Abstract
Background: The inverse association between Mediterranean diet (Med-diet) consumption and insulin resistance or inflammatory markers is well known. However, the extent to which obesity may act directly on or mediate this association is unclear.
Objective: We aimed to investigate whether the associations between Med-diet consumption and markers of insulin resistance and inflammation are mediated by body mass index (BMI) or waist circumference (WC) in a representative US population.
Methods: We used cross-sectional data from 4700 adults aged 20–90 y without any previous diagnosis of cancer, cardiovascular disease, diabetes, or hypertension based on the NHANES III, 1988–1994. A Med-diet score (MDS) was created to assess adherence to the Med-diet. Linear regression models were fitted in conventional and causal mediation analyses comparing extreme MDS tertiles.
Results: Compared with the lowest MDS tertile, the highest tertile of MDS was associated with a 0.77 lower BMI (in kg/m2; P = 0.004) and a 2.7 cm lower WC (P < 0.001) after multivariable adjustment. WC mediated the association of MDS with insulin resistance and glucose intolerance markers (log insulin, log homoeostasis model assessment of insulin resistance, fasting glucose, and glycated hemoglobin) and inflammatory markers (white blood cell count and fibrinogen), whereas BMI mediated the association between MDS and insulin resistance and glucose intolerance markers only (all P < 0.05). The mediated effects of WC were consistently greater than those of BMI for all markers in both conventional and causal mediation analyses. Furthermore, the association between MDS and fasting glucose was fully mediated by adiposity, especially by WC in men aged <45 y and in premenopausal women.
Conclusion: Our results suggest that reducing abdominal obesity may play an important role in the pathway through which Med-diet consumption reduces insulin resistance and inflammation.
Keywords: Mediterranean diet, mediation, waist circumference, body mass index, insulin resistance, inflammation
Introduction
The consumption of a Mediterranean diet (Med-diet)10 is associated with lower cardiometabolic disease risk (1–3). Its beneficial effects on cardiovascular health may be explained by the combined effect of improving metabolic profiles, including lipid profiles, blood pressure (BP), insulin resistance, and inflammatory markers (4).
The consumption of a Med-diet appears to be independently associated with cardiometabolic risk after adjustment for adiposity (5). However, it is unclear to what the extent adjustment for adiposity, such as general obesity measured by BMI or abdominal obesity measured by waist circumference (WC), modifies or attenuates the association between Med-diet consumption and cardiometabolic risk. Mediation analysis could clarify the role of adiposity underlying the relation between Med-diet intake and cardiometabolic risk (6). Furthermore, the degree of mediation may be different between general obesity and abdominal obesity, because abdominal obesity affects metabolic disturbance to a greater extent than general obesity (7).
Mediation analysis is a statistical procedure that can be used to evaluate mechanisms underlying the relation between an exposure and outcome by quantifying the extent to which this relation is mediated by a third variable (8). The traditional approach to mediation analysis proposed by Baron and Kenny (8) compared 2 regression models in which one model was conditioned on the mediator and the other was not. The exposure coefficients in the regression models would be interpreted as a direct effect in the model adjusted for the mediator and as a total effect in the model unadjusted for the mediator (6). This approach to evaluate mediation tends to produce a bias when there is uncontrolled mediator-outcome confounding or an interaction between exposure and mediator. With the use of the counterfactual framework in causal mediation analysis, unbiased valid estimates of direct and indirect effects can be obtained (6, 9).
Limited data exist on the mediating role of adiposity on the relation between Med-diet intake and cardiometabolic risk. Therefore, we examined the relations between Med-diet intake and adiposity with markers of insulin resistance and inflammation. In addition, we investigated whether this association is mediated by BMI and WC with the use of both conventional and causal mediation analyses.
Methods
Study population.
We used data from the NHANES III, 1988–1994. NHANES III was conducted with the use of a complex multistage, stratified, clustered probability sample design to attain a nationally representative sample of the civilian noninstitutionalized US population. The survey included personal interviews, physical examinations, and laboratory measurements.
We included 7871 adults with a BMI (in kg/m2) of 18.5, aged 20–90 y, who had complete data on FFQs, 24-h dietary recalls, BMI, and WC and with blood samples obtained after fasting for ≥10 h. Because dietary habits might change due to chronic illness, we excluded those who reported a history of myocardial infarction, stroke, congestive heart failure, or any previous diagnosis of cancer (n = 988). In addition, we excluded those who were diagnosed or treated for diabetes mellitus and hypertension or who were taking cholesterol-lowering medication (n = 1485). To minimize reverse causation, we additionally excluded the participants who reported changing their dietary patterns due to any medical reason during the previous 12 mo (n = 547). Because one of the main outcomes was the concentration of inflammatory markers, we also excluded those with rheumatoid arthritis or high-sensitivity C-reactive protein (hs-CRP) >10 mg/L. Furthermore, we excluded pregnant or lactating women, those who reported implausible extreme energy intakes (<1st and >99th percentiles of energy intake/d in adults) or those with a BMI >60 (n = 151). Finally, a total of 4700 individuals were analyzed in the present study (Supplemental Figure 1).
Assessment of the Med-diet.
We used an 81-item FFQ and 24-h dietary recall data, validated by the Nutrition Methodology Working Group (10), to evaluate dietary intake in the past month. Adherence to the Med-diet was assessed by using the scoring methodology developed by Panagiotakos et al. (11, 12). The FFQ used in NHANES III did not have records of portion sizes. Thus, we calculated the MDS, assuming that the number of servings per week were equivalent to the number of times that a person consumed a food item per week. In brief, scores of 0–5 were assigned for the weekly consumption of foods that are common in the Med-diet on the basis of the quantity of intake. Foods uncommon to the Med-diet pattern were reversely scored (Table 1). The Mediterranean diet score (MDS) computed by using this method is strongly associated with prevalent cardiometabolic diseases, 10-y cardiovascular disease risk, and inflammation and coagulation markers, in addition to capturing inherent characteristics of the Med-diet pattern (11–13).
TABLE 1.
Assessment of the MDS1
| Score3 |
||||||
| Components of the Mediterranean diet2 | 0 | 1 | 2 | 3 | 4 | 5 |
| Nonrefined cereals (whole-grain) | Never | 1–6 | 7–12 | 13–18 | 19–31 | ≥32 |
| Legumes | Never | <1 | 1–2 | 3–4 | 5–6 | ≥7 |
| Fruit | Never | 1–4 | 5–8 | 9–15 | 16–21 | ≥22 |
| Vegetables | Never | 1–6 | 7–12 | 13–20 | 21–32 | ≥33 |
| Fish | Never | <1 | 1–2 | 3–4 | 5–6 | ≥7 |
| Ratio of MUFAs to SFAs4 | First sextile | Second sextile | Third sextile | Fourth sextile | Fifth sextile | Sixth sextile |
| Red meat and products | ≥11 | 8–10 | 6–7 | 4–5 | 2–3 | ≤1 |
| Poultry | ≥11 | 9–10 | 7–8 | 5–6 | 4–5 | ≤3 |
| Dairy products | ≥31 | 29–30 | 21–28 | 16–20 | 11–15 | ≤10 |
| Alcoholic beverages,5 g/d | 0 or >70 g in men and >28 g in women | Even interval between 2 cutoffs | <28 g in men and <14 g in women | |||
Values are frequencies of consumption (times/week) unless otherwise stated. MDS, Mediterranean diet score.
Food items include cereals, dark breads and rolls, corn bread, muffins, and tortillas for grains; beans, lentils, chickpeas, peanuts, and peanut butter for legumes; citrus fruit, melons, peaches, nectarines, and any other fruit for fruit; carrots, broccoli, Brussels sprouts, cauliflower, tomatoes, spinach, greens, tossed salad, cabbage, coleslaw, sauerkraut, hot red chili peppers, other peppers, and any other vegetables for vegetables; shrimp, clams, and fish for fish; chocolate milk and hot cocoa, milk to drink or on cereal (whole or regular and 2% or low-fat milk), yogurt and frozen yogurt, ice cream, ice milk, milkshakes, cheese, cheese dishes, and butter for dairy products; bacon and sausage or other processed meats, liver and other organ meats, beef, pork, and ham for red meat and products; and chicken and turkey for poultry.
The top score contributes toward the Mediterranean diet and the bottom score contributes against the Mediterranean diet. The total MDS is the sum of individual scores for each of the 10 dietary components making up the MDS. The possible overall MDS ranged from 0 to 50, with higher values indicating greater adherence to the Mediterranean diet.
The ratio was divided into 6 even intervals by using the 24-h dietary recall data.
Daily alcohol consumption was estimated by using the following assumption: 12.8 g for 12 ounces of beer, 11 g for a 4-ounce glass of wine, and 14 g for 1 ounce of liquor based on the questionnaire provided.
Potatoes were excluded in computing the MDS because of differences in preparation methods in the United States and in Europe (14). Because olive oil was not measured in the FFQ, the olive oil component in the MDS was represented by the ratio of MUFAs to SFAs. The ratio was then divided into 6 even intervals by using the 24-h dietary recall data. The MDS ranged from 0 to 50, with higher values indicating greater adherence to the Med-diet (Table 1) (15, 16).
Assessment of markers of insulin resistance and inflammation.
Metabolic variables were measured under quality-control standards of the CDC. BMI was calculated as weight in kilograms divided by height in meters squared; height was measured to the nearest 0.1 cm and weight to the nearest 0.01 kg. WC was measured at the level of the right iliac crest. BP was averaged over 5 separate measurements. Mean arterial pressure was calculated by using the formula of (2 × diastolic BP + systolic BP)/3. Glucose, insulin, glycated hemoglobin (HbA1c), hs-CRP, fibrinogen, homocysteine, and lipoprotein(a) were measured in a standardized way (Supplemental Methods). Postload glucose was measured by using the 2-h oral-glucose-tolerance testing for the participants aged 40–74 y, and fibrinogen was assessed for the participants aged ≥40 y. Thus, analyses for postload glucose and fibrinogen were limited to this age group.
Statistical analysis.
All of the statistical analyses were performed by using SAS 9.4 software (SAS Institute). Skewed variables, such as fasting insulin, HOMA-IR, hs-CRP, homocysteine, and lipoprotein(a), were log-transformed to improve their distribution toward normal. Categorical variables were expressed as percentages with SEs and were compared using Rao-Scott chi-square tests. We used appropriate survey procedures to account for the complex sampling design and weights used in NHANES III. For the subgroups, domain analysis was applied to preserve the complex sampling design in which the entire samples were used for estimating the variance of subpopulations. For the outcome variables in our mediation analyses, fasting insulin, HOMA-IR, fasting glucose, postload glucose, and HbA1c were selected as markers for insulin resistance; hs-CRP, white blood cell (WBC) count, fibrinogen, homocysteine, and lipoprotein(a) were selected for inflammatory markers. For all analyses, individuals in the highest MDS tertile, representing relatively good adherence, were compared with those in the lowest MDS tertile, representing relatively poor adherence.
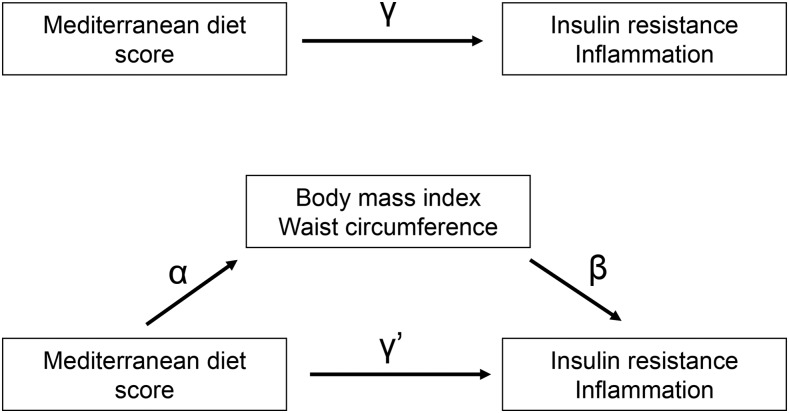
Conventional mediation analysis included the following steps. First, linear regression analyses were applied to examine associations between extreme MDS tertiles (the highest tertile compared with the lowest tertile) with BMI and WC included as outcomes, after adjusting for age, sex, race/ethnicity, educational attainment, living with spouse, income, smoking status, level of physical activity, family history of coronary artery disease, parental history of diabetes mellitus, and total energy intake (the estimate α in Figure 1). Second, linear regression analyses were applied to examine the associations of BMI and WC included as independent variables with markers of insulin resistance or inflammation included as outcomes by using the same covariates in addition to adjusting for MDS (the estimate β in Figure 1). In this step, we added covariates to the model according to the characteristics of outcome variables (mean arterial pressure for markers of insulin resistance; multivitamin use for homocysteine). Third, the simple total effect of Med-diet intake was estimated by regressing the markers of insulin resistance or inflammation (outcomes) on MDS (independent variable) while adjusting for the covariates used in the first step, but without adjusting for BMI or WC (the estimate γ in Figure 1). Fourth, BMI or WC was additionally controlled in the model to estimate the direct effect of Med-diet intake on the markers of insulin resistance or inflammation (the estimate γ′ in Figure 1). Finally, the indirect effect by BMI or WC was estimated by calculating the product of the β-coefficient of the first regression model and the β-coefficient of the second regression model (the estimate α × β in Figure 1) (8, 17). This approach also can be presented by using the following 2 models:
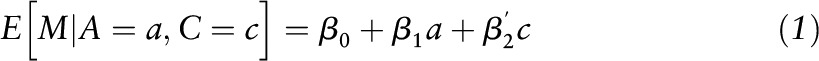 |
and
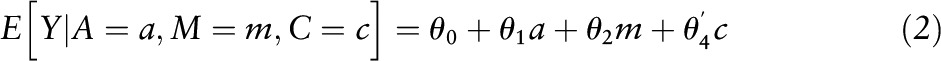 |
where a represents exposure, m represents mediator, and c represents confounders, in which the direct effect was evaluated by estimating θ1; the indirect effect was evaluated by estimating β1 θ2 (18). Next, we divided the indirect effect by its SE and performed a z test under the null hypothesis that the indirect effect is equal to zero (19). In addition, the proportion mediated was calculated by using the indirect effect as a numerator and the total effect as a denominator.
FIGURE 1.
Conventional mediation model for the association between Mediterranean diet intake and the markers of insulin resistance and inflammation with BMI and waist circumference as a mediator. Path α represents the regression coefficient for the association of the MDS with BMI and WC. Path β represents the regression coefficient for the association of BMI and WC with markers of insulin resistance and inflammation. The product of regression coefficients α and β represents the mediated effect of BMI or WC (α × β). Path γ′ represents the direct effect of MDS with markers of insulin resistance and inflammation, after adjustment for BMI or WC. Path γ represents the simple total effect of MDS with markers of insulin resistance and inflammation, without the adjustment for BMI or WC. MDS, Mediterranean diet score; WC, waist circumference.
BMI or WC was considered as a mediator if the estimates α and β were significant (8). Another criterion for determining a mediator would be whether or not the estimate γ was significant (17). However, we did not include this criterion in the present study, because the effect of Med-diet intake on the markers of insulin resistance or inflammation may not be significant when direct and mediated effects have opposite signs (18, 20).
In the causal mediation approach, we assessed the total, direct, and indirect effects of Med-diet intake on markers of insulin resistance or inflammation with BMI or WC as a mediator by using the counterfactual framework (21, 22). In this approach, total effect can be decomposed into direct effect (not mediated by BMI or WC) and indirect effect (mediated by BMI or WC). A causal directed acyclic graph that indicates these effects is presented in Supplemental Figure 2. We applied the SAS macro developed by Valeri and Vanderweele (18) to evaluate the natural direct effect, natural indirect effect, and marginal total effect of Med-diet intake on markers of insulin resistance or inflammation with BMI or WC as mediators.
Natural direct and natural indirect effects can be evaluated conceptually as follows. The natural direct effect is the contrast between the counterfactual outcome if all subjects were exposed at the highest tertile of Med-diet intake and the counterfactual outcome if the same subjects were exposed at the lowest tertile of Med-diet intake, with BMI assuming whatever value it would have taken at the reference value of the lowest tertile of Med-diet intake. The natural indirect effect is the contrast, having set the Med-diet intake at the level of the highest tertile, between the counterfactual outcome if BMI assumed whatever value it would have taken at a value of the highest tertile of Med-diet intake and the counterfactual outcome if BMI assumed whatever value it would have taken at a reference value of the lowest tertile of Med-diet intake. The average natural indirect and direct effects were estimated at the population level on the basis of these approaches (6, 9). Assumptions for this causal mediation analysis and detailed methodologic approaches are given in Supplemental Methods.
In addition, we assessed whether the association of MDS with markers of insulin resistance and inflammation was modified by age group (<45 and ≥45 y for men; before and after menopause for women) (13) or sex by including the interaction terms in the model. Only age group showed a significant interaction in the association between MDS and fasting glucose (P = 0.03). Thus, an additional subgroup analysis was performed by age group for the fasting glucose outcome.
Results
General and clinical characteristics with increasing tertiles of MDS.
General and clinical participant characteristics with increasing tertiles of MDS are shown in Table 2. Higher MDS tertiles were characterized by increased age (P < 0.001); a lower proportion of non-Hispanic black and a higher proportion of Mexican-American individuals (P < 0.001); larger proportions of individuals with high incomes (P < 0.001), high educational levels (P < 0.001), and moderate alcohol consumption (P < 0.001); and larger proportions of individuals who participated in recommended physical activity levels (P < 0.001) and had hypertension (P = 0.02). Individuals in the higher MDS tertiles smoked less (P < 0.001) and had lower BMI (P = 0.01), WC (P < 0.001), HbA1c (P = 0.03), fasting insulin (P < 0.001), log HOMA-IR (P < 0.001), WBC count (P < 0.001), and fibrinogen (P < 0.001). The distributions of consumption of Med-diet components (per week) with increasing tertiles of MDS are shown in Supplemental Table 1. Overall, the consumption in each component of the Med-diet tended to increase with increasing tertiles of MDS (all P < 0.01). Total energy intake was not different across MDS tertiles.
TABLE 2.
Distribution of general and clinical characteristics according to tertiles of the Mediterranean diet score among US adults aged 20–90 y in NHANES III, 1988–19941
| Mediterranean diet score (median, range) |
|||||
| Characteristics | Overall (n = 4700) | Tertile 1 (n = 1729), (22, 8–24) | Tertile 2 (n = 1501), (27, 25–28) | Tertile 3 (n = 1470), (31, 29–42) | P2 |
| Age, y | 38.5 ± 0.5 | 37.3 ± 0.5 | 38.1 ± 0.5 | 40.6 ± 0.8 | <0.001 |
| Men, % | 54.1 ± 0.9 | 54.5 ± 2.2 | 57.0 ± 2.2 | 50.6 ± 2.1 | 0.22 |
| Race/ethnicity, % | <0.001 | ||||
| Non-Hispanic white | 73.9 ± 1.6 | 77.6 ± 2.0 | 71.7 ± 2.4 | 71.1 ± 2.3 | |
| Non-Hispanic black | 11.3 ± 0.7 | 12.0 ± 0.9 | 12.3 ± 1.1 | 9.3 ± 1.0 | |
| Mexican-American | 6.5 ± 0.6 | 3.9 ± 0.4 | 6.5 ± 0.6 | 10.0 ± 1.1 | |
| Other | 8.3 ± 1.2 | 6.5 ± 1.5 | 9.5 ± 1.9 | 9.6 ± 1.8 | |
| Educational attainment, % | <0.001 | ||||
| <12 y | 20.1 ± 1.2 | 23.6 ± 1.6 | 19.8 ± 1.8 | 15.6 ± 1.5 | |
| 12 y | 35.3 ± 1.1 | 41.2 ± 1.7 | 32.9 ± 1.8 | 29.5 ± 1.9 | |
| ≥13 y | 44.7 ± 1.4 | 35.2 ± 2.0 | 47.3 ± 2.4 | 54.9 ± 2.1 | |
| Income, % | <0.001 | ||||
| PIR ≤1.3 | 17.9 ± 1.2 | 20.3 ± 2.2 | 17.2 ± 1.5 | 15.2 ± 1.7 | |
| PIR >1.3–3.5 | 45.5 ± 2.0 | 51.3 ± 3.0 | 44.6 ± 2.3 | 38.2 ± 2.5 | |
| PIR >3.5 | 36.7 ± 2.1 | 28.4 ± 3.3 | 38.3 ± 2.3 | 46.6 ± 2.3 | |
| Smoking status, % | <0.001 | ||||
| Never | 47.2 ± 1.2 | 43.4 ± 1.9 | 50.5 ± 1.8 | 49.0 ± 2.4 | |
| Former | 21.4 ± 0.8 | 19.4 ± 1.3 | 19.3 ± 1.6 | 26.3 ± 1.8 | |
| Current | 31.4 ± 1.3 | 37.3 ± 1.7 | 30.2 ± 1.8 | 24.7 ± 2.2 | |
| Alcohol consumption,3 % | <0.001 | ||||
| Never | 38.5 ± 1.4 | 65.7 ± 1.9 | 26.4 ± 1.7 | 14.0 ± 1.3 | |
| Moderate | 59.1 ± 1.4 | 31.5 ± 2.0 | 71.1 ± 1.8 | 84.2 ± 1.3 | |
| Heavy | 2.4 ± 0.2 | 2.8 ± 0.7 | 2.5 ± 0.6 | 1.9 ± 0.6 | |
| Physical activity,4 % | <0.001 | ||||
| Inactive | 12.0 ± 0.9 | 15.3 ± 1.5 | 10.3 ± 1.2 | 9.2 ± 1.2 | |
| Insufficient activity | 55.1 ± 1.2 | 55.3 ± 1.8 | 58.4 ± 1.8 | 51.2 ± 2.2 | |
| Recommended activity | 32.9 ± 1.3 | 29.4 ± 1.9 | 31.3 ± 1.8 | 39.6 ± 2.3 | |
| CAD family history, % | 15.7 ± 0.8 | 15.9 ± 1.5 | 17.2 ± 1.4 | 13.7 ± 1.7 | 0.34 |
| Diabetes mellitus parental history, % | 16.9 ± 0.9 | 15.6 ± 1.6 | 18.5 ± 1.4 | 17.2 ± 1.7 | 0.42 |
| Diabetes mellitus, % | 2.7 ± 0.3 | 2.5 ± 0.4 | 3.1 ± 0.7 | 2.5 ± 0.6 | 0.63 |
| Hypertension, % | 9.4 ± 0.8 | 8.1 ± 0.9 | 8.2 ± 1.3 | 12.3 ± 1.6 | 0.02 |
| BMI, kg/m2 | 25.8 ± 0.1 | 26.0 ± 0.2 | 26.0 ± 0.2 | 25.3 ± 0.2 | 0.01 |
| WC, cm | 89.1 ± 0.4 | 89.9 ± 0.5 | 90.0 ± 0.6 | 87.2 ± 0.5 | <0.001 |
| Percentage body fat, % | 29.0 ± 0.3 | 29.3 ± 0.5 | 29.2 ± 0.3 | 28.5 ± 0.3 | 0.32 |
| Fasting glucose, mg/dL | 95 ± 0.3 | 95 ± 0.4 | 95 ± 0.4 | 94 ± 0.5 | 0.66 |
| Postload glucose, mg/dL | 116 ± 1.5 | 116 ± 2.4 | 119 ± 3.5 | 114 ± 2.1 | 0.34 |
| HbA1c, % | 5.2 ± 0.02 | 5.2 ± 0.02 | 5.2 ± 0.03 | 5.1 ± 0.03 | 0.03 |
| Fasting insulin, mg/dL | 9.3 ± 0.2 | 9.8 ± 0.2 | 9.4 ± 0.3 | 8.3 ± 0.2 | <0.001 |
| HOMA-IR | 2.21 ± 0.05 | 2.34 ± 0.06 | 2.27 ± 0.08 | 1.98 ± 0.05 | <0.001 |
| hs-CRP, mg/dL | 0.33 ± 0.01 | 0.35 ± 0.01 | 0.32 ± 0.01 | 0.33 ± 0.02 | 0.39 |
| WBC count (×103 cells) | 6.8 ± 0.1 | 7.1 ± 0.1 | 6.8 ± 0.1 | 6.5 ± 0.1 | <0.001 |
| Homocysteine, μmol/L | 9.4 ± 0.2 | 9.5 ± 0.3 | 9.4 ± 0.3 | 9.3 ± 0.4 | 0.94 |
| Fibrinogen, mg/dL | 291 ± 3.2 | 303 ± 4.8 | 287 ± 5.4 | 282 ± 4.4 | <0.001 |
| Lipoprotein(a), mg/dL | 22 ± 1.2 | 24 ± 2.0 | 20 ± 1.4 | 23 ± 1.6 | 0.06 |
Values are means ± SEs or proportions (%) ± SEs. CAD, coronary artery disease; HbA1c, glycated hemoglobin; hs-CRP, high-sensitivity C-reactive protein; MET, metabolic equivalent; PIR, poverty-income ratio; WBC, white blood cell; WC, waist circumference.
P values for continuous variables represent P-trend. P values for fasting insulin, HOMA-IR, hs-CRP, homocysteine, and lipoprotein(a) were calculated after log transformation of each variable.
Consumption ≤28 and 14 g alcohol/d in men and women, respectively, was considered to be moderate alcohol use.
“Recommended” physical activity was designated as a self-reported leisure-time moderate activity (3 ≤ METs < 6) of ≥5 times/wk or leisure-time vigorous activity (METs ≥6) of ≥3 times/wk; “Inactive” indicated no reported leisure-time physical activity; and “Insufficient” physical activity indicated not meeting the criteria for recommended levels of physical activity but not inactive.
Association between MDS, BMI, WC, and markers of insulin resistance and inflammation.
Compared with the lowest tertile of MDS, the highest tertile of MDS was associated with a 0.77 lower BMI (P = 0.004) and 2.67-cm lower WC (P < 0.001) after multivariable adjustment. In addition, when we examined the association of MDS with markers of insulin resistance or inflammation in multivariable models without adjusting for BMI or WC, a significant association representing the total effect was observed in log insulin, log HOMA-IR, fasting glucose, HbA1c, WBC count, and fibrinogen (all P < 0.05) (Table 3).
TABLE 3.
Estimates of regression coefficients (95% CIs) for the association between the Mediterranean diet score, BMI, WC, and markers of insulin resistance and inflammation among US adults aged 20–90 y in NHANES III, 1988–19941
| Estimate2 | 95% CI | P | |
| Mediator | |||
| BMI | −0.770 | −1.284, −0.256 | 0.004 |
| WC | −2.670 | −3.994, −1.347 | <0.001 |
| Outcome | |||
| Log insulin | −0.153 | −0.195, −0.110 | <0.001 |
| Log HOMA-IR | −0.163 | −0.210, −0.116 | <0.001 |
| Fasting glucose | −1.416 | −2.578, −0.253 | 0.02 |
| Postload glucose | −1.720 | −6.866, 3.427 | 0.51 |
| HbA1c | −0.099 | −0.149, −0.049 | <0.001 |
| Log hs-CRP | −0.015 | −0.076, 0.045 | 0.61 |
| WBC count | −0.323 | −0.547, −0.098 | 0.006 |
| Fibrinogen | −17.44 | −26.99, −7.88 | 0.001 |
| Log homocysteine | 0.018 | −0.036, 0.072 | 0.50 |
| Log lipoprotein(a) | −0.006 | −0.251, 0.239 | 0.96 |
HbA1c, glycated hemoglobin; hs-CRP, high-sensitivity C-reactive protein; PIR, poverty-income ratio; WBC, white blood cell; WC, waist circumference.
All estimates were adjusted for age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, or others), educational attainment (<12, 12, or >12 y of education), income [low (PIR ⩽1.3), middle (1.3 < PIR ⩽ 3.5), and high (PIR >3.5)], living with spouse, smoking status (never, former, or current), level of physical activity (inactive, insufficient activity, or recommended activity), family history of coronary artery disease, parental history of diabetes mellitus, mean arterial pressure, and total calories (additionally adjusted for mean arterial pressure in the model for markers of insulin resistance; multivitamin use in the model for homocysteine). Estimates for mediator and outcomes correspond to the regression coefficients α and γ, respectively, in Figure 1. All estimates were calculated for the highest tertile compared with the lowest tertile of Mediterranean diet score.
With increasing BMI and WC, there was a significant increase in log insulin, log HOMA-IR, fasting glucose, postload glucose, HbA1c, log hs-CRP, WBC count, and fibrinogen based on the multivariable model (all P < 0.05). However, no significant association was observed in log homocysteine and log lipoprotein(a) with BMI and WC (Table 4). On the basis of the results from Tables 3 and 4, log insulin, log HOMA-IR, fasting glucose, postload glucose, HbA1c, log hs-CRP, WBC count, and fibrinogen were included as the outcomes in the mediation analysis.
TABLE 4.
Estimates of regression coefficients (95% CIs) for the association between BMI and WC and markers of insulin resistance and inflammation among US adults aged 20–90 y in NHANES III, 1988–19941
| BMI |
WC |
|||||
| Outcomes | Estimate2 (β) | 95% CI | P | Estimate2 (β) | 95% CI | P |
| Log insulin | 0.055 | 0.050, 0.060 | <0.001 | 0.023 | 0.021, 0.024 | <0.0001 |
| Log HOMA-IR | 0.060 | 0.055, 0.066 | <0.001 | 0.025 | 0.023, 0.027 | <0.0001 |
| Fasting glucose | 0.503 | 0.359, 0.646 | <0.001 | 0.229 | 0.168, 0.289 | <0.0001 |
| Postload glucose | 0.814 | 0.112, 1.517 | 0.02 | 0.477 | 0.203, 0.751 | 0.001 |
| HbA1c | 0.015 | 0.010, 0.019 | <0.001 | 0.006 | 0.004, 0.007 | <0.0001 |
| Log hs-CRP | 0.031 | 0.025, 0.037 | <0.001 | 0.012 | 0.010, 0.014 | <0.0001 |
| WBC count | 0.055 | 0.036, 0.074 | <0.001 | 0.020 | 0.013, 0.028 | <0.0001 |
| Fibrinogen | 1.732 | 0.677, 2.786 | 0.002 | 0.625 | 0.194, 1.057 | 0.005 |
| Log homocysteine | 0.001 | −0.004, 0.007 | 0.65 | 0.001 | −0.002, 0.003 | 0.53 |
| Log lipoprotein(a) | 0.002 | −0.016, 0.020 | 0.81 | −0.001 | −0.007, 0.006 | 0.89 |
Direct and indirect effects of the Med-diet on markers of insulin resistance and inflammation with BMI and WC as mediators.
Table 5 shows the total effect, direct effect, indirect effect, and Sobel statistics for testing indirect effects. Overall, the mediated effects of BMI and WC were significant for the associations between MDS and log insulin, log HOMA-IR, fasting glucose, HbA1c, log hs-CRP, WBC count, and fibrinogen, with the exception of postload glucose, for which BMI was a mediator (all P < 0.05). In addition, the mediated effects of WC were greater than those of BMI consistently for all markers. Although the estimates of direct effects were not significant for either marker, the direction of the direct effect and indirect effect were in opposite directions for log hs-CRP; in this case, the proportion of mediation may not be interpretable. Otherwise, the mediated effects by BMI were higher in log HOMA-IR, which showed the highest mediated effects by adiposity. Among the inflammatory markers, WBC count showed the highest mediated effects by adiposity.
TABLE 5.
Direct and indirect effects of Mediterranean diet consumption on markers of insulin resistance and inflammation with BMI and WC as mediators among US adults aged 20–90 y in NHANES III, 1988–19941
| Direct effect (γ′)2 |
Indirect effect (α × β)3 |
|||||||
| Mediator and outcomes | Estimate4 | 95% CI | P | Estimate4 | 95% CI | Sobel test statistic | P | Proportion of mediation, % |
| BMI | ||||||||
| Log insulin | −0.104 | −0.143, −0.065 | <0.001 | −0.042 | −0.070, −0.014 | −2.981 | 0.003 | 27.7 |
| Log HOMA-IR | −0.110 | −0.154, −0.065 | <0.001 | −0.046 | −0.077, −0.016 | −2.982 | 0.003 | 28.4 |
| Fasting glucose | −0.982 | −2.191, 0.227 | 0.11 | −0.387 | −0.661, −0.113 | −2.766 | 0.006 | 27.3 |
| Postload glucose | −0.895 | −6.183, 4.393 | 0.74 | −0.627 | −1.294, 0.040 | −1.842 | 0.07 | 36.5 |
| HbA1c | −0.086 | −0.136, −0.037 | 0.001 | −0.011 | −0.019, −0.003 | −2.731 | 0.006 | 11.5 |
| Log hs-CRP | 0.007 | −0.054, 0.067 | 0.82 | −0.024 | −0.040, −0.008 | −2.880 | 0.004 | NA |
| WBC count | −0.282 | −0.501, −0.062 | 0.01 | −0.042 | −0.073, −0.011 | −2.672 | 0.008 | 13.1 |
| Fibrinogen | −15.98 | −25.93, −6.04 | 0.002 | −1.33 | −2.51, −0.16 | −2.22 | 0.03 | 7.6 |
| WC | ||||||||
| Log insulin | −0.085 | −0.128, −0.043 | <0.001 | −0.061 | −0.090, −0.031 | −4.004 | <0.001 | 39.8 |
| Log HOMA-IR | −0.090 | −0.138, −0.042 | 0.001 | −0.067 | −0.099, −0.034 | −4.010 | <0.001 | 41.0 |
| Fasting glucose | −0.757 | −1.983, 0.469 | 0.22 | −0.611 | −0.945, −0.276 | −3.578 | <0.001 | 43.1 |
| Postload glucose | 0.258 | −5.288, 5.804 | 0.93 | −1.273 | −2.215, −0.331 | −2.649 | 0.008 | NA |
| HbA1c | −0.083 | −0.132, −0.034 | 0.001 | −0.015 | −0.024, −0.006 | −3.408 | 0.001 | 15.3 |
| Log hs-CRP | 0.015 | −0.044, 0.074 | 0.60 | −0.032 | −0.049, −0.016 | −3.809 | <0.001 | NA |
| WBC count | −0.270 | −0.490, −0.049 | 0.02 | −0.054 | −0.088, −0.021 | −3.224 | 0.001 | 16.9 |
| Fibrinogen | −15.21 | −25.21, −5.21 | 0.004 | −1.67 | −3.05, −0.29 | −2.36 | 0.02 | 9.6 |
HbA1c, glycated hemoglobin; hs-CRP, high-sensitivity C-reactive protein; NA, not applicable; WBC, white blood cell; WC, waist circumference.
All estimates for direct effect were adjusted for the same covariates used in Table 3.
Regression coefficients α, β, and γ′ are shown in Figure 1.
All estimates were calculated for the highest tertile compared with the lowest tertile of Mediterranean diet score.
In causal mediation analysis, the overall proportion of mediated effect was lower than that observed with conventional mediation analysis. In addition, the mediated effect of BMI was attenuated and not significant for postload glucose, log hs-CRP, WBC count, and fibrinogen and was attenuated but significant for markers of insulin resistance, including log insulin, log HOMA-IR, fasting glucose, and HbA1c. However, the significance of mediated effects by WC remained (Table 6). In both the conventional approach and the causal mediation approach, the association between MDS and fasting glucose was fully mediated by BMI or WC. In addition, the associations between MDS, postload glucose, and hs-CRP were fully mediated by WC (Tables 5 and 6).
TABLE 6.
Marginal total, natural direct, and natural indirect effects of Mediterranean diet consumption on markers of insulin resistance and inflammation with BMI and WC as mediators by using causal mediation analysis among US adults aged 20–90 y in NHANES III, 1988–19941
| Marginal total effect |
Natural direct effect |
Natural indirect effect |
||||||||
| Mediator and outcomes | Estimate2 | 95% CI | P | Estimate2 | 95% CI | P | Estimate2 | 95% CI | P | Proportion of mediation, % |
| BMI | ||||||||||
| Log insulin | −0.082 | −0.118, −0.045 | <0.001 | −0.061 | 0.000, −0.093 | 0.02 | −0.020 | −0.039, −0.002 | 0.03 | 24.7 |
| Log HOMA-IR | −0.092 | −0.132, −0.051 | <0.001 | −0.070 | −0.105, −0.035 | <0.001 | −0.022 | −0.043, −0.002 | 0.03 | 24.2 |
| Fasting glucose | −1.571 | −3.002, −0.139 | 0.03 | −1.346 | −2.763, 0.072 | 0.06 | −0.225 | −0.437, −0.013 | 0.04 | 14.3 |
| Postload glucose | −5.508 | −12.46, 1.45 | 0.12 | −5.156 | −12.11, 1.80 | 0.15 | −0.352 | −0.745, 0.041 | 0.08 | 6.4 |
| HbA1c | −0.078 | −0.129, −0.028 | 0.003 | −0.071 | −0.122, −0.021 | 0.006 | −0.007 | −0.014, 0.000 | 0.04 | 9.4 |
| Log hs-CRP | −0.043 | −0.088, 0.002 | 0.06 | −0.031 | −0.074, 0.012 | 0.16 | −0.012 | −0.024, 0.000 | 0.05 | 28.2 |
| WBC count | −0.178 | −0.324, −0.032 | 0.02 | −0.161 | −0.307, −0.016 | 0.03 | −0.017 | −0.034, 0.000 | 0.06 | 9.3 |
| Fibrinogen | −9.61 | −18.95, −0.27 | 0.04 | −8.66 | −17.96, 0.64 | 0.07 | −0.944 | −1.927, 0.038 | 0.06 | 9.8 |
| WC | ||||||||||
| Log insulin | −0.082 | −0.118, −0.046 | <0.001 | −0.050 | −0.081, −0.019 | 0.001 | −0.032 | −0.051, −0.013 | 0.001 | 39.0 |
| Log HOMA-IR | −0.093 | −0.133, −0.052 | <0.001 | −0.057 | −0.092, −0.023 | 0.001 | −0.035 | −0.057, −0.014 | 0.001 | 38.3 |
| Fasting glucose | −1.573 | −3.005, −0.142 | 0.03 | −1.187 | −2.601, 0.227 | 0.10 | −0.386 | −0.630, −0.143 | 0.002 | 24.5 |
| Postload glucose | −5.138 | −12.08, 1.81 | 0.15 | −4.327 | −11.27, 2.62 | 0.22 | −0.811 | −1.413, −0.209 | 0.008 | 15.8 |
| HbA1c | −0.079 | −0.130, −0.028 | 0.002 | −0.067 | −0.117, −0.017 | 0.009 | −0.012 | −0.019, −0.004 | 0.002 | 14.8 |
| Log hs-CRP | −0.043 | −0.088, 0.002 | 0.06 | −0.025 | −0.068, 0.018 | 0.26 | −0.018 | −0.030, −0.006 | 0.003 | 42.3 |
| WBC count | −0.179 | −0.325, −0.032 | 0.02 | −0.153 | −0.298, −0.008 | 0.04 | −0.026 | −0.044, −0.008 | 0.005 | 14.4 |
| Fibrinogen | −9.42 | −18.77, −0.07 | 0.05 | −8.03 | −17.36, 1.30 | 0.09 | −1.391 | −2.392, −0.390 | 0.006 | 14.8 |
HbA1c, glycated hemoglobin; hs-CRP, high-sensitivity C-reactive protein; WBC, white blood cell; WC, waist circumference.
All estimates were adjusted for the same covariates used in Table 3 and were calculated for the highest tertile compared with the lowest tertile of Mediterranean diet score.
The association between MDS and fasting glucose was largely mediated by adiposity, especially WC, in the younger age group (Table 7, Supplemental Figure 3). Conventional mediation analysis performed by using a 5-point (1-SD) increment in the MDS gave materially similar results (data not shown).
TABLE 7.
Marginal total, natural direct, and natural indirect effects of Mediterranean diet consumption on fasting glucose with BMI and WC as mediators in younger and older age groups among US adults aged 20–90 y in NHANES III, 1988–19941
| Marginal total effect |
Natural direct effect |
Natural indirect effect |
||||||||
| Age group and outcomes | Estimate2 | 95% CI | P | Estimate2 | 95% CI | P | Estimate2 | 95% CI | P | Proportion of mediation, % |
| Younger | ||||||||||
| BMI | −1.835 | −3.366, −0.304 | 0.02 | −1.559 | −3.076, −0.041 | 0.04 | −0.276 | −0.501, −0.052 | 0.02 | 15.1 |
| WC | −1.832 | −3.363, −0.301 | 0.02 | −1.379 | −2.894, 0.137 | 0.07 | −0.453 | −0.711, −0.194 | 0.001 | 24.7 |
| Older | ||||||||||
| BMI | −1.530 | −4.827, 1.768 | 0.36 | −1.108 | −4.372, 2.156 | 0.51 | −0.422 | −0.938, 0.095 | 0.11 | 27.6 |
| WC | −1.543 | −4.840, 1.754 | 0.36 | −1.046 | −4.297, 2.206 | 0.53 | −0.497 | −1.090, 0.096 | 0.10 | 32.2 |
WC, waist circumference.
All estimates were adjusted for the same covariates used in Table 3 and were calculated for the highest tertile compared with the lowest tertile of Mediterranean diet score.
Discussion
The present study is, to the best of our knowledge, the first to evaluate the role of adiposity in the relation between Med-diet intake and markers of insulin resistance and inflammation in a nationally representative sample of US adults, with the use of both conventional and causal mediation analyses. We observed inverse associations of MDS with markers of insulin resistance, including fasting insulin, HOMA-IR, fasting glucose, and HbA1c and with inflammatory markers such as WBC count and fibrinogen. WC mediated the association of MDS with log insulin, log HOMA-IR, fasting glucose, postload glucose, HbA1c, log hs-CRP, WBC count, and fibrinogen. Furthermore, the mediated effect in this association was greater for WC (abdominal obesity) than for BMI (general obesity) in both conventional and causal mediation approaches.
Several biological mechanisms may explain the inverse association between Med-diet intake and insulin resistance, which is a key pathophysiologic trait of diabetes mellitus. Adherence to the Med-diet is related to increased antioxidant capacity (23), which may protect against oxidative stress accumulation, a key biological process in insulin resistance and β cell dysfunction (24). Other potential mechanisms of action for the effect of the Med-diet may include high intakes of magnesium, which is abundant in vegetables, legumes, and nuts (25); dietary fiber (26); and monounsaturated and polyunsaturated fats (27), as well as the moderate consumption of alcohol (28), all of which may have beneficial effects on insulin sensitivity and glucose metabolism. Furthermore, the inverse relation between Med-diet intake and insulin resistance may be explained by the role of the Med-diet in obesity prevention (29). A potential physiologic role of the Med-diet in preventing obesity has been hypothesized (30). Moreover, a current meta-analysis of clinical trials showed that the Med-diet intake was effective in reducing body weight (31).
WC, which represents abdominal obesity, is a better predictor of diabetes mellitus than BMI, which represents general obesity (7). Increased FFAs are released from expanded adipose tissue, which inhibit insulin-stimulated glucose metabolism in skeletal muscle, stimulate gluconeogenesis in the liver (29), and alter lipid metabolism in the liver (32), all of which contribute to insulin resistance (33). The above-mentioned beneficial health effects of Med-diet intake may play a role in protecting against obesity-induced insulin resistance, especially from abdominal fat. This underlying mechanism is supported by our findings in which a higher MDS was inversely associated with fasting insulin, HOMA-IR, fasting glucose, and HbA1c, with a larger mediated effect of WC than of BMI.
In the present study, the association between MDS and fasting glucose was fully mediated by adiposity, because there was no significant association between the 2 after adjusting for BMI or WC (34). Despite numerical differences in estimates from conventional and causal mediation approaches, the results were qualitatively similar: the mediated effect by WC was consistently observed, especially in men aged <45 y and premenopausal women (Supplemental Figure 3, Table 7), suggesting that the effect of the Med-diet on reducing glucose concentrations might be greater through lowering abdominal rather than general obesity.
Adherence to the Med-diet is associated with decreased inflammation (35). Low-grade chronic inflammation is crucial in the initial phase of developing atherosclerosis, the main cause of coronary artery disease (36). In addition, inflammatory markers such as CRP and IL-6 are associated with the risk of diabetes mellitus (37, 38). Obesity is considered to be a subclinical inflammatory condition that enhances the production of proinflammatory factors that contribute to insulin resistance (39, 40), and abdominal obesity may play a key role in this process (41–43). Thus, the underlying mechanism on the beneficial effect of Med-diet intake on the prevention of inflammation may be supported by our findings in which a higher MDS was inversely associated with WBC count and fibrinogen; the mediated effect in this association was greater with WC than with BMI.
In the present study, Med-diet intake was not associated with hs-CRP, with or without adjustment for BMI or WC. Instead, the indirect effect of hs-CRP was consistently highly significant, especially when WC was a mediator, suggesting that the effect of the Med-diet might be fully mediated by adiposity, especially abdominal obesity. This finding may be supported by a recent study in which combining weight loss with Med-diet intake had no impact on plasma C-reactive protein (CRP), but a subgroup with a substantial decrease in WC showed a reduction in plasma CRP (44).
The main strength of the present study was that the mediated effects of general and abdominal obesity in the associations between Med-diet intake and markers of insulin resistance and inflammation were comprehensively assessed by using conventional and causal mediation analyses. In addition, the analyses included extensive adjustment for potential confounders and exploratory effect modification.
There are also several limitations. First, this cross-sectional study cannot imply a causal and temporal relation between a healthy dietary pattern, adiposity, and markers of insulin resistance and inflammation. We tried to minimize reverse causality by using strict exclusion criteria. However, further prospective studies with long-term follow-up are warranted to confirm our results. Second, because the information on “servings per week” was not available on the NHANES III FFQ, we used “times per week” to represent consumption frequency for MDS calculations. This might cause exposure misclassification, but the direction would be nondifferential. Third, the sextile of a ratio of total MUFAs to total SFAs may not be exactly equal to olive oil consumption as one of the MDS components, because the main sources of MUFAs in the diet are different between the United States and Mediterranean countries. Fourth, the mediated effect of WC may be affected by BMI, or vice versa, because of the high correlation between WC and BMI. This could be addressed by considering 2 mediators (BMI and WC) simultaneously in the model (45). However, this was not feasible in the conventional or causal mediation models with the use of the complex survey design in the present study.
In conclusion, the association between Med-diet intake, obesity, insulin resistance, and inflammation suggests that obesity, especially abdominal obesity, may play a crucial role in the relation between Med-diet intake and decreased insulin resistance and inflammation. Further prospective studies are warranted to better understand the nature and importance of the mediation of these relations by obesity.
Acknowledgments
Y-MP, JZ, SES, TTF, LJH, and ATM conceived of the project and developed the overall research plan; Y-MP, JZ, KH, and ATM performed statistical analyses; Y-MP wrote the manuscript; Y-MP, JZ, SES, TTF, LJH, KH, S-HK, and ATM interpreted the data and critically revised the manuscript for important intellectual content; and Y-MP and ATM had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BP, blood pressure; CRP, C-reactive protein; hs-CRP, high-sensitivity C-reactive protein; MDS, Mediterranean diet score; Med-diet, Mediterranean diet; WBC, white blood cell; WC, waist circumference.
References
- 1.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ 2008;337:a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kastorini C-M, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol 2011;57:1299–313. [DOI] [PubMed] [Google Scholar]
- 3.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 4.Grosso G, Mistretta A, Frigiola A, Gruttadauria S, Biondi A, Basile F, Vitaglione P, D’Orazio N, Galvano F. Mediterranean diet and cardiovascular risk factors: a systematic review. Crit Rev Food Sci Nutr 2014;54:593–610. [DOI] [PubMed] [Google Scholar]
- 5.Kastorini CM, Milionis HJ, Goudevenos JA, Panagiotakos DB. Mediterranean diet and coronary heart disease: is obesity a link? A systematic review. Nutr Metab Cardiovasc Dis 2010;20:536–51. [DOI] [PubMed] [Google Scholar]
- 6.Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol 2013;42:1511–9. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 2005;81:555–63. [DOI] [PubMed] [Google Scholar]
- 8.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–82. [DOI] [PubMed] [Google Scholar]
- 9.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology 1992;3:143–55. [DOI] [PubMed] [Google Scholar]
- 10.US National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. US Department of Health and Human Services, Public Health Service, CDC: Hyattsville (MD); 1994. [Google Scholar]
- 11.Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis 2006;16:559–68. [DOI] [PubMed] [Google Scholar]
- 12.Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med 2007;44:335–40. [DOI] [PubMed] [Google Scholar]
- 13.Carter SJ, Roberts MB, Salter J, Eaton CB. Relationship between Mediterranean Diet Score and atherothrombotic risk: findings from the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Atherosclerosis 2010;210:630–6. [DOI] [PubMed] [Google Scholar]
- 14.Fung TT, McCullough ML, Newby P, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005;82:163–73. [DOI] [PubMed] [Google Scholar]
- 15.Park YM, Steck SE, Fung TT, Zhang J, Hazlett LJ, Han K, Merchant AT. Mediterranean diet and mortality risk in metabolically healthy obese and metabolically unhealthy obese phenotypes. Int J Obes (Lond) 2016;40:1541–9. [DOI] [PubMed] [Google Scholar]
- 16.Park YM, Steck SE, Fung TT, Zhang J, Hazlett LJ, Han K, Lee SH, Kwon HS, Merchant AT. Mediterranean diet, Dietary Approaches to Stop Hypertension (DASH) style diet, and metabolic health in U.S. adults. Clin Nutr 2016. Sep 8 (Epub ahead of print; DOI: 10.1016/j.clnu.2016.08.018). [DOI] [PubMed] [Google Scholar]
- 17.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput 2004;36:717–31. [DOI] [PubMed] [Google Scholar]
- 18.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013;18:137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol 1982;13:290–312. [Google Scholar]
- 20.Rucker DD, Preacher KJ, Tormala ZL, Petty RE. Mediation analysis in social psychology: current practices and new recommendations. Soc Personal Psychol Compass 2011;5:359–71. [Google Scholar]
- 21.VanderWeele TJ. Mediation and mechanism. Eur J Epidemiol 2009;24:217–24. [DOI] [PubMed] [Google Scholar]
- 22.VanderWeele TJ. A three-way decomposition of a total effect into direct, indirect, and interactive effects. Epidemiology 2013;24:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zamora-Ros R, Serafini M, Estruch R, Lamuela-Raventos RM, Martinez-Gonzalez MA, Salas-Salvado J, Fiol M, Lapetra J, Aros F, Covas MI, et al. Mediterranean diet and non enzymatic antioxidant capacity in the PREDIMED study: evidence for a mechanism of antioxidant tuning. Nutr Metab Cardiovasc Dis 2013;23:1167–74. [DOI] [PubMed] [Google Scholar]
- 24.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003;52:1–8. [DOI] [PubMed] [Google Scholar]
- 25.Barbagallo M, Dominguez LJ, Galioto A, Ferlisi A, Cani C, Malfa L, Pineo A, Busardo A, Paolisso G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med 2003;24:39–52. [DOI] [PubMed] [Google Scholar]
- 26.Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 2000;342:1392–8. [DOI] [PubMed] [Google Scholar]
- 27.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res 2009;48:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnet F, Disse E, Laville M, Mari A, Hojlund K, Anderwald CH, Piatti P, Balkau B. Moderate alcohol consumption is associated with improved insulin sensitivity, reduced basal insulin secretion rate and lower fasting glucagon concentration in healthy women. Diabetologia 2012;55:3228–37. [DOI] [PubMed] [Google Scholar]
- 29.Schröder H. Protective mechanisms of the Mediterranean diet in obesity and type 2 diabetes. J Nutr Biochem 2007;18:149–60. [DOI] [PubMed] [Google Scholar]
- 30.Buckland G, Bach A, Serra-Majem L. Obesity and the Mediterranean diet: a systematic review of observational and intervention studies. Obes Rev 2008;9:582–93. [DOI] [PubMed] [Google Scholar]
- 31.Nordmann AJ, Suter-Zimmermann K, Bucher HC, Shai I, Tuttle KR, Estruch R, Briel M. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am J Med 2011;124:841–51.e2. [DOI] [PubMed] [Google Scholar]
- 32.Ginsberg HN, Zhang YL, Hernandez‐Ono A. Metabolic syndrome: focus on dyslipidemia. Obesity (Silver Spring) 2006;14(2S):41S–9S. [DOI] [PubMed] [Google Scholar]
- 33.Lebovitz HE. Insulin resistance—a common link between type 2 diabetes and cardiovascular disease. Diabetes Obes Metab 2006;8:237–49. [DOI] [PubMed] [Google Scholar]
- 34.James LR, Brett JM. Mediators, moderators, and tests for mediation. J Appl Psychol 1984;69:307–21. [Google Scholar]
- 35.Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis 2014;24:929–39. [DOI] [PubMed] [Google Scholar]
- 36.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, Xiao X, Shan ZL, Zhang Y, Yao P, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2013;36:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawada T. Relationship between biological markers, metabolic components, lifestyles, and impaired fasting glucose in male workers. Diabetes Metab J 2015;39:434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bastard J-P, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006;17:4–12. [PubMed] [Google Scholar]
- 40.Ota T. Chemokine systems link obesity to insulin resistance. Diabetes Metab J 2013;37:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim JS, Choi YJ, Kim SK, Huh BW, Lee EJ, Huh KB. Optimal waist circumference cutoff value based on insulin resistance and visceral obesity in Koreans with type 2 diabetes. Diabetes Metab J 2015;39:253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathieu P, Lemieux I, Després JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther 2010;87:407–16. [DOI] [PubMed] [Google Scholar]
- 43.Park YM, Kwon HS, Lim SY, Lee JH, Yoon KH, Son HY, Yim HW, Lee WC. Optimal waist circumference cutoff value reflecting insulin resistance as a diagnostic criterion of metabolic syndrome in a nondiabetic Korean population aged 40 years and over: the Chungju Metabolic Disease Cohort (CMC) study. Yonsei Med J 2010;51:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richard C, Couture P, Desroches S, Lamarche B. Effect of the Mediterranean diet with and without weight loss on markers of inflammation in men with metabolic syndrome. Obesity (Silver Spring) 2013;21:51–7. [DOI] [PubMed] [Google Scholar]
- 45.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 2008;40:879–91. [DOI] [PubMed] [Google Scholar]