Abstract
Background: Poor diet quality is associated with a higher risk of many chronic diseases that are among the leading causes of death in the United States. It has been hypothesized that evolutionary discordance may account for some of the higher incidence and mortality from these diseases.
Objective: We investigated associations of 2 diet pattern scores, the Paleolithic and the Mediterranean, with all-cause and cause-specific mortality in the REGARDS (REasons for Geographic and Racial Differences in Stroke) study, a longitudinal cohort of black and white men and women ≥45 y of age.
Methods: Participants completed questionnaires, including a Block food-frequency questionnaire (FFQ), at baseline and were contacted every 6 mo to determine their health status. Of the analytic cohort (n = 21,423), a total of 2513 participants died during a median follow-up of 6.25 y. We created diet scores from FFQ responses and assessed their associations with mortality using multivariable Cox proportional hazards regression models adjusting for major risk factors.
Results: For those in the highest relative to the lowest quintiles of the Paleolithic and Mediterranean diet scores, the multivariable adjusted HRs for all-cause mortality were, respectively, 0.77 (95% CI: 0.67, 0.89; P-trend < 0.01) and 0.63 (95% CI: 0.54, 0.73; P-trend < 0.01). The corresponding HRs for all-cancer mortality were 0.72 (95% CI: 0.55, 0.95; P-trend = 0.03) and 0.64 (95% CI: 0.48, 0.84; P-trend = 0.01), and for all-cardiovascular disease mortality they were 0.78 (95% CI: 0.61, 1.00; P-trend = 0.06) and HR: 0.68 (95% CI: 0.53, 0.88; P-trend = 0.01).
Conclusions: Findings from this biracial prospective study suggest that diets closer to Paleolithic or Mediterranean diet patterns may be inversely associated with all-cause and cause-specific mortality.
Keywords: Paleolithic diet, Mediterranean diet, diet patterns, cohort study, mortality
Introduction
Cardiovascular disease and cancer are the 2 leading causes of death in the United States (1). Diet is strongly associated with risk for these and other chronic diseases (2–4), and as reviewed elsewhere (5–7), investigations into which dietary constituents may be protective have led to several large studies of the effects of various nutritional supplements hypothesized to lower risk of cardiovascular disease, cancer, and mortality. These trials yielded mostly null, or sometimes adverse, results. Given these findings, nutritional supplements are not generally recommended for preventing cardiovascular disease or cancer (5). There are several potential explanations for the null results from supplementation trials, including an inability of supplements to mimic the complex composition and interacting components of whole foods and diets (3, 8–10).
To assess the totality of diet rather than the influence of single nutrients, foods, or other individual dietary constituents, nutrition researchers have utilized dietary pattern analysis. There are several methods for constructing or representing dietary patterns for analysis, from agnostic data-driven methods, to a priori methods in which food groups and consumption patterns are characterized based on, for example, published dietary quality indexes or current dietary recommendations (3, 11, 12).
A dietary pattern of growing interest is the Paleolithic diet pattern. The consequences of the discrepancies between the diets and lifestyles of Homo sapiens before the agricultural revolution and those during the modern era is referred to as evolutionary discordance, and has been proposed to account for some of the dramatic increase in chronic disease in the past century (13). The Paleolithic diet of preagricultural hunter-gatherer humans was estimated from anthropological evidence from fossils and extant hunter-gather groups (14). The diet pattern is characterized as a predominantly plant food–based diet, with a wide diversity of fruits, nuts, and vegetables, including wild-plant foods that contained high amounts of calcium and other minerals (14). It also includes lean meat, and is low in dairy, grains, sugar, and salt. Although several clinical studies reported beneficial effects of a Paleolithic-like diet on cardiovascular and other metabolic risk biomarkers (15–21), few studies explored associations of a Paleolithic diet pattern with chronic disease endpoints (22).
A more commonly studied dietary pattern with many similarities to the Paleolithic diet is the Mediterranean diet (17, 22–44). It has been strongly and consistently linked with health benefits (45), especially in relation to cardiovascular disease, in randomized controlled trials (46, 47) and observational studies (27–30, 44). These apparent health benefits make the Mediterranean diet pattern a model with which to compare the Paleolithic pattern. The many similarities between the Mediterranean and Paleolithic diet patterns include a high consumption of fruits, vegetables, and nuts and lower intake of added sugar. However, the idealized Mediterranean diet pattern includes grains and moderate amounts of alcohol and dairy foods, whereas the Paleolithic diet includes none (27, 31, 46).
We previously reported similar inverse associations of the Paleolithic and Mediterranean diet scores with incident colorectal adenoma (22) and biomarkers of inflammation and oxidative balance (48). Motivated partly by these results, and by the growing popularity of the Paleolithic diet, we investigated associations of both the Paleolithic diet score and the Mediterranean diet score (as comparison) with all-cause and cause-specific mortality endpoints in the REGARDS (REasons for Geographic and Racial Differences in Stroke) study, a prospective cohort of 30,183 white and black adults predominantly from the Southeastern United States.
Methods
Study population and data collection.
The study design and recruitment for REGARDS was described previously (49–53). Briefly, REGARDS is a prospective cohort study designed to investigate the causes of racial and geographic disparities in stroke. The national cohort (from all lower 48 US states) was assembled by using stratified random sampling with oversampling from the US “stroke belt” (North Carolina, South Carolina, Georgia, Tennessee, Alabama, Mississippi, and Louisiana) and so that there would be equal representation by sex and by white or black ancestry. From January 2003 to October 2007, 30,183 individuals ≥45 y of age were enrolled. Of these participants, 8547 did not complete the FFQ at baseline, and an additional 213 were lost to follow-up and excluded from the present analyses, leaving an analytic cohort of 21,423 individuals. The institutional review boards of all participating institutions approved the study methods, and all participants gave written, informed consent at the time of enrollment.
Computer-assisted telephone interviews were conducted to collect demographic and medical history information, followed 3–4 wk later by an in-home visit during which study staff conducted medical assessments, including anthropometry. At the in-home visit, self-administered questionnaires, including a Block 98 FFQ that included 109 food and beverage items to assess habitual consumption over the past year (54–56), were provided for participants to complete and return by mail.
Assessment of exposures.
The Paleolithic and Mediterranean diet pattern scores were constructed in a similar manner as described previously (22). Briefly, by using the habitual food-consumption information obtained at baseline, each study participant was assigned a quintile rank (a score from 1 to 5) based on the sex-specific distribution of intake for each food group included in the score. As shown in Supplemental Table 1, generally, more points were assigned for higher intakes of foods considered characteristic of a diet pattern, and fewer points were assigned for higher intakes of foods considered uncharacteristic of that pattern. For the Mediterranean diet this scheme was modified in relation to dairy, grain and starch, and alcohol intakes as noted in Supplemental Table 1. For the Paleolithic diet score 2 unique variables were created. The first, a fruit and vegetable diversity score, was created by summing the total number of responses on the fruit and vegetable sections of the FFQ that indicated that the participant ate >1–3 servings of a given food item per month. More diversity was considered desirable. Second, because the Paleolithic diet had little dairy but high amounts of calcium (from wild plant foods) (14), to consider dietary calcium separately from dairy products we used the residuals of a linear regression of total (i.e., dietary plus supplemental) calcium on total dairy intake to represent calcium intake independent of dairy consumption. The points for each of the food groups composing a diet score were then summed, so that the final Paleolithic diet score (14 components) could range from 14 to 70, whereas the Mediterranean diet score (11 components) could range from 11 to 55.
Cohort follow-up.
Study staff contacted cohort participants by telephone every 6 mo to ascertain any stroke events or deaths. If a death occurred, the death certificate and any associated medical records for the 28-d period before death were collected. Identifiers of participants who could not be contacted, and thus considered lost to follow-up, were sent to the social security death index and/or the National Death Index to identify deaths in this subpopulation.
Causes of death.
Cause of death was adjudicated by a committee of physicians using information from death certificates, hospital records, and proxy interviews (57). We defined cardiovascular disease mortality as death from a myocardial infarction, stroke, sudden death, heart failure, pulmonary embolism, other cardiac causes of death, and noncardiac but other cardiovascular disease deaths. We defined cancer mortality as death attributed to any type of cancer. We also created a category that included all other noninjury or accidental deaths, most of which were caused by respiratory illness, infection, liver disease, or kidney failure.
Statistical analysis.
The characteristics of the study population at baseline, by quintile of each diet pattern score, were summarized and compared by using chi-square tests for categorical variables and ANOVA for continuous variables that were normally distributed, or the Kruskal-Wallis nonparametric test for continuous variables that were not. Correlation between the 2 scores was calculated with a Spearman correlation coefficient. HRs and 95% CIs for mortality were estimated with Cox proportional hazards regression models, with participants’ age as the underlying time scale (the results calculated by using time in the study after adjusting for age were similar to those using age as the underlying time scale). We tested each exposure and potential covariate for the proportional hazards assumption using Log-Log Kaplan-Meier curves, goodness-of-fit tests, and extended Cox models.
Based on previous literature and biological plausibility, potential confounding variables considered included sex, race, smoking, BMI (in kg/m2), waist circumference, regular aspirin use, regular other nonsteroidal anti-inflammatory drug use, hormone replacement therapy use (in women), physical activity, income, health insurance coverage, region of residence in the United States, rural or urban classification, personal history of one or more major comorbidities such as cancer or diabetes, education, self-reported health, vitamin or mineral supplement use, total energy intake, and physical activity, all measured at baseline. The criteria for inclusion in the final models included biological plausibility and/or whether inclusion or exclusion of the variable, singly or jointly with other covariates, from the model changed the adjusted HRs for the primary exposure variable by ≥10%. The final, multivariable-adjusted model controlled for sex, race (black or white), total energy intake (kilocalories per day, continuous), BMI [categorized by WHO criteria (58) into underweight, normal, overweight, and obese], physical activity (0, 1–3, or ≥4 exercise sessions on average/wk), smoking (current, former, and never), annual income (refused, <$20,000, $20,000–$34,000, $35,000–$74,000, and ≥$75,000), and hormone replacement therapy use (in women).
To assess potential effect modification by various risk factors, selected based on biological plausibility, separate analyses for all-cause mortality were conducted within categories of race (white or black), sex (male or female), age (≤65 or >65 y), self-reported health at baseline (poor, fair or good, very good, excellent), BMI (underweight and normal or overweight and obese), time since start of follow-up (≤6.25 or >6.25 y), smoking status (never, former, or current), and region of United States (Southern United States or Western, Midwestern, and Eastern United States).
We conducted several analyses to assess the sensitivity of the observed associations to variations in score definition and other considerations. We removed and replaced each individual component one at time from each a priori score to determine whether any one component overly influenced the observed associations. Diet scores were also constructed with the use of the medians rather than the quintiles of the distributions of the components, by using an alternative construction of the fat ratio variable (monounsaturated + polyunsaturated:saturated fats) in the Mediterranean diet score, and by using sex- and race-specific cutoffs of food group consumption to create the final scores. We examined associations excluding BMI from the models because it could be considered both a confounder and a mediator of the association of diet with mortality. Finally, we excluded deaths that occurred during the first 1–3 y of follow-up and excluded participants with comorbidities at baseline to assess the potential influence of premorbid health conditions on diet reporting. We conducted all analyses using SAS statistical software (SAS version 9.3; SAS Statistical Institute). Two-sided tests were considered statistically significant if P ≤ 0.05.
Results
Selected characteristics of the participants by diet score quintiles are presented in Table 1. Compared with those in the lowest quintile of the Paleolithic diet score, those in the highest quintile were, on average, older, and were more likely to be female, white, obese, and a nonsmoker; to exercise more frequently, regularly take aspirin, have a higher annual income, have health insurance, and have been previously diagnosed with cancer or diabetes; and if a woman, to be more likely to currently take hormone replacement therapy. In addition, reported total energy consumption was lower for those in the lowest quintile of the Paleolithic diet score than for those in the highest quintile. The distribution of baseline characteristics across Mediterranean diet score quintiles was similar to that of the Paleolithic diet score, except that age and total energy intakes were more similar across quintiles of the Mediterranean diet score, the proportion of men and women in each quintile was similar, and those in the upper quintile were less likely to reside in the Southern United States or to have previously been diagnosed with cancer or diabetes. The Paleolithic diet scores ranged from 21 to 65 (range of possible scores: 14–70), whereas the Mediterranean diet scores ranged from 14 to 50 (range of possible scores: 11–55). These ranges did not differ appreciably by sex or race, and the 2 scores were moderately correlated (ρ = 0.64, P < 0.01). The concordance of quintile rankings for each diet score is shown in Supplemental Table 2, and the study specific semiquantitative score components’ quintile cutoffs and mean values are presented in Supplemental Table 3.
TABLE 1.
Selected characteristics of participants at baseline in the REGARDS cohort (n = 21,423)1
| Paleolithic diet score quintile2 |
Mediterranean diet score quintile2 |
|||||||
| 1 (n = 5073) | 3 (n = 4137) | 5 (n = 3819) | P3 | 1 (n = 5073) | 3 (n = 4137) | 5 (n = 3819) | P3 | |
| Age,4 y | 63.1 ± 9.45 | 65.2 ± 9.2 | 66.1 ± 8.8 | <0.01 | 64.3 ± 9.5 | 65.0 ± 9.3 | 65.3 ± 8.9 | 0.05 |
| Male | 2344 (46.2) | 1837 (44.4) | 1581 (41.4) | <0.01 | 2127 (44.0) | 2058 (44.3) | 1611 (44.4) | 0.99 |
| White | 3180 (62.7) | 2733 (66.1) | 2695 (70.6) | <0.01 | 3014 (62.31) | 3095 (66.7) | 2647 (72.9) | <0.01 |
| Current smoker | 1215 (24.1) | 516 (12.5) | 193 (5.1) | <0.01 | 1062 (22.1) | 589 (12.7) | 213 (5.9) | <0.01 |
| BMI,6 kg/m2 | ||||||||
| Underweight (<18.5) | 75 (1.5) | 35 (0.9) | 31 (0.8) | 73 (1.5) | 46 (1.0) | 19 (0.5) | ||
| Normal (18.5–24.9) | 1200 (23.8) | 952 (23.2) | 1060 (27.9) | 1143 (23.8) | 1047 (22.7) | 1032 (28.6) | ||
| Overweight (25–29.9) | 1802 (35.8) | 1598 (38.9) | 1483 (39.1) | 1676 (34.9) | 1812 (39.3) | 1475 (40.8) | ||
| Obese (≥30) | 1959 (38.9) | 1521 (37.0) | 1223 (32.2) | <0.01 | 1906 (39.7) | 1704 (37.0) | 1089 (30.1) | <0.01 |
| Exercise sessions/wk6 | ||||||||
| None | 2039 (40.9) | 1343 (32.9) | 884 (23.4) | 1953 (41.1) | 1454 (31.8) | 811 (22.5) | ||
| 1–3 | 1666 (33.4) | 1539 (37.7) | 1473 (39.0) | 1573 (33.1) | 1761 (38.5) | 1442 (40.1) | ||
| ≥4 | 1279 (25.7) | 1198 (29.4) | 1423 (37.7) | <0.01 | 1231 (25.9) | 1357 (29.7) | 1345 (37.4) | <0.01 |
| Regular aspirin user6,7 | 2009 (39.6) | 1803 (43.6) | 1822 (47.7) | <0.01 | 2045 (42.3) | 2069 (44.6) | 1677 (46.3) | <0.01 |
| Regular NSAID user6,7 | 784 (15.5) | 636 (15.4) | 548 (14.4) | 0.58 | 725 (15.0) | 704 (15.2) | 531 (14.7) | 0.94 |
| Income <$20,000/y | 1110 (21.9) | 639 (15.5) | 388 (10.2) | <0.01 | 1109 (22.9) | 656 (14.1) | 310 (8.5) | <0.01 |
| Relationship status | ||||||||
| Married | 2946 (58.1) | 2589 (62.6) | 2464 (64.5) | 2685 (55.5) | 2864 (61.7) | 2436 (67.1) | ||
| Widowed | 861(17.0) | 731 (17.7) | 633 (16.6) | <0.01 | 967 (20.0) | 832 (17.9) | 519 (14.3) | <0.01 |
| Has health insurance4 | 4608 (90.9) | 3920 (94.8) | 3665 (96.1) | <0.01 | 4446 (92.0) | 4388 (94.6) | 3493 (96.3) | <0.01 |
| Region | ||||||||
| Southern United States | 2872 (56.6) | 2367 (57.2) | 2090 (54.7) | 2866 (59.3) | 2597 (56.0) | 1875 (51.7) | ||
| Western, Midwestern, Eastern United States | 2201 (43.4) | 1770 (42.8) | 1729 (45.3) | 0.19 | 1971 (40.8) | 2044 (44.0) | 1754 (48.3) | <0.01 |
| Ever diagnosed with cancer | 381 (12.4) | 374 (15.2) | 363 (16.3) | <0.01 | 414 (14.5) | 441 (15.9) | 335 (15.6) | 0.44 |
| Diabetes at baseline | 854 (17.4) | 786 (19.7) | 758 (20.5) | <0.01 | 997 (21.4) | 891 (19.8) | 509 (14.5) | <0.01 |
| HRT use (in women) | 1485 (54.6) | 1323 (57.8) | 1384 (62.0) | <0.01 | 1475 (54.7) | 1509 (58.7) | 1290 (64.1) | <0.01 |
| Total energy intake,4 kcal/d | 1905 ± 723 | 1676 ± 736 | 1540 ± 591 | <0.01 | 1628 ± 736 | 1725 ± 732 | 1776 ± 625 | <0.01 |
| Fiber, g/d | 13.3 ± 6.2 | 15.5 ± 8.4 | 19.8 ± 9.8 | <0.01 | 11.7 ± 6.6 | 16.2 ± 8.1 | 21.2 ± 9.0 | <0.01 |
| Alcohol, g/wk | 115 ± 236 | 91 ± 193 | 63 ± 150 | <0.01 | 92 ± 233 | 93 ± 199 | 86 ± 156 | <0.01 |
| Protein, g/d | 63.9 ± 27.8 | 61.4 ± 31.2 | 61.3 ± 27.4 | <0.01 | 56.4 ± 28.2 | 62.9 ± 20.4 | 68.7 ± 27.3 | <0.01 |
| Carbohydrates, g/d | 229 ± 90 | 200 ± 90 | 183 ± 76 | <0.01 | 198 ± 94 | 205 ± 90 | 209 ± 76 | 0.03 |
| Saturated fat, g/d | 22.4 ± 11.4 | 20.2 ± 10.6 | 16.8 ± 8.3 | <0.01 | 21.3 ± 11.5 | 20.7 ± 10.9 | 19.3 ± 8.9 | <0.01 |
Values are n (%) unless otherwise indicated. Diet score quintile ranges were as follows—Paleolithic: quintile 1, 21–37; quintile 3, 41–43; quintile 5, 48–65; Mediterranean: quintile 1, 14–26; quintile 3, 30–32; quintile 5, 36–50. HRT, hormone replacement therapy; NSAID, nonsteroidal anti-inflammatory drug; REGARDS, REasons for Geographic and Racial Differences in Stroke.
Unequal sample sizes in quintiles because of ranking ties.
P values were calculated by using chi-square tests for categorical variables and ANOVA for continuous variables unless otherwise noted.
P values were calculated by using the Kruskal-Wallis nonparametric test.
Mean ± SD (all such values).
Missing data: smoking status (n = 82), BMI (n = 140), exercise sessions (n = 292), regular aspirin use (n = 10), regular NSAID use (n = 69), insurance status (n = 14).
Regular was defined as ≥2 times/wk.
During the 11 y of follow-up (median: 6.25 y), 2513 participants died. Adjusting only for age, sex, race, and total energy intake, there were statistically significant trends for decreasing risk for all-cause mortality with increasing Paleolithic and Mediterranean diet scores. Among those in the upper relative to those in the lowest quintiles of the Paleolithic and Mediterranean diet scores, risk was statistically significantly 41% (HR: 0.59; 95% CI: 0.51, 0.67) and 51% (HR: 0.49; 95% CI: 0.42, 0.56) lower, respectively (Table 2). After additional adjustment for potential confounders, particularly smoking status, the association of each score with all-cause mortality was somewhat attenuated [to 23% (HR: 0.77; 95% CI: 0.67, 0.89) and 36% (HR: 0.64; 95% CI: 0.55, 0.74) lower risk for those in the upper relative to the lowest quintiles of the Paleolithic and Mediterranean diet scores, respectively], but the point estimates for the extreme quintile comparisons and the tests for trend remained statistically significant. For each diet score, the findings for cardiovascular-specific, cancer-specific, and other noninjury or accident mortality were similar to those for all-cause mortality.
TABLE 2.
Associations of Paleolithic and Mediterranean diet scores with total and cause-specific mortality in the REGARDS cohort (n = 21,423)1
| Paleolithic diet score quintile |
Mediterranean diet score quintile |
|||||||||||||
| Cause of death | n | 1 (n = 5073) | 2 (n = 3728) | 3 (n = 4137) | 4 (n = 4666) | 5 (n = 3819) | P-trend2 | n | 1 (n = 4829) | 2 (n = 4584) | 3 (n = 4651) | 4 (n = 3782) | 5 (n = 3577) | P-trend2 |
| All causes | ||||||||||||||
| Deaths | 2513 | 626 (24.9) | 482 (19.2) | 500 (19.9) | 536 (21.3) | 369 (14.7) | 2513 | 706 (28.1) | 595 (23.7) | 525 (20.9) | 398 (15.8) | 289 (11.5) | ||
| Minimally adjusted HR (95% CI)3 | 1.00 | 0.89 (0.78, 1.01) | 0.80 (0.71, 0.91) | 0.71 (0.63, 0.80) | 0.59 (0.51, 0.67) | <0.01 | 1.00 | 0.82 (0.73, 0.93) | 0.72 (0.64, 0.81) | 0.65 (0.57, 0.74) | 0.49 (0.42, 0.56) | <0.01 | ||
| Fully adjusted HR (95% CI)4 | 1.00 | 0.95 (0.84, 1.08) | 0.94 (0.83, 1.07) | 0.87 (0.77, 0.99) | 0.77 (0.67, 0.89) | <0.01 | 1.00 | 0.90 (0.80, 1.02) | 0.82 (0.72, 0.92) | 0.79 (0.69, 0.9) | 0.64 (0.55, 0.74) | <0.01 | ||
| Cardiovascular5 | ||||||||||||||
| Deaths | 863 | 199 (23.1) | 154 (17.8) | 198 (22.9) | 186 (21.6) | 126 (14.6) | 863 | 230 (26.7) | 205 (23.8) | 190 (22.0) | 139 (16.1) | 99 (11.5) | ||
| Minimally adjusted HR (95% CI)3 | 1.00 | 0.95 (0.76, 1.19) | 1.04 (0.84, 1.29) | 0.79 (0.64, 0.98) | 0.63 (0.49, 0.80) | <0.01 | 1.00 | 0.85 (0.69, 1.04) | 0.85 (0.7, 1.05) | 0.71 (0.57, 0.89) | 0.54 (0.42, 0.69) | <0.01 | ||
| Fully adjusted HR (95% CI)4 | 1.00 | 1.01 (0.80, 1.27) | 1.18 (0.95, 1.46) | 0.94 (0.75, 1.18) | 0.78 (0.61, 1.00) | 0.06 | 1.00 | 0.92 (0.75, 1.13) | 0.94 (0.76, 1.15) | 0.86 (0.68, 1.08) | 0.68 (0.53, 0.88) | 0.01 | ||
| Cancer | ||||||||||||||
| Deaths | 728 | 184 (25.3) | 151 (20.7) | 134 (18.4) | 166 (22.8) | 93 (12.8) | 728 | 206 (28.3) | 159 (21.8) | 147 (20.2) | 129 (17.7) | 87 (12.0) | ||
| Minimally adjusted HR (95% CI)3 | 1.00 | 0.91 (0.72, 1.14) | 0.73 (0.57, 0.92) | 0.75 (0.60, 0.94) | 0.52 (0.40, 0.67) | <0.01 | 1.00 | 0.76 (0.61, 0.95) | 0.68 (0.54, 0.85) | 0.72 (0.57, 0.91) | 0.48 (0.36, 0.63) | <0.01 | ||
| Fully adjusted HR (95% CI)4 | 1.00 | 1.00 (0.80, 1.27) | 0.87 (0.69, 1.11) | 0.96 (0.76, 1.21) | 0.72 (0.55, 0.95) | 0.03 | 1.00 | 0.84 (0.68, 1.06) | 0.78 (0.62, 0.98) | 0.88 (0.70, 1.12) | 0.64 (0.48, 0.84) | 0.01 | ||
| Other cause of death | ||||||||||||||
| Deaths | 822 | 221 (26.9) | 160 (19.5) | 148 (18.0) | 160 (19.5) | 133 (16.2) | 822 | 240 (29.2) | 208 (25.3) | 167 (20.3) | 120 (14.6) | 87 (10.6) | ||
| Minimally adjusted HR (95% CI)3 | 1.00 | 0.80 (0.64, 1.00) | 0.64 (0.51, 0.80) | 0.58 (0.47, 0.72) | 0.58 (0.46, 0.73) | <0.001 | 1.00 | 0.86 (0.71, 1.05) | 0.64 (0.51, 0.79) | 0.56 (0.44, 0.70) | 0.42 (0.32, 0.55) | <0.001 | ||
| Fully adjusted HR (95% CI)4 | 1.00 | 0.84 (0.67, 1.05) | 0.76 (0.61, 0.96) | 0.71 (0.57, 0.89) | 0.77 (0.60, 0.98) | 0.01 | 1.00 | 0.95 (0.77, 1.16) | 0.73 (0.59, 0.91) | 0.67 (0.53, 0.86) | 0.56 (0.43, 0.74) | <0.001 | ||
Values are n (%) unless otherwise indicated. Diet score quintile ranges were as follows—Paleolithic: quintile 1, 21–37; quintile 2, 38–40; quintile 3, 41–43; quintile 4, 44–47; quintile 5, 48–65; Mediterranean: quintile 1, 14–26; quintile 2, 27–29; quintile 3, 30–32; quintile 4, 33–35; quintile 5, 36–50. REGARDS, REasons for Geographic and Racial Differences in Stroke.
P-trend was calculated by assigning the median of each diet score quintile to each quintile and treating this quintile exposure as continuous.
Adjusted for sex, race (black or white), and total energy intake (kilocalories per day) at baseline in an age-as-time-scale Cox model.
Adjusted for sex, race (black or white), total energy intake (kilocalories per day), BMI [kg/m2, categorized by WHO criteria (58) into underweight, normal, overweight, or obese], physical activity (0, 1–3, or ≥4 exercise sessions/wk), smoking (current, former, or never), annual income (refused, <$20,000, $20,000–$34,000, $35,000–$74,000, or ≥$75,000/y), and hormone replacement therapy use (in women) at baseline in an age (in months)-as-time-scale model.
Cardiovascular disease deaths include deaths attributed to myocardial infarction, stroke, sudden death, heart failure, pulmonary embolism, other cardiac causes of death, and other noncardiac cardiovascular disease deaths.
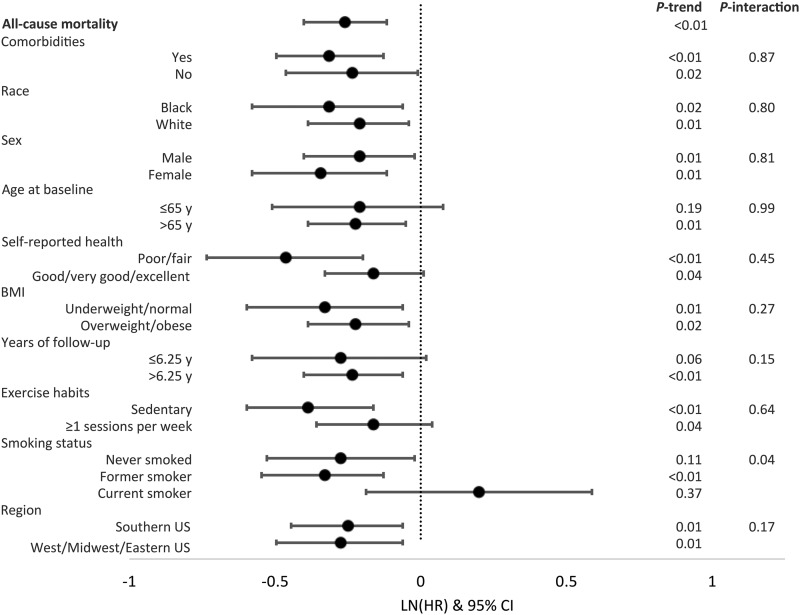
The associations of the Paleolithic diet score with all-cause mortality stratified by various participant characteristics at baseline are summarized in Figure 1. The associations comparing those in the upper with those in the lowest quintile differed minimally across the various strata. One exception was that among never or former smokers the score was inversely associated with mortality, but directly associated among current smokers. Although the test for multiplicative interaction for this finding was statistically significant (P = 0.04), the 95% CIs for the point estimates for the strata of current smokers included 1.0.
FIGURE 1.
Associations of the Paleolithic diet score with all-cause mortality, according to selected participant characteristics at baseline in the REGARDS cohort (n = 21,423). From the Cox model, only the comparison of the fifth relative to the first quintile of each diet score with all-cause mortality is shown; model covariates included sex, race (black or white), total energy intake (kilocalories per day), BMI [kg/m2, categorized by WHO criteria (58) into underweight, normal, overweight, or obese], physical activity (0, 1–3, or ≥4 exercise sessions/wk), smoking (current, former, or never), annual income (refused, <$20,000, $20,000–$34,000, $35,000–$74,000, or ≥$75,000/y), and hormone replacement therapy use (in women) at baseline in an age-as-time-scale. P-trend was calculated by assigning the median of each diet score quintile to each quintile, and treating this quintile exposure as continuous. Comorbidities include history of any cancer, kidney failure, type II diabetes, stent, heart surgery, aneurysm, or myocardial infarction. Comorbidities: yes (n = 7436; Q1, n = 1643; Q5, n = 1348), no (n = 13,987; Q1, n = 3425; Q5, n = 2433); race: black (n = 7163; Q1, n = 1882; Q5, n = 1127), white (n = 14,260; Q1, n = 3186; Q5, n = 2654); sex: male (n = 9457; Q1, n = 2346; Q5, n = 1566), female (n = 11,966; Q1, n = 2722; Q5, n = 2215); age at baseline: ≤65 y (n = 11,663; Q1, n = 3128; Q5, n = 1875), >65 y (n = 9760; Q1, n = 1940; Q5, n = 1906); self-reported health: poor or fair (n = 3435; Q1, n = 1010; Q5, n = 416), good, very good, or excellent (n = 17,953; Q1, n = 4049; Q5, n = 3360); BMI: underweight or normal (n = 5480; Q1, n = 1263; Q5, n = 1082), overweight or obese (n = 15,803; Q1, n = 3768; Q5, n = 2679); years of follow-up: ≤6.25 y (n = 10,773; Q1, n = 2640; Q5, n = 1807), >6.25 y (n = 10,649; Q1, n = 2428; Q5, n = 1974); exercise habits: sedentary (n = 6924; Q1, n = 2031; Q5, n = 888), ≥1 session/wk (n = 14,207; Q1, n = 2949; Q5, n = 2855); smoking status: never smoked (n = 9607; Q1, n = 1925; Q5, n = 1978), former smoker (n = 8818; Q1, n = 1910; Q5, n = 1602), current smoker (n = 2916; Q1, n = 1210; Q5, n = 189); region: Southern United States (n = 12,050; Q1, n = 2861; Q5, n = 2075), Western, Midwestern, or Eastern United States (n = 9373; Q1, n = 2207; Q5, n = 1706). LN, natural logarithm; Q, quintile; REGARDS, REasons for Geographic and Racial Differences in Stroke.
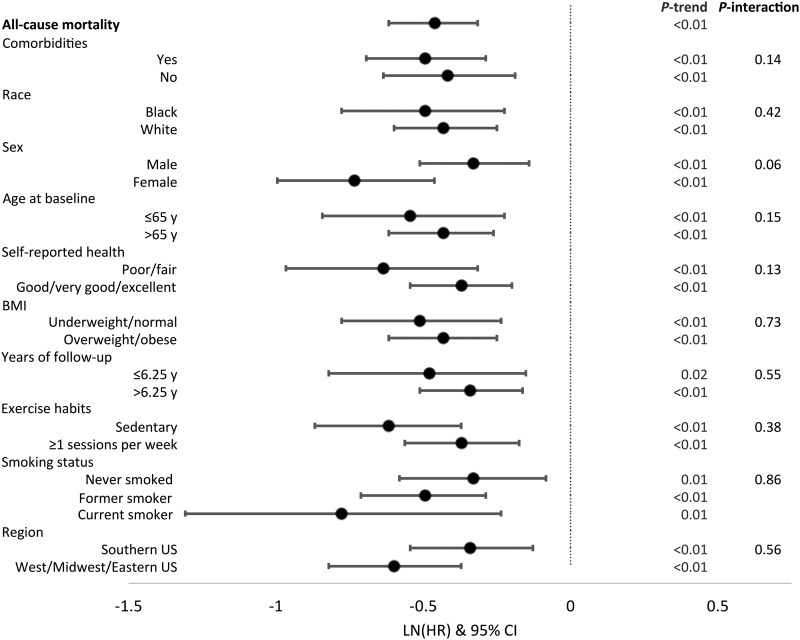
The associations of the Mediterranean diet score with all-cause mortality according to selected participant characteristics are summarized in Figure 2. The findings for those in the highest relative to those in the lowest quintile differed minimally across the various strata except that the inverse association tended to be stronger among men than among women (P-interaction = 0.10). Associations of both diet scores with all-cause and select cause–specific mortality across race were similar (Supplemental Tables 4 and 5).
FIGURE 2.
Associations of the Mediterranean diet score with all-cause mortality, according to selected participant characteristics at baseline in the REGARDS cohort (n = 21,423). From the Cox model, only the comparison of the fifth relative to the first quintile of each diet score with all-cause mortality is shown; model covariates included sex, race (black or white), total energy intake (kilocalories per day), BMI [kg/m2, categorized by WHO criteria (58) into underweight, normal, overweight, and obese], physical activity (0, 1–3, or ≥4 exercise sessions/wk), smoking (current, former, or never), annual income (refused, <$20,000, $20,000–$34,000, $35,000–$74,000, or ≥$75,000/y), and hormone replacement therapy use (in women) at baseline in an age-as-time-scale. P-trend was calculated by assigning the median of each diet score quintile to each quintile, and treating this quintile exposure as continuous. Comorbidities include history of any cancer, kidney failure, type II diabetes, stent, heart surgery, aneurysm, or myocardial infarction. Comorbidities: yes (n = 7436; Q1, n = 1791; Q5, n = 1077), no (n = 13,987; Q1, n = 3038; Q5, n = 2500); race: black (n = 7163; Q1, n = 1802; Q5, n = 974), white (n = 14,260; Q1, n = 3027; Q5, n = 2603); sex: male (n = 9457; Q1, n = 2130; Q5, n = 1584), female (n = 11,966; Q1, n = 2699; Q5, n = 1993); age at baseline: ≤65 y (n = 11,663; Q1, n = 2729; Q5, n = 1884), >65 y (n = 9760; Q1, n = 2100; Q5, n = 1693); self-reported health: poor/fair (n = 3435; Q1, n = 1094; Q5, n = 338), good/very good/excellent (n = 17,953; Q1, n = 3724; Q5, n = 3235); BMI: underweight/normal (n = 5480; Q1, n = 1222; Q5, n = 1039), overweight/obese (n = 15,803; Q1, n = 3568; Q5, n = 2523); years of follow-up: ≤6.25 y (n = 10,773; Q1, n = 2611; Q5, n = 1642), >6.25 y (n = 10,649; Q1, n = 2218; Q5, n = 1934); exercise habits: sedentary (n = 6924; Q1, n = 1944; Q5, n = 815), ≥1 session/wk (n = 14,207; Q1, n = 2806; Q5, n = 2730); smoking status: never smoked (n = 9607; Q1, n = 1932; Q5, n = 1797), former smoker (n = 8818; Q1, n = 1813; Q5, n = 1549), current smoker (n = 2916; Q1, n = 1059; Q5, n = 215); region: Southern United States (n = 12,050; Q1, n = 2863; Q5, n = 1848), Western/Midwestern/Eastern United States (n = 9373; Q1, n = 1966; Q5, n = 1729). LN, natural logarithm; Q, quintile; REGARDS, REasons for Geographic and Racial Differences in Stroke.
In sensitivity analyses, removal of individual score components one at a time did not materially alter the results. However, for the Paleolithic diet score, removal of the nuts and the red and processed meats components attenuated the observed associations for all-cause mortality for those in the fifth relative to those in the first quintile by 15.6% and 14.3%, respectively, to HR: 0.89 (95% CI: 0.77, 1.02; P-trend = 0.04) and HR: 0.88 (95% CI: 0.76, 1.02; P-trend < 0.01), respectively. Removal of nuts and red and processed meats from the Mediterranean diet score also attenuated the observed associations, but more modestly, by 8.6% and 4.5%, respectively (Supplemental Tables 6 and 7). Modifying the construction of the scores by using 2 rather than 5 categories for each component, by using sex- and race-specific quintile cutoffs, or for the Mediterranean diet by using an alternative fat ratio variable did not materially alter the associations. Finally, excluding persons with chronic diseases at baseline, removing BMI from the models, or excluding those who died during the first 1–3 y of follow-up did not materially affect the associations.
Discussion
Our results suggest that diets that are more Paleolithic- or Mediterranean-like may be associated with a lower risk of all-cause, cardiovascular-specific, cancer-specific, and other noninjury or accident-specific mortality, although the observed inverse associations were slightly stronger for the Mediterranean diet pattern. To our knowledge, the current study is the first investigation of an association of a Paleolithic diet pattern with all-cause or cause-specific mortality.
The Paleolithic and Mediterranean diets may reduce the risk of chronic disease mortality by several potential mechanisms. The foods that characterize both diets, including high intakes of fruits, vegetables, fish, and nuts, are associated with lower inflammation and less oxidative stress, biochemical processes that are associated with cardiovascular disease and cancer (45, 59). Also, the Paleolithic and Mediterranean diets, relative to the Western diet (60), feature more low energy–dense foods, thereby facilitating a healthier energy balance. Indeed, in previous pilot trials it was found that participants on a Paleolithic-like diet relative to a standard diet reported greater satiety (61) and had a greater release of anorectic gut hormone (21). Adiposity is associated with higher systemic inflammation and oxidative stress and may influence chronic disease risk by other, independent mechanisms (62, 63). To address this, in the present study, we included BMI in the models for our primary analyses as a potential confounder but also excluded it in our sensitivity analyses because it could also be a mediator of the associations. Inclusion or exclusion of BMI from the models had no material impact on the estimated associations, suggesting that the possible effects of the Paleolithic and Mediterranean diets on chronic disease mortality may indeed include mechanisms beyond improving energy balance, such as reducing inflammation and improving oxidative balance.
There have been few reported studies on the health effects of following a Paleolithic diet pattern. Reported studies include 7 small pilot trials and one slightly larger, long-term weight-loss trial (15–19, 21). Most of the pilot trials examined the effects of the diet on cardiovascular and glycemic control biomarkers, including glycated hemoglobin, plasma insulin, and serum lipids as well as on satiety; in general, all reported improved values and effects (15–22), although only 4 of the trials had a control group. A larger study (n = 70) of the effects of a Paleolithic diet on long-term weight loss in postmenopausal women found statistically significant weight loss at 6 mo relative to those in the control group, but this difference became attenuated and was no longer statistically significant after 24 mo (20).
The Mediterranean diet has been associated with lower mortality (64), mainly through lower risk of cardiovascular disease (30, 46, 47, 61, 65–69) and possibly cancer (40, 70). There have been several trials of a Mediterranean diet to improve health and prevent cardiovascular disease. The Lyon Diet Heart study (47) randomly assigned 605 free-living individuals who previously had a recent myocardial infarction to receive either dietary advice to follow a Mediterranean-type diet or standard care, which included the general recommendation to follow a “prudent diet” for the secondary prevention of myocardial infarction. After a mean follow-up of 46 mo, 58 recurrent nonfatal and fatal cardiac events occurred—14 in the Mediterranean diet group and 44 in the control group (RR: 0.28; 95% CI: 0.15, 0.53) (47). Also, a second, larger trial (n = 7447) of the Mediterranean diet for cardiovascular disease primary prevention in at-risk individuals, PREDIMED (Prevención con Dieta Mediterránea), found reduced cardiovascular disease risk in the 2 study intervention arms, which included following a Mediterranean diet and supplying participants with either nuts (83 events; HR: 0.72; 95% CI: 0.54, 0.96) or olive oil (96 events; HR: 0.70; 95% CI: 0.54, 0.92), relative to the control arm (46). The results of the current study support these earlier trial results. Given the well-known inverse association of moderate alcohol consumption with cardiovascular disease and total mortality (71), one might expect alcohol consumption to contribute to our finding of a slightly stronger inverse association of cardiovascular disease mortality with the Mediterranean diet than with the Paleolithic; however, when we removed alcohol from the Mediterranean diet score, it did not change the HRs appreciably.
We found no substantial differences in associations of the diet patterns with all-cause mortality within subgroups of participants except for an estimated direct Paleolithic diet score–mortality association among current smokers. However, this latter finding was not statistically significant, although the test for multiplicative interaction was, and there were multiple comparisons and few smokers in the upper quintile of the diet, and any possible biological plausibility is unclear, suggesting that it resulted from chance.
Although our findings support our hypothesis that multiple aspects of diet, via multiple mechanisms, collectively affect risk for chronic disease–related mortality more strongly than does any single aspect of diet, we do note that we found that higher consumption of nuts and lower consumption of lower red and processed meats more strongly contributed to the inverse associations of the scores—especially the Paleolithic diet score—with mortality than did other individual components of the scores. Higher nut and lower red and processed meat consumptions have both been associated with lower mortality (72–75), possibly via their contributions to intakes of a healthier balance of FAs. Further exploration of Paleolithic diet components and their relation to each other as well as to the overall score is needed.
This study has several strengths and limitations. Strengths include that it is the first investigation of associations of a Paleolithic-like diet pattern with all-cause or cause-specific mortality, its prospective design, the diverse study population, and the rigorous ascertainment of causes of death (57). The Paleolithic and Mediterranean diet pattern scores were constructed by using similar methods so that differences in the observed diet-mortality associations between the 2 diets would be attributable to differences in what was included in each score and how components common to both scores were weighted rather than to the score-construction methods. Limitations include the potential for residual confounding from insufficiently characterized or unmeasured aspects of a healthy lifestyle and that the results may not be generalizable to the entire US population (76). In addition, because we used relative scoring rather than absolute cutoffs, few participants’ diets were likely to have been strongly concordant with either diet pattern (i.e., few people were eating a true Mediterranean or Paleolithic diet), likely resulting in underestimating the potential of the diets for reducing risk for chronic disease mortality. Although using self-reported FFQs in prospective cohort studies is well established, and the commonly used Block FFQ was used in the REGARDS cohort to assess various diet-outcome associations (49, 77, 78), FFQs have known limitations, such as reliance on participant memory and generally, as in our study, are not specifically designed to probe adherence to a particular diet pattern. In addition, FFQs contain a limited number of specific food items, limited differentiation of food types (e.g., fat content of meat, types of nuts and oils), limited ability to capture food growing or grazing conditions, and limited information on food cooking methods. Finally, while cause-specific mortality is subject to competing risks, all-cause mortality is not, and the consistency of our findings across types of mortality outcomes lends support to the validity of the cause-specific associations.
In conclusion, our findings, taken together with those from previous studies, suggest that diets that are more Paleolithic- or Mediterranean-like may be associated with lower risk of all-cause, cardiovascular-specific, cancer-specific, and other noninjury or accident-specific mortality.
Acknowledgments
KAW, SJ, MLM, WDF, TJH, and RMB designed the research; KAW analyzed the data; and KAW and RMB wrote the manuscript and had primary responsibility for the final content. All authors read and approved the final manuscript.
References
- 1.Heron M. Deaths: leading causes for 2010. Natl Vital Stat Rep 2013;62:1–96. [PubMed] [Google Scholar]
- 2.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 2009;169:659–69. [DOI] [PubMed] [Google Scholar]
- 3.Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health 2014;36:e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012;62:30–67. [DOI] [PubMed] [Google Scholar]
- 5.Fortmann SP, Burda BU, Senger CA, Lin JS, Beil TL, O’Connor E, Whitlock EP. US preventive services task force evidence syntheses, formerly systematic evidence reviews. In: Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: a systematic evidence review for the US Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. [PubMed] [Google Scholar]
- 6.NIH State-of-the-science conference statement on multivitamin/mineral supplements and chronic disease prevention. NIH Consens State Sci Statements 2006;23:1–30. [PubMed] [Google Scholar]
- 7.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 2012;3:CD007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yusof AS, Isa ZM, Shah SA. Dietary patterns and risk of colorectal cancer: a systematic review of cohort studies (2000–2011). Asian Pac J Cancer Prev 2012;13:4713–7. [DOI] [PubMed] [Google Scholar]
- 9.Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer 2010;62:413–24. [DOI] [PubMed] [Google Scholar]
- 10.Millen BE, Quatromoni PA, Copenhafer DL, Demissie S, O’Horo CE, D’Agostino RB. Validation of a dietary pattern approach for evaluating nutritional risk: the Framingham Nutrition Studies. J Am Diet Assoc 2001;101:187–94. [DOI] [PubMed] [Google Scholar]
- 11.Jacques PF, Tucker KL. Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr 2001;73:1–2. [DOI] [PubMed] [Google Scholar]
- 12.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 13.Konner M, Eaton SB. Paleolithic nutrition: twenty-five years later. Nutr Clin Pract 2010;25:594–602. [DOI] [PubMed] [Google Scholar]
- 14.Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 1985;312:283–9. [DOI] [PubMed] [Google Scholar]
- 15.Frassetto LA, Schloetter M, Mietus-Synder M, Morris RC Jr., Sebastian A. Metabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type diet. Eur J Clin Nutr 2009;63:947–55. [DOI] [PubMed] [Google Scholar]
- 16.Jönsson T, Granfeldt Y, Ahrén B, Branell UC, Pålsson G, Hansson A, Söderström M, Lindeberg S. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol 2009;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindeberg S, Jonsson T, Granfeldt Y, Borgstrand E, Soffman J, Sjostrom K, Ahren B. A Palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia 2007;50:1795–807. [DOI] [PubMed] [Google Scholar]
- 18.Boers I, Muskiet FA, Berkelaar E, Schut E, Penders R, Hoenderdos K, Wichers HJ, Jong MC. Favourable effects of consuming a Palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis 2014;13:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osterdahl M, Kocturk T, Koochek A, Wandell PE. Effects of a short-term intervention with a paleolithic diet in healthy volunteers. Eur J Clin Nutr 2008;62:682–5. [DOI] [PubMed] [Google Scholar]
- 20.Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, Larsson C, Olsson T, Lindahl B. Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr 2014;68:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bligh HF, Godsland IF, Frost G, Hunter KJ, Murray P, MacAulay K, Hyliands D, Talbot DC, Casey J, Mulder TP, et al. Plant-rich mixed meals based on Palaeolithic diet principles have a dramatic impact on incretin, peptide YY and satiety response, but show little effect on glucose and insulin homeostasis: an acute-effects randomised study. Br J Nutr 2015;113:574–84. [DOI] [PubMed] [Google Scholar]
- 22.Whalen KA, McCullough M, Flanders WD, Hartman TJ, Judd S, Bostick RM. Paleolithic and Mediterranean diet pattern scores and risk of incident, sporadic colorectal adenomas. Am J Epidemiol 2014;180:1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev 2006;64:S27–47. [DOI] [PubMed] [Google Scholar]
- 24.Gaskins AJ, Rovner AJ, Mumford SL, Yeung E, Browne RW, Trevisan M, Perkins NJ, Wactawski-Wende J, Schisterman EF; BioCycle Study Group. Adherence to a Mediterranean diet and plasma concentrations of lipid peroxidation in premenopausal women. Am J Clin Nutr 2010;92:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai J, Jones DP, Goldberg J, Ziegler TR, Bostick RM, Wilson PW, Manatunga AK, Shallenberger L, Jones L, Vaccarino V. Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr 2008;88:1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abiemo EE, Alonso A, Nettleton JA, Steffen LM, Bertoni AG, Jain A, Lutsey PL. Relationships of the Mediterranean dietary pattern with insulin resistance and diabetes incidence in the Multi-Ethnic Study of Atherosclerosis (MESA). Br J Nutr 2013;109:1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Fernández E, Rico-Cabanas L, Rosgaard N, Estruch R, Bach-Faig A. Mediterranean diet and cardiodiabesity: a review. Nutrients 2014;6:3474–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alkerwi A, Vernier C, Crichton GE, Sauvageot N, Shivappa N, Hebert JR. Cross-comparison of diet quality indices for predicting chronic disease risk: findings from the observation of cardiovascular risk factors in Luxembourg (ORISCAV-LUX) study. Br J Nutr 2015;113:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agnoli C, Krogh V, Grioni S, Sieri S, Palli D, Masala G, Sacerdote C, Vineis P, Tumino R, Frasca G, et al. A priori-defined dietary patterns are associated with reduced risk of stroke in a large Italian cohort. J Nutr 2011;141:1552–8. [DOI] [PubMed] [Google Scholar]
- 30.de Lorgeril M. Mediterranean diet and cardiovascular disease: historical perspective and latest evidence. Curr Atheroscler Rep 2013;15:370. [DOI] [PubMed] [Google Scholar]
- 31.Verberne L, Bach-Faig A, Buckland G, Serra-Majem L. Association between the Mediterranean diet and cancer risk: a review of observational studies. Nutr Cancer 2010;62:860–70. [DOI] [PubMed] [Google Scholar]
- 32.Bosetti C, Turati F, Dal Pont A, Ferraroni M, Polesel J, Negri E, Serraino D, Talamini R, La Vecchia C, Zeegers MP. The role of Mediterranean diet on the risk of pancreatic cancer. Br J Cancer 2013;109:1360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couto E, Sandin S, Lof M, Ursin G, Adami HO, Weiderpass E. Mediterranean dietary pattern and risk of breast cancer. PLoS One 2013;8:e55374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couto E, Boffetta P, Lagiou P, Ferrari P, Buckland G, Overvad K, Dahm CC, Tjonneland A, Olsen A, Clavel-Chapelon F, et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br J Cancer 2011;104:1493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckland G, Agudo A, Lujan L, Jakszyn P, Bueno-de-Mesquita HB, Palli D, Boeing H, Carneiro F, Krogh V, Sacerdote C, et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European prospective investigation into cancer and nutrition (EPIC) cohort study. Am J Clin Nutr 2010;91:381–90. [DOI] [PubMed] [Google Scholar]
- 36.Cade JE, Taylor EF, Burley VJ, Greenwood DC. Does the Mediterranean dietary pattern or the Healthy Diet Index influence the risk of breast cancer in a large British cohort of women? Eur J Clin Nutr 2011;65:920–8. [DOI] [PubMed] [Google Scholar]
- 37.Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and dietary approaches to stop hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr 2010;92:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bamia C, Lagiou P, Buckland G, Grioni S, Agnoli C, Taylor AJ, Dahm CC, Overvad K, Olsen A, Tjonneland A, et al. Mediterranean diet and colorectal cancer risk: results from a European cohort. Eur J Epidemiol 2013;28:317–28. [DOI] [PubMed] [Google Scholar]
- 39.Demetriou CA, Hadjisavvas A, Loizidou MA, Loucaides G, Neophytou I, Sieri S, Kakouri E, Middleton N, Vineis P, Kyriacou K. The mediterranean dietary pattern and breast cancer risk in Greek-Cypriot women: a case-control study. BMC Cancer 2012;12:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giacosa A, Barale R, Bavaresco L, Gatenby P, Gerbi V, Janssens J, Johnston B, Kas K, La Vecchia C, Mainguet P, et al. Cancer prevention in Europe: the Mediterranean diet as a protective choice. Eur J Cancer Prev 2013;22:90–5. [DOI] [PubMed] [Google Scholar]
- 41.Zazpe I, Sanchez-Tainta A, Toledo E, Sanchez-Villegas A, Martinez-Gonzalez MA. Dietary patterns and total mortality in a Mediterranean cohort: the SUN project. J Acad Nutr Diet 2014;114:37–47. [DOI] [PubMed] [Google Scholar]
- 42.Sjögren P, Becker W, Warensjö E, Olsson E, Byberg L, Gustafsson IB, Karlström B, Cederholm T. Mediterranean and carbohydrate-restricted diets and mortality among elderly men: a cohort study in Sweden. Am J Clin Nutr 2010;92:967–74. [DOI] [PubMed] [Google Scholar]
- 43.Bonaccio M, Di Castelnuovo A, Costanzo S, Persichillo M, De Curtis A, Donati MB, de Gaetano G, Iacoviello L; MOLI-SANI study Investigators. Adherence to the traditional Mediterranean diet and mortality in subjects with diabetes. Prospective results from the MOLI-SANI study. Eur J Prev Cardiol 2016;23:400–7. [DOI] [PubMed] [Google Scholar]
- 44.Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr 2014;17:2769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katz DL, Meller S. Can we say what diet is best for health? Annu Rev Public Health 2014;35:83–103. [DOI] [PubMed] [Google Scholar]
- 46.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 47.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 1999;99:779–85. [DOI] [PubMed] [Google Scholar]
- 48.Whalen KA, McCullough ML, Flanders WD, Hartman TJ, Judd S, Bostick RM. Paleolithic and Mediterranean diet pattern scores are inversely associated with biomarkers of inflammation and oxidative balance in adults. J Nutr 2016;146:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsivgoulis G, Psaltopoulou T, Wadley VG, Alexandrov AV, Howard G, Unverzagt FW, Moy C, Howard VJ, Kissela B, Judd SE. Adherence to a Mediterranean diet and prediction of incident stroke. Stroke 2015;46:780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 51.Wadley VG, McClure LA, Howard VJ, Unverzagt FW, Go RC, Moy CS, Crowther MR, Gomez CR, Howard G. Cognitive status, stroke symptom reports, and modifiable risk factors among individuals with no diagnosis of stroke or transient ischemic attack in the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Stroke 2007;38:1143–7. [DOI] [PubMed] [Google Scholar]
- 52.Tsivgoulis G, Alexandrov AV, Wadley VG, Unverzagt FW, Go RC, Moy CS, Kissela B, Howard G. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology 2009;73:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wadley VG, Unverzagt FW, McGuire LC, Moy CS, Go R, Kissela B, McClure LA, Crowe M, Howard VJ, Howard G. Incident cognitive impairment is elevated in the stroke belt: the REGARDS study. Ann Neurol 2011;70:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–69. [DOI] [PubMed] [Google Scholar]
- 55.Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr 2006;9:84–93. [DOI] [PubMed] [Google Scholar]
- 56.Johnson BA, Herring AH, Ibrahim JG, Siega-Riz AM. Structured measurement error in nutritional epidemiology: applications in the pregnancy, infection, and nutrition (PIN) study. J Am Stat Assoc 2007;102:856–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halanych JH, Shuaib F, Parmar G, Tanikella R, Howard VJ, Roth DL, Prineas RJ, Safford MM. Agreement on cause of death between proxies, death certificates, and clinician adjudicators in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Epidemiol 2011;173:1319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i–xii. [PubMed] [Google Scholar]
- 59.Bosma-den Boer MM, van Wetten ML, Pruimboom L. Chronic inflammatory diseases are stimulated by current lifestyle: how diet, stress levels and medication prevent our body from recovering. Nutr Metab (Lond) 2012;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaiser KA, Brown AW, Bohan Brown MM, Shikany JM, Mattes RD, Allison DB. Increased fruit and vegetable intake has no discernible effect on weight loss: a systematic review and meta-analysis. Am J Clin Nutr 2014;100:567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jonsson T, Granfeldt Y, Lindeberg S, Hallberg AC. Subjective satiety and other experiences of a Paleolithic diet compared to a diabetes diet in patients with type 2 diabetes. Nutr J 2013;12:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 2014;56:369–81. [DOI] [PubMed] [Google Scholar]
- 63.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington (DC): AICR; 2007. [Google Scholar]
- 64.Trichopoulou A, Bamia C, Trichopoulos D. Anatomy of health effects of Mediterranean diet: greek EPIC prospective cohort study. BMJ 2009;338:b2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, Guidollet J, Touboul P, Delaye J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994;343:1454–9. [DOI] [PubMed] [Google Scholar]
- 66.de Lorgeril M, Salen P. Dietary prevention of coronary heart disease: the Lyon diet heart study and after. World Rev Nutr Diet 2005;95:103–14. [DOI] [PubMed] [Google Scholar]
- 67.Ibarrola-Jurado N, Bullo M, Guasch-Ferre M, Ros E, Martinez-Gonzalez MA, Corella D, Fiol M, Warnberg J, Estruch R, Roman P, et al. Cross-sectional assessment of nut consumption and obesity, metabolic syndrome and other cardiometabolic risk factors: the PREDIMED study. PLoS One 2013;8:e57367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nordmann AJ, Suter-Zimmermann K, Bucher HC, Shai I, Tuttle KR, Estruch R, Briel M. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am J Med 2011;124:841–51.e2. [DOI] [PubMed] [Google Scholar]
- 69.Shen J, Wilmot KA, Ghasemzadeh N, Molloy DL, Burkman G, Mekonnen G, Gongora MC, Quyyumi AA, Sperling LS. Mediterranean dietary patterns and cardiovascular health. Annu Rev Nutr 2015;35:425–49. [DOI] [PubMed] [Google Scholar]
- 70.Toledo E, Salas-Salvado J, Donat-Vargas C, Buil-Cosiales P, Estruch R, Ros E, Corella D, Fito M, Hu FB, Aros F, et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern Med 2015;175:1752–60. [DOI] [PubMed] [Google Scholar]
- 71.Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW Jr, Doll R. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med 1997;337:1705–14. [DOI] [PubMed] [Google Scholar]
- 72.Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Association of nut consumption with total and cause-specific mortality. N Engl J Med 2013;369:2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu Z, Malik VS, Keum N, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS, Bao Y. Associations between nut consumption and inflammatory biomarkers. Am J Clin Nutr 2016;104:722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjonneland A, Nailler L, Boutron-Ruault MC, Clavel-Chapelon F, Krogh V, et al. Meat consumption and mortality–results from the European prospective investigation into cancer and nutrition. BMC Med 2013;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Newby PK, Noel SE, Grant R, Judd S, Shikany JM, Ard J. Race and region have independent and synergistic effects on dietary intakes in black and white women. Nutr J 2012;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Booth JN III, Levitan EB, Brown TM, Farkouh ME, Safford MM, Muntner P. Effect of sustaining lifestyle modifications (nonsmoking, weight reduction, physical activity, and mediterranean diet) after healing of myocardial infarction, percutaneous intervention, or coronary bypass (from the REasons for Geographic and Racial Differences in Stroke Study). Am J Cardiol 2014;113:1933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsivgoulis G, Judd S, Letter AJ, Alexandrov AV, Howard G, Nahab F, Unverzagt FW, Moy C, Howard VJ, Kissela B, et al. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology 2013;80:1684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]