgically fitted with gastric catheters as previously described (16) and housed in individual cages. All mouse procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Diet and infusion treatments.
After recovery to presurgery body weight (5 d postsurgery), mice were randomly assigned to 1 of 7 dietary treatment groups (n = 9) described below. We have shown that for maximal growth, C57BL/6J mice have an arginine requirement (17). Thus, to meet this arginine requirement, a base (control) diet was formulated by adding l-arginine (2.5 g arginine/kg diet; l-arginine, free base; Research Diets) to an elemental arginine-free diet (Research Diets) formulated based on Teklad 9020 (17). Over and above the base diet arginine content, the diet was supplemented with 3 levels of either l-arginine or l-citrulline (2.5, 7.5, or 12.5 g/kg diet) (Sigma) (Table 1). Because arginine and citrulline have similar molecular weights (174 and 175 g/mol, respectively), diets were also considered to provide supplemental (quasi)isomolar amounts of arginine and citrulline. The powdered diets were consumed ad libitum from glass jars for 2 wk; feed intake and body weight were determined 2 times/wk.
TABLE 1.
Arginine and citrulline concentrations of diets fed to mice and arginine and citrulline infusion rates of the different treatments
| Treatment diets and infusions |
|||||||
| Arginine-supplemented |
Citrulline-supplemented |
||||||
| Control | A1 | A3 | A5 | C1 | C3 | C5 | |
| Contents, g/kg diet | |||||||
| Basal arginine | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Supplemental arginine | 0 | 2.5 | 7.5 | 12.5 | 0 | 0 | 0 |
| Supplemental citrulline | 0 | 0 | 0 | 0 | 2.5 | 7.5 | 12.5 |
| Infusion, μmol · kg−1 · h#x22121 | |||||||
| Basal arginine | 75 | 75 | 75 | 75 | 75 | 75 | 75 |
| Supplemental arginine | 0 | 75 | 225 | 375 | 0 | 0 | 0 |
| Supplemental citrulline | 0 | 0 | 0 | 0 | 75 | 225 | 375 |
On the day of the infusion after a food deprivation of 3 h beginning at 0700, mice were implanted with a tail-vein catheter, and the intragastric and intravenous catheters were connected to separate syringe infusion pumps as previously described (9). After a 1-h bolus priming dose, continuous intravenous (5 mL · kg−1 · h−1) and intragastric (22 mL · kg−1 · h−1) infusions were carried out for 4 h before collection of blood samples. We showed previously that plateau isotopic enrichment of the tracers infused, as well as their products, are achieved within this period (18).
The intragastric infusate was an elemental diet formulated to mimic the respective treatment diets (Supplemental Table 1), delivering 75 μmol arginine · kg−1 · h−1 (control) and supplemented with 75, 225, or 375 μmol · kg−1 · h−1 of arginine or citrulline for the arginine and citrulline treatments, respectively (Table 1). In addition, the intragastric infusate contained U-[13C6] arginine in order to estimate the FPM of arginine (the priming dose and rate are shown in Supplemental Table 2). The intravenous infusate contained U-[15N4] arginine, (ureido)[15N] citrulline, 5,5-[2H2] ornithine, (ring)[2H5] phenylalanine, and 3,3-[2H2] tyrosine, which were used to determine fluxes and interconversions (the priming doses and rates are shown in Supplemental Table 2).
Blood and tissue sampling.
Blood samples were collected after the 4-h infusion by cheek puncture and centrifuged immediately at 4000 × g for 10 min at 4°C. Mice were killed with carbon dioxide and liver tissue was collected for expression analysis and snap-frozen in liquid nitrogen. Plasma and liver samples were stored at −80°C until analysis.
Amino acid analysis.
Amino acid enrichments in plasma were determined as their dansyl derivatives with the use of LC–tandem MS (TSQ Vantage; ThermoFisher Scientific) by monitoring the ions as previously described (19). In addition, plasma amino acid concentrations were determined with the use of l-homoarginine as an internal standard. The following precursor-product ion transitions were monitored: arginine (m/z 408→170, 410→170, 411→170, 412→170, 413→170, 414→170, 408→391, and 409→391), citrulline (m/z 409→170, 411→170, 412→170, 414→170, 409→392, and 410→392), ornithine (m/z 599→170, 601→170, 602→170, and 604→170), phenylalanine (m/z 399→120 and 404→125), tyrosine (m/z 415→136, 417→138, and 419→140), and homoarginine (m/z 422→170).
Tissue sample analysis.
Liver samples (n = 5/treatment) were homogenized in guanidinium thiocyanate-phenol-chloroform extraction solution (TRIzol; Life Technologies), and RNA was isolated with the use of Qiagen RNeasy MinElute columns. After cDNA synthesis, PCR amplification and gene differentiation expression for arginase1, cationic amino acid transporter (CAT) 1, CAT2A, CAT2B, ornithine transcarbamylase, and ornithine aminotransferase (OAT) (primer sequences are shown in Supplemental Table 3) were performed with SYBR Green1 on a multicolor real-time PCR detection system (CFX96; BioRad Laboratories). GAPDH expression was used to normalize the expression of the genes of interest.
Calculations.
Rates of appearance and conversion, as well as splanchnic FPM, were calculated as described elsewhere (16). In brief, the rate of appearance was calculated based on the isotopic dilution of the tracer infused and the rate of conversion based on the transfer of the label from the precursor to the product. Arginine FPM was calculated based on the disappearance of the intragastric tracer in comparison with the intravenously infused tracer. Arginine FPM was expressed as a rate (micromoles per kilogram per hour) and as percentage of the total arginine intragastric infusion rate. The clearance of the different amino acids, the virtual volume of plasma cleared per unit of time, was calculated for each individual amino acid by dividing the amino acid flux by the corresponding plasma amino acid concentration.
Statistics.
All data are reported as means ± SEMs. Linear regression analyses were performed to determine the relation between the supplementation level [4 supplemental levels for arginine supplementation (control and 3 arginine concentrations) and 4 supplemental levels for citrulline supplementation (control and 3 citrulline concentrations)] and the different variables. Depending on the response variable, the independent variable was the dietary content of arginine or citrulline (e.g., weight gain and feed intake) or the intragastric infusion rate of arginine or citrulline (e.g., fluxes, interconversions, clearances, or FPM). To determine the differences between arginine and citrulline supplementation, the dummy variable technique was used (20). To compare linear and nonlinear models (quadratic), goodness of fit was evaluated with the use of the extra sum of squares F test. All statistical analyses were performed with the use of RStudio, version 0.99.484 (21). Differences were considered to be significant at P < 0.05.
Results
All mice recovered to their presurgery body weight within 5 d postsurgery. Over the 2-wk feeding of experimental diets, mouse weight gain (2.0 ± 0.13 g/2 wk) and feed intake (3.5 ± 0.15 g/d) were not different (P > 0.24) between the different experimental diets. Because of the design of the feeders, some mice lost their intragastric catheters; the final number of mice is shown in the corresponding tables and figures.
Amino acid fluxes, concentrations, and clearance rates.
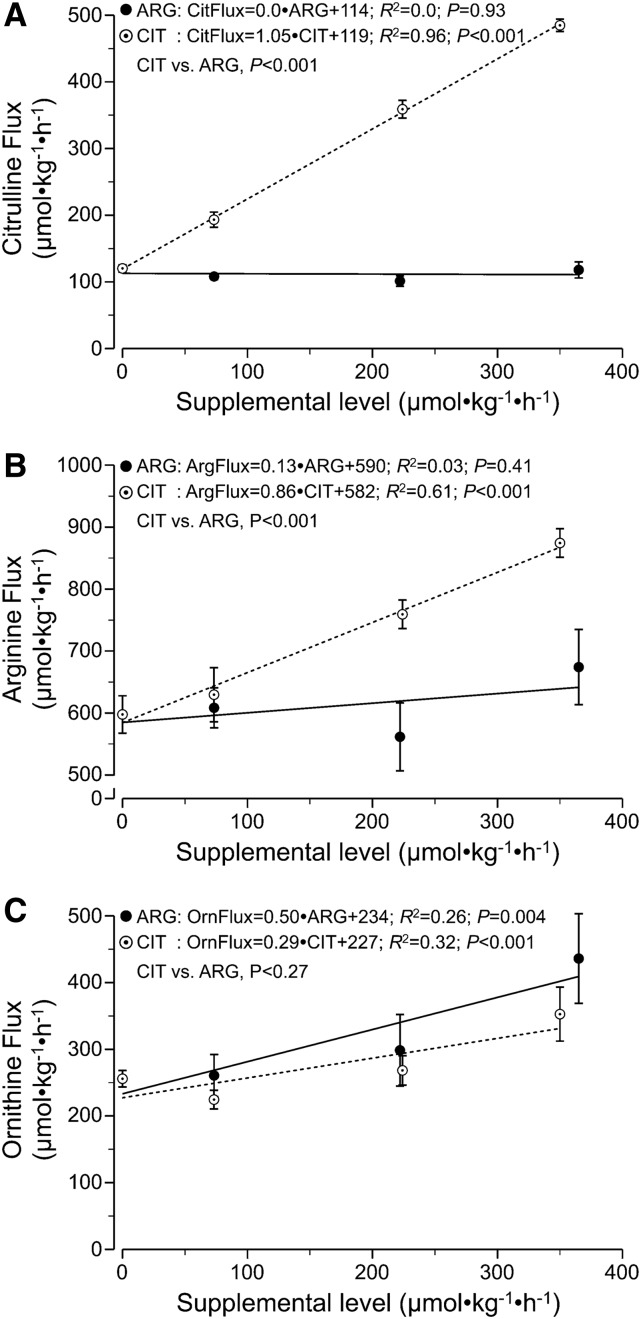
Citrulline supplementation resulted in a linear increase (P < 0.001) in citrulline and arginine fluxes (Figure 1A and B). In contrast, arginine supplementation did not increase the peripheral arginine flux (P = 0.41) (Figure 1B). All of the supplemental citrulline appeared in plasma (slope = 1.05 ± 0.07; R2 = 0.96), which resulted in a marginal increase of 86% on the arginine flux (i.e., the increase in arginine flux per unit of supplemental citrulline; slope = 0.86 ± 0.21; R2 = 0.61) (Figure 1B). The plasma concentration of citrulline increased linearly with increasing citrulline supplementation levels (P < 0.01), but arginine supplementation had no effect on citrulline concentration (P = 0.31). Both citrulline and arginine supplementation increased plasma arginine concentration (P < 0.01); however, citrulline supplementation resulted in a larger increase (P < 0.01) (Table 2). Both arginine and citrulline supplementation resulted in a linear increase in the flux and plasma concentration of ornithine (P < 0.01), but there was no difference between the 2 supplements (P = 0.27 and P = 0.83, respectively) (Figure 1C). The fluxes and plasma concentrations of phenylalanine and tyrosine were not affected by the different experimental diets fed to the mice (P > 0.20) (Supplemental Figure 1 and Table 1). Plasma clearances were not different for the amino acids studied (Table 3), with the exception of plasma arginine clearance, which decreased with increasing citrulline supplementation levels.
FIGURE 1.
Fluxes of citrulline (A), arginine (B), and ornithine (C) in mice infused intragastrically with a dextrose/amino acid mixture containing different amounts of CIT and ARG. Values are means ± SEMs, n = 6–9. ARG, supplemental arginine; ArgFlux, flux of arginine; CIT, supplemental citrulline; CitFlux, flux of citrulline; OrnFlux, flux of ornithine.
TABLE 2.
Selected plasma amino acid concentrations of mice fed diets with various levels of supplemental arginine or citrulline for 2 wk1
| Treatment diets |
||||||||||
| Arginine-supplemented |
Citrulline-supplemented |
|||||||||
| Plasma concentration, μM | Control | A1 | A3 | A5 | C1 | C3 | C5 | PArg | PCit | PCit-Arg2 |
| n | 9 | 7 | 7 | 9 | 9 | 6 | 7 | |||
| Arginine | 109 ± 11 | 126 ± 7 | 141 ± 15 | 159 ± 14 | 131 ± 11 | 169 ± 5 | 214 ± 12 | 0.002 | <0.001 | 0.008 |
| Citrulline | 74 ± 2 | 73 ± 2 | 73 ± 3 | 71 ± 2 | 119 ± 4 | 224 ± 8 | 283 ± 19 | 0.31 | <0.001 | <0.001 |
| Ornithine | 94 ± 2 | 140 ± 7 | 145 ± 9 | 199 ± 11 | 123 ± 8 | 165 ± 8 | 186 ± 18 | <0.001 | <0.001 | 0.83 |
| Phenylalanine | 186 ± 4 | 184 ± 7 | 171 ± 8 | 183 ± 6 | 187 ± 6 | 188 ± 9 | 185 ± 9 | 0.52 | 0.98 | 0.66 |
| Tyrosine | 114 ± 6 | 114 ± 7 | 109 ± 7 | 111 ± 5 | 124 ± 9 | 117 ± 8 | 123 ± 13 | 0.63 | 0.78 | 0.62 |
Values are means ± SEMs. Control, 75 μmol arginine · kg−1 · h−1; A1, A3, and A5 provided 75, 225, and 375 μmol supplemental arginine · kg−1 · h−1, respectively; C1, C3, and C5 provided 75, 225, and 375 μmol supplemental citrulline · kg−1 · h−1, respectively.
Comparison between citrulline and arginine; slopes differed for P < 0.05.
TABLE 3.
Selected plasma amino acid clearances of mice fed diets with various levels of supplemental arginine or citrulline for 2 wk1
| Treatment diets |
||||||||||
| Arginine-supplemented |
Citrulline-supplemented |
|||||||||
| Clearance rate, L · kg−1 · h−1 | Control | A1 | A3 | A5 | C1 | C3 | C5 | PArg | PCit | PCit-Arg2 |
| n | 9 | 7 | 7 | 9 | 9 | 6 | 7 | |||
| Arginine | 6.0 ± 0.2 | 4.9 ± 0.4 | 4.5 ± 0.4 | 5.4 ± 0.5 | 4.7 ± 0.4 | 4.8 ± 0.3 | 4.1 ± 0.3 | 0.44 | 0.012 | 0.21 |
| Citrulline | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.1 | 0.66 | 0.18 | 0.42 |
| Ornithine | 2.6 ± 0.1 | 1.9 ± 0.2 | 2.2 ± 0.5 | 2.0 ± 0.3 | 1.9 ± 0.2 | 1.8 ± 0.2 | 2.1 ± 0.4 | 0.39 | 0.09 | 0.58 |
| Phenylalanine | 2.8 ± 0.1 | 2.7 ± 0.1 | 2.3 ± 0.2 | 2.5 ± 0.2 | 2.4 ± 0.2 | 2.8 ± 0.2 | 2.5 ± 0.1 | 0.09 | 0.86 | 0.39 |
| Tyrosine | 3.8 ± 0.2 | 4.0 ± 0.3 | 3.4 ± 0.2 | 3.6 ± 0.3 | 3.4 ± 0.3 | 3.7 ± 0.4 | 3.3 ± 0.4 | 0.16 | 0.71 | 0.63 |
Values are means ± SEMs. Control, 75 μmol arginine · kg−1 · h−1; A1, A3, and A5 provided 75, 225, and 375 μmol supplemental arginine · kg−1 · h−1, respectively; C1, C3, and C5 provided 75, 225, and 375 μmol supplemental citrulline · kg−1 · h−1, respectively.
Comparison between citrulline and arginine; slopes differed for P < 0.05.
Amino acid interconversions.
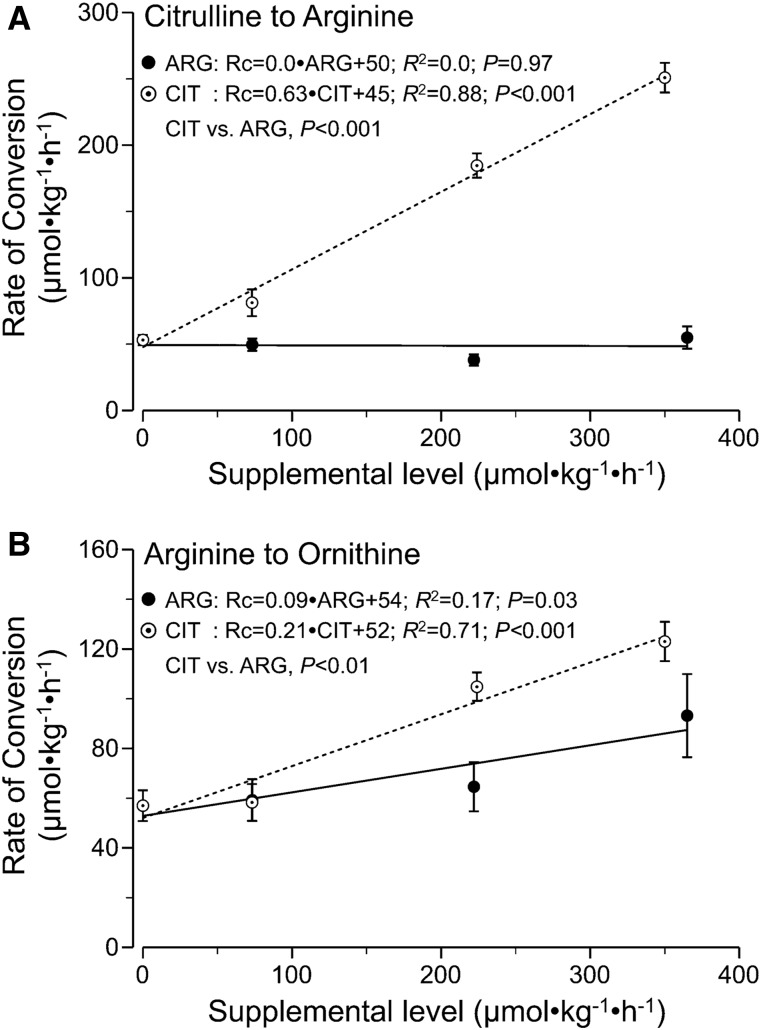
The rate of conversion of citrulline to arginine (de novo arginine synthesis) increased linearly with increasing concentration of citrulline supplementation (P < 0.001), whereas it remained unaffected by arginine supplementation (P = 0.97) (Figure 2A). The rate of conversion of circulating arginine to ornithine increased linearly with citrulline and arginine supplementation (P < 0.03) (Figure 2B). However, citrulline supplementation resulted in a greater increase in the rate of conversion of arginine into ornithine (P < 0.01). Neither arginine nor citrulline supplementation had an effect on the rates of conversion of phenylalanine to tyrosine (P > 0.40) (Supplemental Figure 2).
FIGURE 2.
Rc of citrulline to arginine (de novo arginine synthesis) (A) and circulating arginine to ornithine (B) in mice infused intragastrically with a dextrose/amino acid mixture containing different amounts of CIT and ARG. Values are means ± SEMs, n = 6–9. ARG, supplemental arginine; CIT, supplemental citrulline; Rc, rate of conversion.
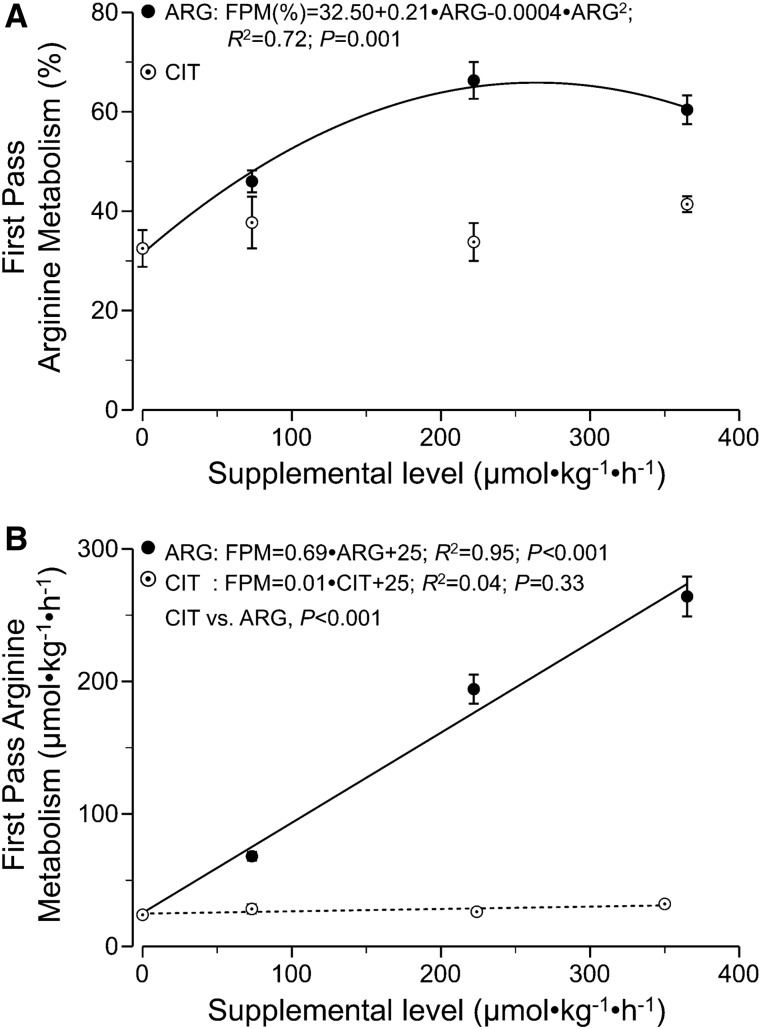
Splanchnic FPM of arginine.
Arginine FPM increased from 32.5% for mice on the control diet to 60.4% for mice on the highest arginine supplementation concentration (Figure 3A). A quadratic model was a better fit (P < 0.001) for the arginine FPM data than was a linear model (quadratic R2 = 0.72; linear R2 = 0.60). Citrulline supplementation, in contrast, had no effect on arginine FPM (P = 0.54). Increasing amounts of arginine were metabolized by the splanchnic tissue (slope = 0.69 ± 0.04; R2 = 0.95) (Figure 3B) with increasing arginine supplementation levels, indicating that 69% of the supplemental arginine disappeared during FPM. As a consequence, only 31% ± 3% of the supplemental arginine escaped first-pass splanchnic extraction and entered the general circulation.
FIGURE 3.
FPM of arginine as a percentage of total arginine infused intragastrically (A) or as an absolute rate (B) in mice infused intragastrically with a dextrose/amino acid mixture containing different amounts of CIT and ARG. The slope of FPM in panel A for citrulline was not different from zero (P = 0.50; not shown for clarity of presentation). Values are means ± SEMs, n = 6–9. ARG, supplemental arginine; CIT, supplemental citrulline; FPM, first-pass metabolism.
Liver expression of arginase and other enzymes related to arginine metabolism.
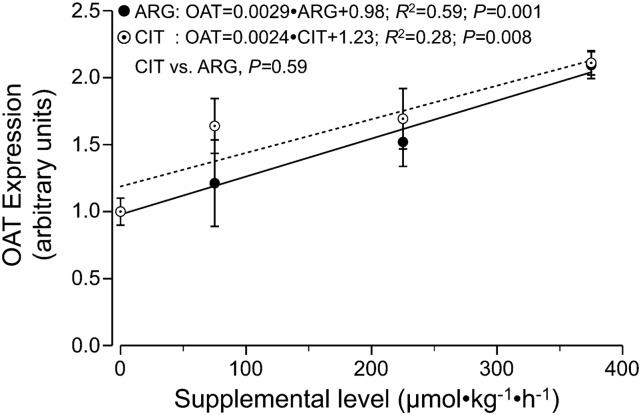
No differences in liver expression were detected for arginase 1, ornithine transcarbamylase, or arginine transporters (CAT1, CAT2A and CAT2B) from arginine or citrulline supplementation (results not shown). OAT expression, however, increased linearly with arginine and citrulline supplementation (P < 0.01) (Figure 4).
FIGURE 4.
OAT expression in the liver of mice fed diets containing different amounts of CIT and ARG. Values are means ± SEMs, n = 5. ARG, supplemental arginine; CIT, supplemental citrulline; OAT, ornithine aminotransferase.
Discussion
Citrulline, the endogenous precursor for the synthesis of arginine, has been proposed as an alternative to arginine supplementation (14, 22, 23). Because of the reduced FPM of citrulline and the fact that the only known fate of this amino acid is its conversion to arginine, citrulline has the potential not only to circumvent the adverse side effects of arginine supplementation, but also to be a more efficient option to increase arginine availability.
To directly compare the metabolic fate and the efficiency of arginine and citrulline supplementation, we used a strain of mice (C57BL/6J) in which we had previously defined the arginine requirements for growth (2.5 g/kg diet) (17). This arginine requirement was associated with the ability of this strain to produce citrulline, which is the rate-limiting step in the endogenous synthesis of arginine (24), rather than the ability of converting citrulline into arginine. The supplementation levels were designed to provide 1 time, 3 times, and 5 times the arginine requirement. This was to mimic the mean human intake of arginine (∼4 g/d) (25) and common arginine supplemental regimens used in humans (low: 4 g/d, medium: 12 g/d, and high: 20 g/d) (15, 26, 27). It is noteworthy to mention that regular rodent maintenance unpurified feed (e.g., Teklad Global 2014 or LabDiet 5V5R) contains ∼8 g arginine/kg diet (similar to our A5 diet), and complete life-cycle diets (e.g., LabDiet 5001) contain up to ∼16 g arginine/kg diet. Because arginine is in excess of requirements, the basal diet in many supplementation studies already contains excess arginine (28, 29); thus, those comparisons are not between unsupplemented and supplemented diets, but rather between high and higher arginine supplement levels. For this reason, the lack of response to arginine supplementation in some studies (30) could be due to the fact that the arginine contained in the regular unpurified diet had already maximized the physiologic response.
Citrulline escapes first-pass splanchnic extraction.
Our data demonstrate that whereas supplemental citrulline escaped FPM, a large fraction (∼70%) of the supplemental arginine underwent extraction by the gut and the liver. The negligible splanchnic FPM of citrulline is consistent with previous reports (12) and with the early observation by Krebs and Henseleit (31) that liver slices were poorly permeable to citrulline. More recently, this has been questioned in patients undergoing hepatectomy because of colorectal metastasis (13), in which citrulline uptake by the liver was observed. It is unclear whether these observations were due to species differences, the arterio-venous method used to determine uptake, or the hepatic tumors present in these subjects. Here, we have shown that the escape of citrulline into circulation resulted in an ≤4-fold increase in flux and plasma concentration; however, the citrulline clearance remained unchanged (∼1.6 L · kg−1 · h−1) regardless of the citrulline suppplementation amount, suggesting that even at the highest supplementation level, there is still spare ability to convert citrulline into arginine. This excess capacity for the disposal of citrulline was first postulated in rats (24); however, a large oral citrulline dose may at least temporarily overwhelm the ability of the kidney and other tissues to convert citrulline into arginine (32). Of note is that whereas the continuous delivery of citrulline here and in research by Dhanakoti et al. (24) resulted in an ∼4-fold increase in plasma citrulline concentration, the single citrulline dose administered in research by Moinard et al. (32) raised plasma citrulline >100-fold.
Arginine extraction by the gut and liver.
Arginine FPM has been described in many species, including humans (8, 9, 33). We showed previously in C57BL/6 mice consuming an 8 g arginine/kg diet that the FPM of this amino acid was 75%; a large fraction of this FPM seems to take part in the liver, because only a moderate reduction in arginine extraction (65% FPM) was observed in mice lacking arginase II (9). However, other pathways for arginine utilization by the gut (e.g., protein synthesis) have not been quantified, and their contribution to FPM remains unknown. Here, we have shown that arginine FPM is not a fixed amount or fraction, and that it varies depending on arginine intake. When the arginine content of the diet was just above requirements, the FPM of arginine was 32% for the control diet or 37% for the citrulline-supplemented diets. These values are remarkably similar to the ones reported by Castillo et al. (8) in humans on a 1 g protein · kg−1 · d−1 diet (32% and 38%). Thus, it seems that previous reports in different species reflect differences in arginine intake and arginine demand, rather than true species differences. The marginal arginine FPM of 70% resulted in an increase in the fractional FPM with increasing arginine supplementation levels that agrees with our previous observation in mice consuming a regular unpurified diet (9, 16). Despite the >10-fold increase in arginine extraction during first pass in the arginine-supplemented mice between the control diet and the highest arginine supplementation concentration, we were unable to detect changes in the expression of arginase 1 or in arginine transporters (CAT1, CAT2A, and CAT2B) in the liver. However, the main hepatic arginine transporter (CAT2A) is a low-affinity, high-capacity transporter (34), with the highest Michaelis-Menten constant (3.5 mmol/L) of the CAT family (e.g., that for CAT1 is 0.12 mmol/L) (35). This implies that transporter activity is very responsive to increased portal arginine concentration, which is consistent with the linear increase in arginine disappearance observed during first pass. This low affinity of the transporter has been postulated as a mechanism to protect arginine against high concentrations of hepatic arginase (36).
Supplementation increases arginine availability.
Only ∼30% of the supplemented arginine escaped splanchnic FPM and reached peripheral circulation, increasing plasma arginine concentrations; however, arginine supplementation failed to increase the flux of arginine. In contrast, citrulline supplementation not only resulted in an increase in arginine flux, but also in a greater increase in plasma arginine concentration than arginine supplementation itself. For these reasons, citrulline was a more efficient supplement than arginine in increasing arginine availability. Although we were able to account for only a fraction (86%) of the supplemental citrulline as an increase in arginine flux, and that the estimation of the de novo arginine synthesis was 63% of the citrulline flux, it is likely that all the supplemented citrulline was converted into arginine. This is due to the fact that the only known fate of citrulline is its conversion into arginine and because citrulline losses in the urine are minimal (even when large citrulline doses are administered as a single bolus) (32). The widespread expression of argininosuccinate synthase and lyase suggests that most tissues are able to use citrulline for arginine synthesis to meet their local needs for this amino acid (37, 38). However, because this arginine does not enter the circulation, it cannot be detected in the blood and thus it is not accounted for in the de novo arginine synthesis estimation.
Arginine disposal.
Although arginine is used in the synthesis of many different compounds (protein, creatine, polyamines, and NO), quantitatively, the fate of excess arginine is its disposal through arginase and the production of ornithine. Here, we demonstrated that both arginine and citrulline supplementation did increase the flux of ornithine as expected. Whereas arginine supplementation increased the disposal of arginine and subsequent conversion to ornithine before entering the peripheral circulation, citrulline supplementation increased the disposal of plasma arginine into ornithine. Regardless, the 2 supplements resulted in a similar increase in the expression of hepatic OAT. This enzyme is the main route for ornithine disposal (39), yielding glutamate semialdehyde and glutamate. The deamination of glutamate results in α-ketoglutarate, which can then enter the tricarboxylic cycle, where it is oxidized to generate energy.
In conclusion, citrulline supplementation resulted in an increase in arginine flux and a greater increase in plasma arginine concentration than did arginine supplementation itself. Whereas citrulline escaped FPM, 70% of the supplemental arginine disappeared before reaching the peripheral circulation. This extensive extraction by the splanchnic organs limits the ability of arginine supplementation to increase arginine availability. This is likely the reason why supplementation with citrulline resulted in a greater response in NO production (14, 15).
Acknowledgments
UA analyzed the samples and data, wrote an initial draft of the manuscript, and reviewed the article; ICD conducted the research and prepared the samples for analysis; YY and XW analyzed the tissue samples; and JCM designed and conducted the research, performed surgeries, analyzed the data, wrote the final manuscript, and had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CAT, cationic amino acid transporter; FPM, first-pass metabolism; OAT, ornithine aminotransferase.
References
- 1.Becker RM, Wu G, Galanko JA, Chen W, Maynor AR, Bose CL, Rhoads JM. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J Pediatr 2000;137:785–93. [DOI] [PubMed] [Google Scholar]
- 2.Argaman Z, Young VR, Noviski N, Castillo-Rosas L, Lu XM, Zurakowski D, Cooper M, Davison C, Tharakan JF, Ajami A, et al. Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit Care Med 2003;31:591–7. [DOI] [PubMed] [Google Scholar]
- 3.Luiking YC, Poeze M, Dejong CH, Ramsay G, Deutz NE. Sepsis: an arginine deficiency state? Crit Care Med 2004;32:2135–45. [DOI] [PubMed] [Google Scholar]
- 4.Kohli R, Meininger CJ, Haynes TE, Yan W, Self JT, Wu GY. Dietary L-arginine supplementation enhances endothelial nitric oxide synthesis in streptozotocin-induced diabetic rats. J Nutr 2004;134:600–8. [DOI] [PubMed] [Google Scholar]
- 5.Castillo L, Beaumier L, Ajami AM, Young VR. Whole body nitric oxide synthesis in healthy men determined from [15N]arginine-to-[15N]citrulline labeling. Proc Natl Acad Sci USA 1996;93:11460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr 2007;27:241–61. [DOI] [PubMed] [Google Scholar]
- 7.Seiler N, Daune G, Bolkenius FN, Knodgen B. Ornithine aminotransferase activity, tissue ornithine concentrations and polyamine metabolism. Int J Biochem 1989;21:425–32. [DOI] [PubMed] [Google Scholar]
- 8.Castillo L, Chapman TE, Yu YM, Ajami A, Burke JF, Young VR. Dietary arginine uptake by the splanchnic region in adult humans. Am J Physiol 1993;265:E532–9. [DOI] [PubMed] [Google Scholar]
- 9.Marini JC, Keller B, Didelija IC, Castillo L, Lee B. Enteral arginase II provides ornithine for citrulline synthesis. Am J Physiol Endocrinol Metab 2011;300:E188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimble GK. Adverse gastrointestinal effects of arginine and related amino acids. J Nutr 2007;137:1693S–701S. [DOI] [PubMed] [Google Scholar]
- 11.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr 2003;27:355–73. [DOI] [PubMed] [Google Scholar]
- 12.Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol 1981;241:E473–80. [DOI] [PubMed] [Google Scholar]
- 13.van de Poll MCG, Siroen MPC, van Leeuwen PAM, Soeters PB, Melis GC, Boelens PG, Deutz NEP, Dejong CHC. Interorgan amino acid exchange in humans: consequences for arginine and citrulline metabolism. Am J Clin Nutr 2007;85:167–72. [DOI] [PubMed] [Google Scholar]
- 14.El-Hattab AW, Hsu JW, Emrick LT, Wong LJC, Craigen WJ, Jahoor F, Scaglia F. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Mol Genet Metab 2012;105:607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, Spickler W, Schulze F, Boger RH. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol 2008;65:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marini JC. Arginine and ornithine are the main precursors for citrulline synthesis in mice. J Nutr 2012;142:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marini JC, Agarwal U, Didelija IC. Dietary arginine requirements for growth are dependent on the rate of citrulline production in mice. J Nutr 2015;145:1227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marini JC, Didelija IC, Castillo L, Lee B. Glutamine: precursor or nitrogen donor for citrulline synthesis? Am J Physiol Endocrinol Metab 2010;299:E69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marini JC. Quantitative analysis of 15N-labeled positional isomers of glutamine and citrulline via electrospray ionization tandem mass spectrometry of their dansyl derivatives. Rapid Commun Mass Spectrom 2011;25:1291–6. [DOI] [PubMed] [Google Scholar]
- 20.Gujarati D. Use of dummy variables in testing for equality between sets of coefficients in two linear regressions: a note. Am Stat 1970;24:50–2. [Google Scholar]
- 21.RStudio Team. RStudio: integrated development for R. RStudio, Inc. Boston; 2015. [cited 2016 Jan 15]. Available from: http://www.rstudio.com/.
- 22.Orozco-Gutiérrez JJ, Castillo-Martínez L, Orea-Tejeda A, Vázquez-Díaz O, Valdespino-Trejo A, Narváez-David R, Keirns-Davis C, Carrasco-Ortiz O, Navarro-Navarro A, Sánchez-Santillán R. Effect of L-arginine or L-citrulline oral supplementation on blood pressure and right ventricular function in heart failure patients with preserved ejection fraction. Cardiol J 2010;17:612–8. [PubMed] [Google Scholar]
- 23.Wijnands KAP, Vink H, Briedé JJ, van Faassen EE, Lamers WH, Buurman WA, Poeze M. Citrulline a more suitable substrate than arginine to restore NO production and the microcirculation during endotoxemia. PLoS One 2012;7:e37439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME. Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol 1990;259:E437–42. [DOI] [PubMed] [Google Scholar]
- 25.King DE, Mainous III AG, Geesey ME. Variation in L-arginine intake follow demographics and lifestyle factors that may impact cardiovascular disease risk. Nutr Res 2008;28:21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, Arigoni F, Zhang W. Effect of oral l-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J 2011;162:959–65. [DOI] [PubMed] [Google Scholar]
- 27.De Luis DA, Izaola O, Cuellar L, Terroba MC, Martin T, Ventosa M. A randomized double-blind clinical trial with two different doses of arginine enhanced enteral nutrition in postsurgical cancer patients. Eur Rev Med Pharmacol Sci 2010;14:941–5. [PubMed] [Google Scholar]
- 28.Coburn LA, Gong X, Singh K, Asim M, Scull BP, Allaman MM, Williams CS, Rosen MJ, Washington MK, Barry DP, et al. L-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS One 2012;7:e33546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dasgupta T, Hebbel RP, Kaul DK. Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radic Biol Med 2006;41:1771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You H, Gao T, Cooper TK, Morris SM Jr, Awad AS. Diabetic nephropathy is resistant to oral L-arginine or L-citrulline supplementation. Am J Physiol Renal Physiol 2014;307:F1292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krebs HA, Henseleit K. Untersuchungen uber die Harnstoffbildung im Tierkorper. [Studies on urea formation in the animal organism.] Hoppe Seylers Z Physiol Chem 1932;210:33–66 (in German). [Google Scholar]
- 32.Moinard C, Nicolis I, Neveux N, Darquy S, Benazeth S, Cynober L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: the Citrudose pharmacokinetic study. Br J Nutr 2008;99:855–62. [DOI] [PubMed] [Google Scholar]
- 33.Marini JC, Stoll B, Didelija IC, Burrin DG. De novo synthesis is the main source of ornithine for citrulline production in neonatal pigs. Am J Physiol Endocrinol Metab 2012;303:E1348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Closs EI, Albritton LM, Kim JW, Cunningham JM. Identification of a low affinity, high capacity transporter of cationic amino acids in mouse liver. J Biol Chem 1993;268:7538–44. [PubMed] [Google Scholar]
- 35.Closs EI, Simon A, Vekony N, Rotmann A. Plasma membrane transporters for arginine. J Nutr 2004;134:2752S–9S. [DOI] [PubMed] [Google Scholar]
- 36.Wu JY, Robinson D, Kung HJ, Hatzoglou M. Hormonal regulation of the gene for the type C ecotropic retrovirus receptor in rat liver cells. J Virol 1994;68:1615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marini JC, Didelija IC, Fiorotto ML. Extrarenal citrulline disposal in mice with impaired renal function. Am J Physiol Renal Physiol 2014;307:F660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marini JC, Didelija IC. Arginine depletion by arginine deiminase does not affect whole protein metabolism or muscle fractional protein synthesis rate in mice. PLoS One 2015;10:e0119801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alonso E, Rubio V. Participation of ornithine aminotransferase in the synthesis and catabolism of ornithine in mice. Studies using gabaculine and arginine deprivation. Biochem J 1989;259:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]