Abstract
Background: Glutamine is considered the main precursor for citrulline synthesis in many species, including humans. The transfer of 15N from 2-[15N]-glutamine to citrulline has been used as evidence for this precursor-product relation. However, work in mice has shown that nitrogen and carbon tracers follow different moieties of glutamine and that glutamine contribution to the synthesis of citrulline is minor. It is unclear whether this small contribution of glutamine is also true in other species.
Objective: The objective of the present work was to determine the contribution of glutamine to citrulline production by using nitrogen and carbon skeleton tracers in multiple species.
Methods: Humans (n = 4), pigs (n = 5), rats (n = 6), and mice (n = 5) were infused with l-2-[15N]- and l-[2H5]-glutamine and l-5,5-[2H2]-citrulline. The contribution of glutamine to citrulline synthesis was calculated by using different ions and fragments: glutamine M+1 to citrulline M+1, 2-[15N]-glutamine to 2-[15N]-citrulline, and [2H5]-glutamine to [2H5]-citrulline.
Results: Species-specific differences in glutamine and citrulline fluxes were found (P < 0.001), with rats having the largest fluxes, followed by mice, pigs, and humans (all P < 0.05). The contribution of glutamine to citrulline as estimated by using glutamine M+1 to citrulline M+1 ranged from 88% in humans to 46% in pigs. However, the use of 2-[15N]-glutamine and 2-[15N]-citrulline as precursor and product yielded values of 48% in humans and 28% in pigs. Furthermore, the use of [2H5]-glutamine to [2H5]-citrulline yielded lower values (P < 0.001), resulting in a contribution of glutamine to the synthesis of citrulline of ∼10% in humans and 3% in pigs.
Conclusions: The recycling of the [15N]-glutamine label overestimates the contribution of glutamine to citrulline synthesis compared with a tracer that follows the carbon skeleton of glutamine. Glutamine is a minor precursor for the synthesis of citrulline in humans, pigs, rats, and mice.
Keywords: amino acid, glutamine, citrulline, stable isotope, tracer kinetics
Introduction
The pioneering work of Windmueller and Spaeth (1) established the role of glutamine as a respiratory substrate for the small intestine and the gut as the main site for citrulline production. Many researchers have interpreted these findings as evidence that glutamine is the (main) precursor for citrulline synthesis (2–4). This is despite the fact that Windmueller and Spaeth, in a follow-up review article, clearly stated that “intestinally derived citrulline [is] an end product of glutamine nitrogen metabolism” (5). In vitro studies that used swine enterocytes seemed to support the role of glutamine as the main precursor for citrulline synthesis (6), because the inclusion of glutamine in the culture media increased the production of citrulline many-fold. Furthermore, glutamine supplementation was shown to increase plasma citrulline concentrations in rats (7) and in humans (8). However, because glutamine is a main energy source for enterocytes, it is unclear in these studies whether glutamine acted as precursor for citrulline synthesis or just provided energy for its synthesis. Therefore, to establish a precursor-product relation between glutamine and citrulline, labeled glutamine was used in mice (9) and humans (10). The transfer of the 15N label from 2-[15N]-glutamine to citrulline seemed to confirm that glutamine is the main precursor for citrulline synthesis in mice (11), accounting for ∼85% of circulating citrulline in humans (4).
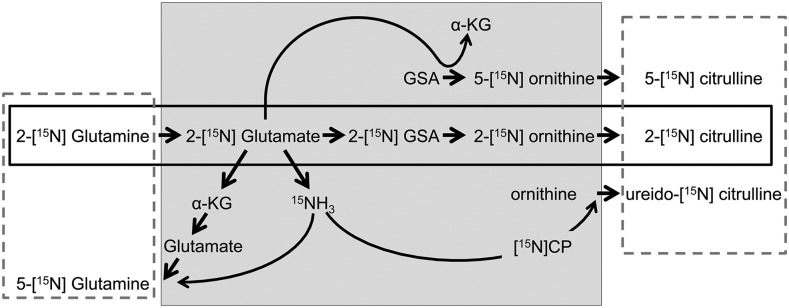
These findings firmly established the role of glutamine as the precursor for citrulline synthesis in the literature. However, our work with 15N- and 13C-labeled glutamine in mice (12) and pigs (13) indicated that the contribution of glutamine to the synthesis of citrulline is rather modest. Considerable debate since then has ensued (14–19), with some authors attributing the difference in glutamine utilization for citrulline synthesis to species differences (17). Furthermore, central to this debate is the meaning of precursor. The synthesis of citrulline requires different molecules that provide the carbon skeleton, the α (2-) and δ (5-) nitrogen groups, and the ureido carbon and nitrogen. These last 2 originate from carbon dioxide and ammonia, and thus are not specific to any particular precursor because they can originate during the oxidation of many compounds. Furthermore, the amino group of glutamate (after glutamine deamidation) is easily exchangeable via glutamate dehydrogenase with the ammonia pool (which is unlikely to be limiting). In our view, the term “precursor” should be reserved for those molecules that contribute to the carbon skeleton of citrulline (C1–C5).
Glutamine supplementation has been proposed to mitigate the deleterious effects of ischemia reperfusion (20) and has also been postulated as an immunonutrient in critically ill patients (21). It has been suggested that most of the beneficial effects of glutamine supplementation are partly mediated by providing additional precursors for citrulline synthesis and the de novo production of arginine (22). A recent large, multicenter, randomized controlled trial in critically ill patients, however, not only failed to find any benefit of glutamine supplementation but rather showed an increase in mortality (23). For these reasons, a better understanding of the mechanism by which glutamine exerts its (putative) action is needed. The goal of the present work is to establish the relation between glutamine and citrulline in multiple species with the use of carbon and nitrogen tracers.
Methods
Whole-body glutamine and citrulline kinetics were determined in humans, pigs, rats, and mice. All of the species were primed and continuously infused for 4 h with l-2,3,3,4,4-[2H5]-glutamine, l-2-[15N]-glutamine, and l-5,5-[2H2]-citrulline (Table 1) to determine the rate of appearance (Ra) of glutamine and citrulline and the contribution of glutamine carbon and nitrogen to the synthesis of citrulline. A 4-h infusion is sufficient to reach steady state isotopic enrichment in humans, pigs, rats, and mice (Supplemental Figures 1–3 and reference 12, respectively). In addition, 2 sets of mice were infused with either l-2,3,3,4,4-[2H5]- or U-[13C5]-glutamine to validate the use of the deuterated tracer (Table 1). A third group of mice was infused with U-[13C6]-glucose to show the potential for carbon recycling and the incorporation of 13C into glutamine and citrulline. Infusions were performed in the fasting state; infusion rates for the different species are reported in Table 1. The human studies were approved by the Institutional Review Board for Investigations in Human Subjects of the Baylor College of Medicine (protocol H-37869). All of the animal procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee (protocols AN-4496, 6418, and 6988) and followed the NRC’s guide for the care and use of laboratory animals.
TABLE 1.
Intravenous infusion rates of the different tracers in humans, pigs, rats, and mice1
| Species, μmol ⋅ kg−1 ⋅ h−1 |
||||
| Humans | Pigs | Rats | Mice | |
| Tracer validation | ||||
| l-2,3,3,4,4-[2H5]-glutamine | — | — | — | 156.0 |
| l-U-[13C5]-glutamine | — | — | — | 156.0 |
| U-[13C6]-glucose | — | — | — | 690.0 |
| Main study | ||||
| l-2,3,3,4,4-[2H5]-glutamine | 6.0 | 16.1 | 128.0 | 156.0 |
| l-2-[15N]-glutamine | 15.0 | 37.1 | 128.0 | 156.0 |
| l-5,5-[2H2]-citrulline | 0.5 | 1.2 | 5.0 | 7.0 |
Stable isotopes were purchased from Cambridge Isotopic Laboratories. The priming dose was the equivalent to a 1-h infusion.
Human subjects.
Healthy male [n = 2; aged 53 y (74.3 kg) and 29 y (64.9 kg)] and female [n = 2; aged 54 y (41.1 kg) and 31 y (53.5 kg)] subjects were enrolled in the study. After informed consent, a detailed physical examination was performed and blood was drawn for assessment of hematologic indexes, electrolytes, blood urea nitrogen, creatinine, calcium, glucose, bilirubin, albumin, aspartate and alanine aminotransferase concentrations, and alkaline phosphatase activity (data not shown). All 4 subjects had no clinically significant laboratory abnormalities and had normal synthetic hepatic function. After an overnight fast (no food after midnight), the subjects reported to the Metabolic Research Unit (Children’s Nutrition Research Center, Houston, TX). Peripheral venous catheters were placed in one forearm or hand for infusion and in the contralateral forearm or hand for sampling (Insyte Autogard; BD). Blood samples were obtained from the sampling catheter at 0, 30, 60, 90, 120, 150, 180, 210, and 240 min after the start of the infusion.
Pigs.
Five sows (35 d old; 8.5 ± 0.3 kg) were obtained from Rosenbaum Farms. Pigs were fed a liquid diet for the duration of the study (50 g diet dissolved in 240 mL water ⋅ kg−1 ⋅ d−1; Soweena Litter Life; Merrick’s). Three days after arrival, pigs underwent carotid and jugular catheterization. After a 4-d recovery period and 8 h of feed deprivation, pigs were infused with the tracers. Blood samples were obtained from the carotid catheter at 0, 30, 60, 90, 120, 150, 180, 210, and 240 min after the start of the infusion.
Rats.
Six male Sprague-Dawley rats (48 d old; 149 ± 2 g) were obtained from Charles River Laboratories. Rats were fed a pelleted diet for the duration of the study (5V5R PicoLab Select Rodent 50 IF/6F; PMI Nutrition). After a 7-d acclimation period and 4 h of feed deprivation, rats were infused with the use of a tail vein catheter (Insyte Autogard; BD). Blood samples were obtained from the contralateral tail vein at 210 and 240 min after the start of the infusion.
Mice.
Five male C57BL/6J mice (55 d old; 23.3 ± 0.5 g) were obtained from The Jackson Laboratory. Mice were fed a pelleted diet for the duration of the study (5V5R PicoLab Select Rodent 50 IF/6F). After a 7-d acclimation period and 4 h of feed deprivation, mice were infused with the use of a tail vein catheter as previously described (12). Blood samples were obtained from the submandibular bundle by using a lancet at the end of the infusion. An identical protocol and sampling technique were followed for the mice infused with l-2,3,3,4,4-[2H5]- (n = 7) or l-U-[13C5]-glutamine (n = 7) in the tracer validation study and for the mice (n = 6) infused with U-[13C6]-glucose.
Sample collection.
Blood samples were collected in EDTA tubes, immediately put on ice, and centrifuged (10 min, 4000 × g at 4°C). Plasma was then placed into aliquots and transferred to prelabeled cryovials and stored at −80°C until analysis.
Sample analysis.
Plasma enrichments of glutamine and citrulline were measured by LC–tandem MS as previously described (24). Briefly, 20 μL plasma was reacted with 20 μL 20-mM dansyl-HCl in 15 μL 0.1-M sodium tetraborate buffer (pH 9.2). After 30 min of incubation, 300 μL ice-cold acetonitrile was added to precipitate the protein. Samples were then centrifuged and the supernatant recovered. After drying the supernatant in a vacuum concentrator (Savant; Thermo Scientific), the residue was resuspended in 60 μL solvent A (water + 0.1% formic acid). The analyses were performed by LC–tandem MS (TSQ Vantage; Thermo Scientific) by injecting 10 μL on an RP MAX column (Phenomenex). Elution was performed by using solvents A and B (acetonitrile + 0.1% formic acid). Different fragments were monitored to determine the carbon skeleton contribution of glutamine to citrulline and the site-specific incorporation of 15N into citrulline, as well as the glutamine and citrulline M+1 ions (Table 2). Due to the in vivo partial loss of 1 deuterium atom (25), M+5 glutamine and citrulline were calculated by adding M+5 and M+4 (see tracer validation below). Glucose enrichments were determined by gas chromatography–mass spectrometry (6890/5973; Agilent Technologies) in positive chemical ionization mode as their penta-acetate derivative monitoring m/z 331 and 337.
TABLE 2.
Parent-daughter ions monitored to determine isotopic enrichments for glutamine and citrulline1
| M0, m/z |
M+n, m/z |
||||
| Parent | Daughter | Parent | Daughter | Enrichment determined | |
| Glutamine | 380 | 842 | 381 | 85 | Amino N |
| 380 | 3633 | 381 | 363 | Amido N | |
| 380 | 1704 | 381 | 170 | Whole molecule M+1 | |
| 380 | 170 | 381–3855 | 170 | Carbon skeleton (M+1−M+5) | |
| Citrulline | 409 | 702 | 410 | 71 | Amino N |
| 409 | 70 | 411 | 72 | 5,5 2H2 | |
| 409 | 3923 | 410 | 392 | Ureido N | |
| 409 | 392 | 410 | 393 | Amino N + δ N | |
| 409 | 170 | 410 | 170 | Whole molecule M+1 | |
| 409 | 170 | 410–4145 | 170 | Carbon skeleton (M+1−M+5) | |
M0, base ion; M+n, isotopologue of interest.
Fragments m/z 84 and 70 contain 2-N and 2,3,4 and 5-C of glutamine and citrulline, respectively.
Fragments m/z 363 and 392 contain the whole dansylated molecule after the loss of ammonia from the amido or ureido group of glutamine and citrulline, respectively.
The fragment m/z 170 represents the dimethyl amino naphthalene fragment of the dansylated molecule.
The reporter ion m/z 170 was used to determine the isotope distribution of glutamine and citrulline.
Calculations.
The flux or Ra of glutamine and citrulline was determined by the isotope dilution of the infused tracers as follows:
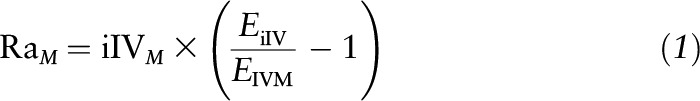 |
where RaM is the Ra of the unlabeled metabolite M (μmol ⋅ kg−1 ⋅ h−1), iIVM is the intravenous infusion rate of the tracer (μmol ⋅ kg−1 ⋅ h−1), EiIV is the enrichment of the infused intravenous tracer, and EIVM is the plasma enrichment of metabolite M at isotopic plateau enrichment [mole percent excess (mpe)].
The extent of intramolecular glutamine 15N recycling from the 2- (amino) to the 5- (amido) position was calculated as follows:
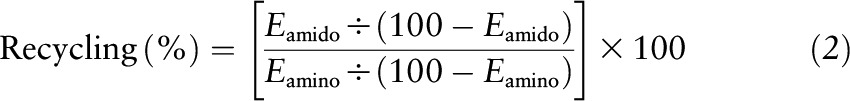 |
where Eamino and Eamido are the enrichments of 2-[15N]- and 5-[15N]-glutamine, respectively. Similarly, the enrichments of the δ and ureido nitrogen of citrulline were expressed in relation to the enrichment of the amino group of this amino acid.
The contribution of glutamine to citrulline synthesis was determined by the transfer of the label from the precursor to the product as follows:
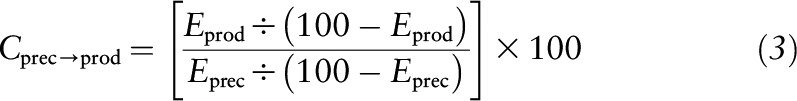 |
where Cprec→prod is the contribution of glutamine to citrulline synthesis (%) and Eprec and Eprod are the respective plasma enrichments of the precursor and product due to the infusion of the labeled precursor. The absolute rate of conversion of glutamine to citrulline can be calculated by multiplying the Cprec→prod by the flux of citrulline determined from the steady state enrichments of the intravenously infused 5,5-[2H2]-citrulline. Due to the potential of the 15N label of the infused 2-[15N]-glutamine to recycle and label the amido (5-) group of glutamine and any of the 3 nitrogen groups of citrulline (Figure 1), multiple precursor-product pairs exist. Because the goal of the present study was not only to compare different species but also to compare our results with others reported in the literature, we performed different precursor-product calculations between glutamine and citrulline. These were as follows: glutamine M+1 to citrulline M+1, as reported by Ligthart-Melis et al. (4) and Buijs et al. (22); 2-[15N]-glutamine to 2-[15N]-citrulline, as reported by Tomlinson et al. (2) and Kao et al. (3); in addition, glutamine M+5 to citrulline M+5 was determined to measure the contribution of the carbon skeleton of glutamine to the synthesis of citrulline.
FIGURE 1.
Pathway of glutamine utilization for the synthesis of citrulline. The infusion of 2-[15N]-glutamine has the potential to label the amido (5-) group of glutamine and the amino (2-), δ (5-) and ureido nitrogen of citrulline. The contribution of glutamine to citrulline synthesis was determined as the conversion of glutamine M+1 to citrulline M+1 (dashed boxes), the conversion of 2-[15N]-glutamine to 2-[15N]-citrulline (solid rectangle), and the conversion of [2H5]-glutamine to [2H5] citrulline (not shown for clarity). The reactions shown in the gray-shaded center box take place in multiple organs and cell types. However, the synthesis of CP and citrulline occurs in enterocytes, the site of production of most of the circulating citrulline. CP, carbamoyl phosphate; GSA, glutamate semialdehyde; α-KG, α-ketoglutarate.
Data analysis.
The Ra (fluxes) of glutamine and citrulline, as well as the contribution of glutamine to citrulline synthesis, were analyzed statistically by using the PROC MIXED procedure of SAS (version 9.2; SAS Institute) with species as the fixed effect. Post hoc pairwise comparisons (Tukey’s procedure) were also conducted. The comparison between glutamine fluxes determined by using 2,3,3,4,4-[2H5]-glutamine and 2-[15N]-glutamine as well as the different precursor-product relations between glutamine and citrulline were analyzed by using the PROC MIXED procedure with tracer or “precursor-product” pair as the fixed effect and species and subject or animal within species as the random effects of the model. For the contribution of 2,3,3,4,4-[2H5]-glutamine or U-[13C5]-glutamine to citrulline synthesis, no random effect was used because determinations were made in different animals. Values presented in the text are least-squares means ± SEs and were tested for significance at the 5% level.
Results
Tracer validation.
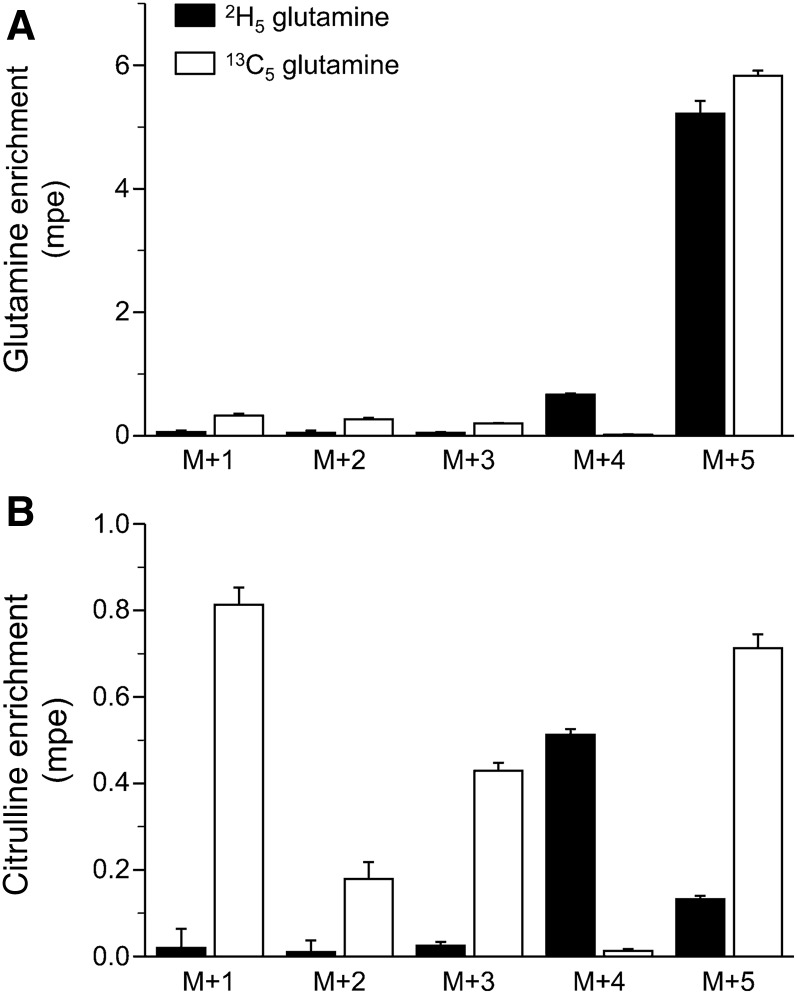
Both the infused l-2,3,3,4,4-[2H5]- and l-U-[13C5]-glutamine tracers, nominally M+5, contained M+4 ions (12% ± 0.2% and 3.4% ± 0.1%, respectively), which agrees with the isotopic purity determined by the manufacturer for these tracers (97% for deuterium and 99% for 13C). After correcting for the infusion of these M+4 species, the infusion of l-2,3,3,4,4-[2H5]-glutamine resulted in negligible M+1, M+2, and M+3 plasma glutamine enrichments but in substantial M+5 and M+4 enrichments (Figure 2A). The extent of the glutamine M+4 was 12.9% ± 0.2% of the M+5 signal for the deuterated tracer. In contrast, the infusion of U-[13C5]-glutamine resulted mainly in lower mass ions (M+1, M+2, and M+3) and M+5 ions (Figure 2A).
FIGURE 2.
Plasma enrichment of glutamine (A) and citrulline (B) after the continuous infusion of 2,3,3,4,4-[2H5]- or [13C5]-glutamine in mice. Values are means ± SEMs; n = 7. The addition of the M+4 and M+5 of glutamine due to the infusion of [2H5]-glutamine was not different than the M+5 enrichment due to the infusion of [13C5]-glutamine (5.89 compared with 5.85 mpe, respectively; P = 0.44). Likewise, citrulline M+4 + M+5 enrichment due to the infusion of the deuterated or carbon-labeled glutamine did not differ (0.68 and 0.71 mpe; P = 0.28). mpe, mole percent excess.
Although the infusion of U-[13C]-labeled glutamine resulted in different citrulline isotopologs (M+1: 0.81 ± 0.04 mpe; M+2: 0.18 ± 0.04 mpe; M+3: 0.43 ± 0.02 mpe; M+4: not detected; and M+5: 0.71 ± 0.03 mpe; Figure 2B), the deuterated glutamine tracer resulted in only citrulline M+4 and M+5 ions (0.51 ± 0.01 and 0.13 ± 0.01 mpe, respectively; Figure 2B). The use of M+4 and M+5 ions to calculate the fractional contribution of 2,3,3,4,4-[2H5]-glutamine to the synthesis of citrulline yielded a value of 13.1% ± 0.6%, a value not different (P = 0.28) than that obtained with U-[13C5]-glutamine (13.6% ± 0.6%).
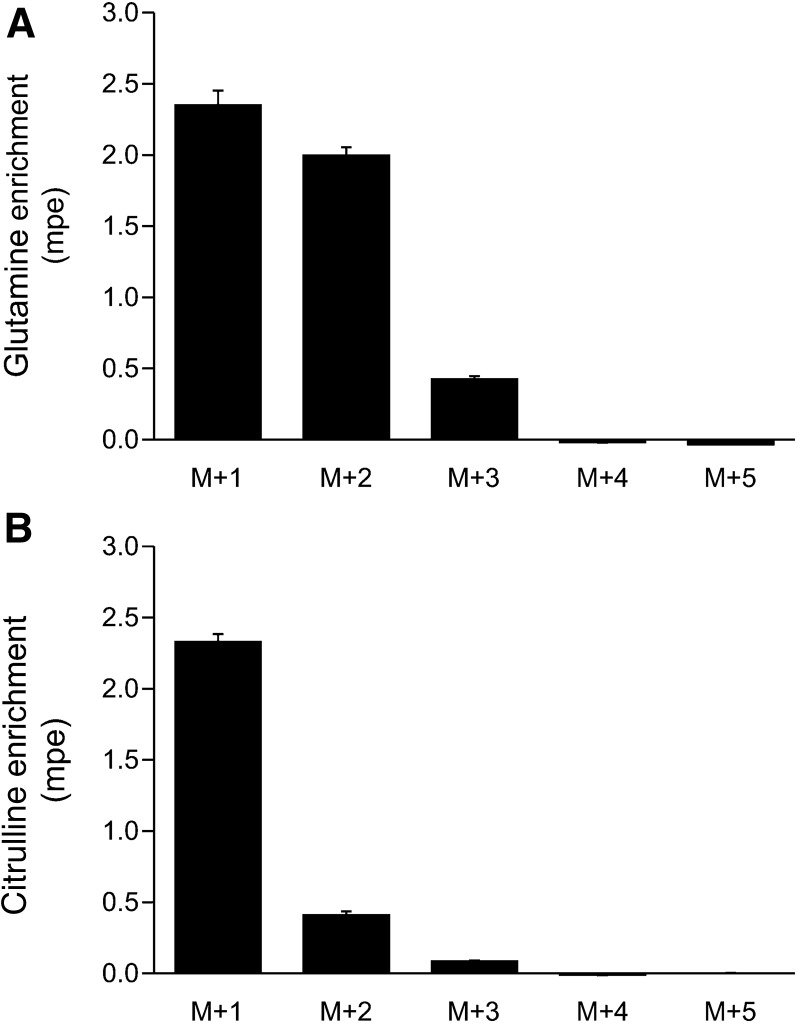
Glucose carbon incorporation into citrulline.
The infusion of U-[13C6]-glucose resulted in a glucose enrichment of 9.5 ± 0.7 mpe; the estimated glucose flux was 6576 ± 430 μmol ⋅ kg−1 ⋅ h−1. The infusion of this glucose tracer resulted in the incorporation of ≤3 carbon atoms in glutamine and citrulline (Figure 3).
FIGURE 3.
Plasma enrichment of glutamine (A) and citrulline (B) after the continuous infusion of [13C6]-glucose in mice. Values are means ± SEMs; n = 6. mpe, mole percent excess.
Rates of appearance of glutamine and citrulline.
The Ra of glutamine ranged from 320 to 3800 μmol ⋅ kg−1 ⋅ h−1 depending on the species studied and tracer used (Table 3). There were significant interspecies differences (P < 0.001), and rats had the highest (P < 0.001) fluxes, followed by mice, pigs, and humans (all different from each other, P < 0.05). The Ra of glutamine by using the infused [2H5] tracer gave values 30–60% greater than those obtained by using the [15N] tracer (P < 0.001). The Ra of citrulline ranged from 10 to 200 μmol ⋅ kg−1 ⋅ h−1 across species, and again, rats had the highest citrulline flux (P < 0.001) followed by mice, pigs, and humans (all different from each other, P < 0.05; Table 3).
TABLE 3.
Glutamine and citrulline fluxes and contribution of glutamine to citrulline synthesis in humans, pigs, rats, and mice1
| Species |
|||||
| Humans (n = 4) | Pigs (n = 5) | Rats (n = 6) | Mice (n = 5) | P | |
| Flux,2 μmol ⋅ kg−1 ⋅ h−1 | |||||
| Glutamine ([2H5] tracer) | 423 ± 252,d | 882 ± 39c | 3789 ± 162a | 3228 ± 89b | <0.001 |
| Glutamine (2-[15N] tracer) | 320 ± 13d | 663 ± 31c | 2565 ± 109a | 1989 ± 54b | <0.001 |
| Citrulline | 10.6 ± 2.3d | 46.5 ± 3.1c | 197.5 ± 11a | 81.1 ± 4.7b | <0.001 |
| Glutamine contribution to citrulline synthesis,3 % of citrulline flux | |||||
| Glutamine M+1 to citrulline M+1 | 87.7 ± 8.4a | 46.3 ± 3.3b | 56.5 ± 3.0b | 59.6 ± 3.7b | <0.001 |
| 2-[15N]-glutamine to 2-[15N] | 48.3 ± 5.0a | 28.0 ± 2.3b | 35.0 ± 2.9a,b | 28.9 ± 4.0b | <0.001 |
| [2H5] glutamine to [2H5]-citrulline | 9.7 ± 0.9b | 3.4 ± 0.4c | 16.7 ± 0.8a | 14.4 ± 0.8a | <0.001 |
Values are means ± SEMs. Means in the same row with different superscript letters differ, P < 0.05.
Glutamine flux determined with the [2H5] tracer was greater (P < 0.001) than that calculated by using the [2-15N] tracer.
The contribution of glutamine to citrulline synthesis differed (P < 0.001) depending on the tracer and fragment used to determine isotope enrichment.
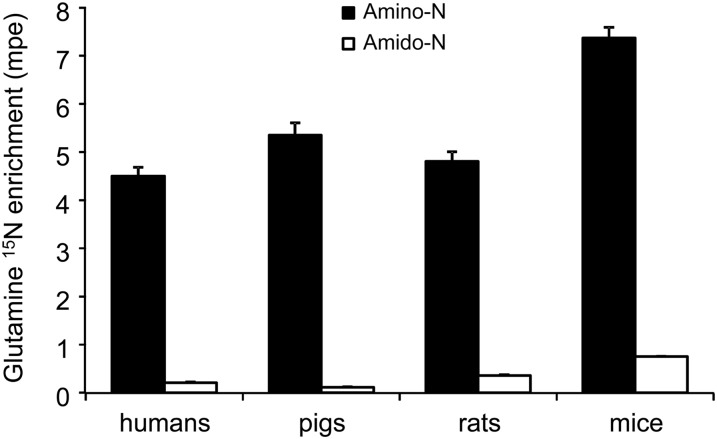
Recycling of the 15N label into the glutamine amido position.
The infusion of 2-[15N]-glutamine also resulted in the labeling of the amido nitrogen of glutamine (Figure 4). The extent of this intramolecular nitrogen recycling ranged from 2.1% ± 0.3% in pigs to 10.3% ± 0.6% in mice (compared with the enrichment of the infused 2-[15N]-glutamine), with intermediate values in humans (4.6% ± 0.4%) and rats (7.4% ± 0.6%).
FIGURE 4.
Plasma glutamine amino (2-) and amido (5-) 15N enrichments after continuous infusion of [2-15N]-glutamine in humans, pigs, rats, and mice. Values are means ± SEMs; n = 4 (humans), 5 (pigs), 6 (rats), and 5 (mice). The amino enrichment was greater (P < 0.001) than the amido enrichment in all species; species differences were not statistically analyzed because different infusion rates were performed. mpe, mole percent excess.
15N labeling of citrulline.
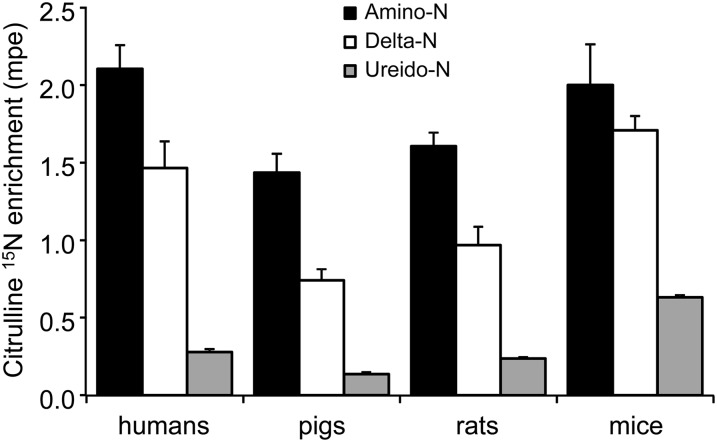
The 3 nitrogen groups of citrulline were labeled by the 2-[15N]-glutamine tracer (Figure 5). The highest enrichment was found in the amino position of citrulline, followed by the δ nitrogen and, last, the ureido group. Humans and mice had a similar δ nitrogen enrichment (66% ± 6% and 73% ± 8%, respectively) compared with the amino nitrogen enrichment, whereas pig and rat relative enrichments were lower (54% ± 10% and 53% ± 7%, respectively). The relative (compared with the amino nitrogen) enrichment of the ureido group ranged from 9% ± 2% in pigs to 35% ± 6% in mice, with humans and rats being intermediate (14% ± 1% and 15% ± 2%, respectively).
FIGURE 5.
Plasma citrulline amino (2-), δ (5-), and ureido 15N enrichment after continuous infusion of [2-15N]-glutamine in humans, pigs, rats, and mice. Values are means ± SEMs; n = 4 (humans), 5 (pigs), 6 (rats), and 5 (mice). The amino enrichment was greater (P < 0.001) than the δ enrichment, which was greater than the ureido enrichment in all species; species differences were not statistically analyzed because different infusion rates were performed. mpe, mole percent excess.
Glutamine contribution to citrulline synthesis.
When measured as the rate of conversion of glutamine M+1 to citrulline M+1, the contribution of glutamine to citrulline synthesis was higher in humans than in the other species (P < 0.05), which did not differ from one another (Table 3). The use of 2-[15N]-glutamine to 2-[15N]-citrulline to determine the rate of conversion gave values ∼50% lower than the use of M+1 ions, with a contribution of glutamine to the synthesis of citrulline ranging from 48.3% in humans to ∼28% in pigs and mice (Table 3). However, the contribution of glutamine to citrulline synthesis was only a fraction of the above values when the [2H5]-glutamine tracer was used to calculate this process (Table 3). Thus, the use of different tracers and ions resulted in different (P < 0.001) apparent contributions of glutamine to citrulline synthesis.
Discussion
We recently discussed some of the issues with regard to the determination of precursor-product relations between glutamine and citrulline (14). Briefly, the choice of tracer, the analytical determination of these metabolites, and the mathematical model used to integrate the tracer data can result in artifacts that overestimate the contribution of glutamine to citrulline synthesis. It has been argued that our initial observations in mice (12) were likely due to species differences (17). For this reason, the present work was conducted in 4 different species, including humans, with the use of tracers that follow the fate of the nitrogen and carbon skeleton of glutamine. To trace the carbon skeleton of glutamine, we used a deuterated tracer instead of 13C-labeled glutamine; glutamine oxidation has the potential to produce 13CO2, which can be incorporated into the ureido group of citrulline, resulting in M+1 ions that would interfere with the determination of 15N labeling. In fact, any 13C-labeled substrate (e.g., glucose) that is oxidized in enterocytes has the potential to label citrulline this way. Furthermore, the recycling of the carbon label followed by the generation of M+1, M+2, and M+3 citrulline ions precludes the co-infusion of carbon-labeled glutamine with other glutamine and citrulline tracers. The advantage of a glutamine-deuterated tracer, compared with a carbon-labeled tracer, is that if the glutamine carbon skeleton were to enter the tricarboxylic acid cycle, the deuterium atoms would be lost and no lower isotopologs would be formed. For these reasons, and because we wanted to simultaneously determine the flux of citrulline and the contribution of glutamine nitrogen and carbon skeleton to the synthesis of citrulline, we used a deuterated glutamine tracer.
Unfortunately, the available 2,3,3,4,4-[2H5]-glutamine tracer has 1 deuterium atom at the C2 position, which is semilabile (25). Furthermore, glutamate semialdehyde, an intermediate in the utilization of glutamine for citrulline synthesis (Figure 1), is spontaneously converted and in equilibrium with pyrroline-5-carboxylic acid. The opening and closing of the pyrroline ring result in further loss of the deuterium in the C2 position (25). After making the appropriate corrections, however, we showed here that 2,3,3,4,4-[2H5]- and U-[13C5]-glutamine tracers provide a similar estimation of the contribution of glutamine to citrulline synthesis.
Rates of appearance of glutamine and citrulline.
The importance of tracer selection is further shown by the differences in glutamine fluxes determined by using [2H5]-glutamine and 2-[15N]-glutamine. Whereas the former tracer follows the carbon backbone of the glutamine molecule, the latter follows (to some degree) the amino nitrogen. The difference between the 2 fluxes is the extent to which the nitrogen moiety is recycled. Similar recycling of the amido nitrogen was determined in humans by simultaneously using 5-[15N]- and 1-[13C]-glutamine (26). Our estimates of glutamine flux measured with the 2-[15N] tracer were similar to those measured previously with the same tracer in healthy humans (3, 4, 27). Pig glutamine fluxes measured with the [2H5] tracer were similar to those measured previously by us with the use of [13C5]-glutamine (13). Our previous determination of glutamine flux in mice (of a different genetic background) with the use of [2H5]-glutamine (28) was higher than those reported here. Likewise, the glutamine fluxes in rats were higher than previously reported (29).
The flux of citrulline in humans was similar to those reported in the literature in healthy subjects (3, 4, 30). Citrulline fluxes in mice were in the range of previous determinations for the same mouse strain (31) and in pigs were similar to previous determinations in animals of the same age (32). To the best of our knowledge, the citrulline fluxes reported here represent the first determination in rats with the use of stable isotopes.
Recycling of the 15N label into the glutamine amido position.
One potential source for the overestimation of the contribution of glutamine to citrulline synthesis is the extent of the recycling of the 15N label of glutamine from the infused amino (2-) position to the amido position. Our previous work in mice infused intragastrically with 2-[15N]-glutamine showed that the plasma enrichment of the amido nitrogen was ∼35% of that of the amino group (12). In the present work, the infusion of the same tracer intravenously showed a 10% recycling in mice. In humans, the observed recycling was more modest (∼5%), whereas other researchers found it to be insignificant (27). However, the delivery of the tracer orally in humans can result in a recycling of ∼20% (2).
Glutamine is a main fuel source for enterocytes, and the extensive utilization of glutamine during the first pass results in the 15N labeling of the ammonia pool in the mitochondria, where citrulline is synthesized (33). Furthermore, the reincorporation of the label into the glutamine amido position, and the utilization of glutamine during the second pass by the gut, adds uncertainty to what the precursor (amino or amido group of glutamine) for citrulline synthesis is. This explains, at least in part, the differences in citrulline enrichment seen when the tracer was given orally rather than intravenously in mice and humans (10, 11).
Glutamine contribution to citrulline synthesis.
The analytical methods used by most researchers are unable to distinguish the position of the 15N label in glutamine and citrulline. However, the advent of tandem-MS technology allows for the fragmentation of molecules and the ability to measure site-specific isotopic enrichments (24).
Our data show that when glutamine M+1 is used as the precursor and citrulline M+1 as the product, the apparent contribution of glutamine to the synthesis of citrulline in humans results in values similar (85%) to those reported by Ligthart-Melis et al. (4). In theory, 2-[15N]-glutamine should label to the same extent the amino and δ nitrogen of citrulline because both nitrogen groups originate from glutamate (Figure 1) (34); however, this has never been considered when doing this precursor-product calculation. In practice, we observed that for the different species studied the enrichment of the δ nitrogen of citrulline was 50–85% of the enrichment of the amino group. Regardless, even when using 2-[15N]-glutamine as the precursor and 2-[15N]-citrulline as the product, the contribution of glutamine to citrulline synthesis was overestimated when compared with the results that used [2H5]-glutamine. The contribution of glutamine to citrulline with the use of the carbon and deuterated tracers in the tracer validation and the main experiment in mice provided a similar estimate (13% and 14%, respectively), albeit slightly higher than our previous determination with the use of [13C5]-glutamine (9%) (35). In contrast, the present estimate in young pigs (3%) was lower than our previous determination in neonatal pigs (8%) (13), which may be due to the younger age of these pigs. Thus, depending on what tracer is used and what fragments are measured, the contribution of glutamine to citrulline production in humans may range from 9.7% to almost 88%.
In an attempt to follow the carbon skeleton of glutamine and determine its contribution to citrulline synthesis, Tomlinson et al. (2) administered 1-[13C]-glutamine orally. The rationale behind the use of this tracer was that “There is no known pathway whereby the label of 1-[13C] glutamine can be incorporated into the carbon skeleton of glutamate, ornithine, citrulline, and arginine other than in the 1-C position.” However, the oxidation of 1-[13C]-glutamine yields 13CO2, which can be incorporated into the ureido group of citrulline. Unfortunately, the enrichment of 1-C and ureido carbon could not be distinguished by the fragment (m/z 70) measured by these authors and thus no “precursor-product” relation should be established with the use of the 1-[13C] tracer in this study.
The main reason for using 2-[15N]-glutamine to determine the contribution of glutamine to citrulline synthesis was economical, because the cost of this tracer is just a fraction of the cost of [2H5]- or [13C5]-glutamine. However, the underlying assumption when using 2-[15N]-glutamine is that the N atom follows the carbon skeleton and it is only incorporated at the 2- (amino) position of citrulline. We have shown here that this is not the case. For this reason, the use of the 15N label does not provide the answer sought and results in an overestimation of the contribution of glutamine to the synthesis of citrulline. To trace the carbon skeleton of glutamine into citrulline, the best alternative is U-[13C5]-glutamine; however, the appearance of multiple isotopologues precludes the co-infusion with other tracers. Therefore, U-[13C5]-glutamine is not a useful tracer within the context of determining the contribution of glutamine to citrulline synthesis and citrulline flux. Here we have shown that, provided the proper corrections are made, the use of 2,3,3,4,4 [2H5]-glutamine yields the same result as the U-[13C5]-glutamine tracer and permits the simultaneous infusion of a citrulline tracer.
In conclusion, the contribution of glutamine to citrulline synthesis has been greatly overestimated in the past when “M+1” was used to determine the precursor-product relation between these 2 amino acids. Even when site-specific 15N labeling of glutamine and citrulline was considered, glutamine contribution was still overestimated. This overestimation occurs to a similar degree in all 4 species studied. The increase in plasma citrulline concentration after glutamine supplementation observed by others warrants further research, but the mechanism for this increase does not appear to be due to increased precursor availability.
Acknowledgments
We thank Jean Hsu for preparing the stable isotope infusates for the human study. JCM and SCSN had full access to all data in the study, wrote the manuscript, and took responsibility for the integrity of the data and the accuracy of the data analysis; JCM designed the research; and JCM, UA, ICD, MA, BS, and SCSN were involved in the conduct of the research and data analysis. All authors read and approved the final manuscript.
References
- 1.Windmueller HG, Spaeth AE. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem 1974;249:5070–9. [PubMed] [Google Scholar]
- 2.Tomlinson C, Rafii M, Ball RO, Pencharz P. Arginine synthesis from enteral glutamine in healthy adults in the fed state. Am J Physiol Endocrinol Metab 2011;301:E267–73. [DOI] [PubMed] [Google Scholar]
- 3.Kao C, Hsu J, Bandi V, Jahoor F. Alterations in glutamine metabolism and its conversion to citrulline in sepsis. Am J Physiol Endocrinol Metab 2013;304:E1359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ligthart-Melis GC, van de Poll MCG, Boelens PG, Dejong CHC, Deutz NEP, van Leeuwen PAM. Glutamine is an important precursor for de novo synthesis of arginine in humans. Am J Clin Nutr 2008;87:1282–9. [DOI] [PubMed] [Google Scholar]
- 5.Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol 1981;241:E473–80. [DOI] [PubMed] [Google Scholar]
- 6.Wu G, Knabe DA, Flynn NE. Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 1994;299:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houdijk APJ, Vanleeuwen PAM, Teerlink T, Flinkerbusch EL, Boermeester MA, Sauerwein HP, Wesdorp RIC. Glutamine-enriched enteral diet increases renal arginine production. JPEN J Parenter Enteral Nutr 1994;18:422–6. [DOI] [PubMed] [Google Scholar]
- 8.Houdijk APJ, Visser JJ, Rijnsburger ER, Teerlink T, van Leeuwen PAM. Dietary glutamine supplementation reduces plasma nitrate levels in rats. Clin Nutr 1998;17:11–4. [DOI] [PubMed] [Google Scholar]
- 9.Boelens PG, van Leeuwen PA, Dejong CH, Deutz NE. Intestinal renal metabolism of L-citrulline and L-arginine following enteral or parenteral infusion of L-alanyl-L-[2,15N]glutamine or L-[2,15N]glutamine in mice. Am J Physiol Gastrointest Liver Physiol 2005;289:G679–85. [DOI] [PubMed] [Google Scholar]
- 10.Ligthart-Melis GC, van de Poll MCG, Dejong CHC, Boelens PG, Deutz NEP, van Leeuwen PAM. The route of administration (enteral or parenteral) affects the conversion of isotopically labeled L-[2-15N]glutamine into citrulline and arginine in humans. JPEN J Parenter Enteral Nutr 2007;31:343–8. [DOI] [PubMed] [Google Scholar]
- 11.Boelens PG, Melis GC, van Leeuwen PA, ten Have GA, Deutz NE. Route of administration (enteral or parenteral) affects the contribution of L-glutamine to de novo L-arginine synthesis in mice: a stable-isotope study. Am J Physiol Endocrinol Metab 2006;291:E683–90. [DOI] [PubMed] [Google Scholar]
- 12.Marini JC, Didelija IC, Castillo L, Lee B. Glutamine: precursor or nitrogen donor for citrulline synthesis? Am J Physiol Endocrinol Metab 2010;299:E69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marini JC, Stoll B, Didelija IC, Burrin DG. De novo synthesis is the main source of ornithine for citrulline production in neonatal pigs. Am J Physiol Endocrinol Metab 2012;303:E1348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marini JC. Interrelationships between glutamine and citrulline metabolism. Curr Opin Clin Nutr Metab Care 2016;19:62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marini JC, Didelija IC, Lee B. Reply to Ligthart-Melis et al [letter]. Am J Physiol 2010;299:E684. [Google Scholar]
- 16.Ligthart-Melis GC, Marini JC, Engelen MPKJ, Deutz NEP. Glutamine supplementation, citrulline production, and de novo arginine synthesis: is there a relation? Am J Clin Nutr 2015;101:890–2. [DOI] [PubMed] [Google Scholar]
- 17.Ligthart-Melis GC, Deutz NEP. Is glutamine still an important precursor of citrulline? Am J Physiol Endocrinol Metab 2011;301:E264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ligthart-Melis GC, Vermeulen MAR, Van Leeuwen PAM, Deutz NEP. Glutamine: precursor or nitrogen donor for citrulline synthesis? Am J Physiol Endocrinol Metab 2010;299:E683. [DOI] [PubMed] [Google Scholar]
- 19.Buijs N, Brinkmann SJ, Oosterink JE, Luttikhold J, Schierbeek H, Wisselink W, Beishuizen A, van Goudoever JB, Houdijk AP, van Leeuwen PA, et al. Reply to GC Ligthart-Melis et al [letter]. Am J Clin Nutr 2015;101:892–3. [DOI] [PubMed] [Google Scholar]
- 20.Brinkmann SJH, Buijs N, Vermeulen MAR, Oosterink E, Schierbeek H, Beishuizen A, de Vries JPPM, Wisselink W, van Leeuwen PAM. Perioperative glutamine supplementation restores disturbed renal arginine synthesis after open aortic surgery: a randomized controlled clinical trial. Am J Physiol Renal Physiol 2016;311:F567–75. [DOI] [PubMed] [Google Scholar]
- 21.Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001;286:944–53. [DOI] [PubMed] [Google Scholar]
- 22.Buijs N, Brinkmann SJH, Oosterink JE, Luttikhold J, Schierbeek H, Wisselink W, Beishuizen A, van Goudoever JB, Houdijk AP, van Leeuwen PA, et al. Intravenous glutamine supplementation enhances renal de novo arginine synthesis in humans: a stable isotope study. Am J Clin Nutr 2014;100:1385–91. [DOI] [PubMed] [Google Scholar]
- 23.Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013;368:1489–97. Erratum in: N Engl J Med 2013;368:1853. [DOI] [PubMed] [Google Scholar]
- 24.Marini JC. Quantitative analysis of 15N-labeled positional isomers of glutamine and citrulline via electrospray ionization tandem mass spectrometry of their dansyl derivatives. Rapid Commun Mass Spectrom 2011;25:1291–6. [DOI] [PubMed] [Google Scholar]
- 25.Rittenberg D, Keston AS, Schoenheimer R, Foster GL. Deuterium as an indicator in the study of intermediate metabolism. XIII. The stability of hydrogen in amino acids. J Biol Chem 1938;125:1–12. [Google Scholar]
- 26.Van Acker BAC, Hulsewé KWE, Wagenmakers AJM, Deutz NEP, Van Kreel BK, Halliday D, Matthews DE, Soeters PB, Von Meyenfeldt MF. Absence of glutamine isotopic steady state: implications for the assessment of whole-body glutamine production rate. Clin Sci 1998;95:339–46. [PubMed] [Google Scholar]
- 27.Darmaun D, Matthews DE, Bier DM. Glutamine and glutamate kinetics in humans. Am J Physiol 1986;251:E117–26. [DOI] [PubMed] [Google Scholar]
- 28.Marini JC, Lee B, Garlick PJ. Ornithine restores ureagenesis capacity and mitigates hyperammonemia in Otc(spf-ash) mice. J Nutr 2006;136:1834–8. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S, Lanza-Jacoby S, Stein TP. Leucine and glutamine metabolism in septic rats. Biochem J 1991;276:405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castillo L, Chapman TE, Sanchez M, Yu YM, Burke JF, Ajami AM, Vogt J, Young VR. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc Natl Acad Sci USA 1993;90:7749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marini JC, Agarwal U, Didelija IC. Dietary arginine requirements for growth are dependent on the rate of citrulline production in mice. J Nutr 2015;145:1227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marini JC, Agarwal U, Robinson JL, Yuan Y, Didelija IC, Stoll B, Burrin DG. Citrulline and arginine synthesis in perinatal and young pigs. In: Skomial J, Lappierre H, editors. Energy and protein metabolism and nutrition. EAAP Scientific Series, Vol. 137. Wageningen (Netherlands): Wageningen Academic Publishers; 2016. p. 157–8. [Google Scholar]
- 33.Wanders RJA, Van Roermund CWT, Meijer AJ. Analysis of the control of citrulline synthesis in isolated rat-liver mitochondria. Eur J Biochem 1984;142:247–54. [DOI] [PubMed] [Google Scholar]
- 34.Markova M, Peneff C, Hewlins MJE, Schirmer T, John RA. Determinants of substrate specificity in omega-aminotransferases. J Biol Chem 2005;280:36409–16. [DOI] [PubMed] [Google Scholar]
- 35.Marini JC. Arginine and ornithine are the main precursors for citrulline synthesis in mice. J Nutr 2012;142:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]