Abstract
Background: Oxidative stress and reduced antioxidants may be a trigger for liver fibrogenesis. Reducing oxidative stress through higher antioxidant concentration may be a potential antifibrotic target.
Objective: We aimed to investigate longitudinally whether plasma zinc, an antioxidant, is related to mitochondrial oxidative stress and the progression of liver fibrosis in the Miami Adult Studies in HIV (MASH) cohort.
Methods: A prospective observational cohort study was conducted in 487 predominantly African American HIV-monoinfected and HIV/hepatitis C virus (HCV)–coinfected adults with a mean ± SD age of 47.08 ± 7.67 y from the MASH cohort and followed for a median of 34 mo. Blood was collected for plasma zinc and measures were used to calculate the fibrosis-4 (FIB-4) score (aspartate amino transferase, alanine aminotransferase, and platelets). Plasma zinc deficiency was defined as <0.75 mg/L. Total DNA was extracted from peripheral blood mononuclear cells and mitochondrial DNA (mtDNA) 8-hydroxyguanosine (8-oxo-dG) was determined. Adjusted mixed models were used to assess the relations between zinc, stage of liver disease, and oxidative stress over time and compared between HIV and HIV/HCV groups.
Results: Zinc concentrations (β: −0.368, SE = 0.172; P = 0.033) and deficiency were associated with lower FIB-4 scores over time (β: 0.381, SE = 0.118; P = 0.001). Compared with those who were not zinc deficient, zinc-deficient participants had an increased risk of having more-progressed liver disease (OR: 1.91; 95% CI: 1.15, 3.16; P = 0.012). Higher mtDNA 8-oxo-dG was associated with zinc deficiency (β: 0.049, SE = 0.024; P = 0.044) and higher FIB-4 scores over time (β: 0.597, SE = 0.168, P < 0.001).
Conclusions: Lower plasma zinc concentrations were associated with liver fibrosis progression and mitochondrial oxidative stress in the HIV and HIV/HCV groups. Zinc may play a role in the impact of liver disease outcomes.
Keywords: zinc, HIV, HIV/HCV coinfection, mtDNA 8-oxo-dG, oxidative stress
Introduction
Liver disease in people living with HIV is a major public health issue because it is one of the most common causes of non-AIDS–related death (1). It is estimated that approximately one-quarter of HIV-infected adults in the United States are coinfected with hepatitis C virus (HCV)9 (2). HIV and HCV coinfection has been associated with faster progression of liver disease to cirrhosis and hepatocellular carcinoma (3, 4). Liver disease progression includes fibrosis, which involves a process of accumulation of collagen and other extracellular matrix proteins that eventually weaken the structure of the liver and reduce the amount of functional tissue (5). Liver fibrosis is a continuous process marked by molecular-, tissue-, and cellular-level activities that are influenced by oxidative stress and lower antioxidant defenses (6). HIV and HCV, in their destructive interaction with immune mechanisms systemically and locally, greatly increase oxidative stress, which contributes to liver fibrogenesis (7, 8).
Zinc is an essential nutrient and an antioxidant that is part of >300 enzymes in the body. It is needed for proper immune function, and its deficiency has been associated with oxidative stress (9). A considerable amount of zinc is found in the nucleus, where it plays a role in the stability of genes and their expression (10). Studies have shown that >50% of people living with HIV have low plasma zinc concentrations (11–13). We and others have shown that zinc has been low not only in HIV-infected cohorts, but also in those with HCV infections, as well as in those with HIV/HCV coinfection (14–16). Zinc may directly and indirectly affect liver fibrosis. Zinc affects the activity and availability of proteins, including the enzymes needed for the production and destruction of collagen, thus directly affecting the process of fibrosis (17–20). Zinc administration during in vitro and in vivo studies diminished the action of one of the essential enzymes for collagen formation, prolyl hydroxylase (17, 21). Zinc possesses anti-inflammatory and antioxidant characteristics that may indirectly affect hepatic stellate cells (22). In vitro zinc deficiency activated hepatic stellate cells to form collagen (20) and augmented the oxidative stress stimulated by hepatotoxins (23). Zinc therapy in both HIV and HCV separately improved disease outcome measures (24, 25).
Oxidative stress may be generated through mitochondrial alterations. Mitochondrial DNA (mtDNA) is more susceptible to oxidative damage than is nuclear DNA because it lacks introns, the structural protection and repairing capabilities of nuclear DNA. The mtDNA position is contiguous to the electron transport system, which is a major source of reactive oxygen species (ROSs); in addition, it lacks protective histones and nucleotide excision repair capacity (26, 27). 8-Hydroxyguanosine (8-oxo-dG), a common DNA lesion, is considered to be a marker of DNA damage (28, 29), and higher concentrations of mtDNA 8-oxo-dG have been associated with higher mutation, deletion, fragmentation of DNA, and diminished mtDNA (30, 31). A greater amount of 8-oxo-dG lesions has been shown to be produced from mtDNA than from nuclear DNA (32, 33). Mitochondrial dysfunction in HIV is commonly present as a result of both HIV itself and antiretroviral therapy (34).
Although oxidative stress appears to be one of the triggers of fibrogenesis, studies on the effect of antioxidants are lacking. To our knowledge, most of the studies conducted on the relation between zinc status and liver disease have been observational and cross-sectional, and the majority of the zinc supplementation trials in HIV and HCV have been of too short a duration to observe an effect on liver fibrosis. Therefore, long-term longitudinal studies are needed to elucidate the role of zinc status in generating excess oxidative stress and its effect on liver fibrosis. Because the reduction of oxidative stress has the potential to be an antifibrotic target for interventions, we longitudinally investigated the association between plasma zinc concentrations and mitochondrial oxidative stress and liver fibrosis in those who were HIV monoinfected and HIV/HCV coinfected in the Miami Adult Studies in HIV cohort.
Methods
Study design
After we obtained informed consent, a total of 487 participants were enrolled into observational studies from October 2009 through October 2012 and followed for a median of 34 mo. Participants were eligible if they were HIV monoinfected or HIV/HCV coinfected by clinical documentation; were >18–60 y of age; had a BMI (in kg/m2) >18 but <40; had controlled comorbid diseases (diabetes, symptomatic cardiovascular disease, hyperlipidemia, and metabolic syndrome); were free of hepatitis B virus and hepatic encephalopathy, carcinoma, or cirrhosis; were not heavy tobacco smokers; and were not pregnant. The study protocol was approved by the Florida International University Institutional Review Board.
Assessments
Study visits were completed every 3 mo and data were collected on demographics, medications, medical history, and alcohol and substance abuse. Alcohol use information was obtained with the use of the validated and standardized Alcohol Use Disorders Identification Test questionnaire that detects frequency of use, hazardous and dependent drinking, and binge drinking (35). Fasting blood was collected at baseline and every 6 mo for metabolic panel, oxidative stress (mtDNA 8-oxo-dG), and plasma zinc. Plasma samples for zinc concentrations and peripheral mononuclear cells (PBMCs) for mtDNA 8-oxo-dG were stored in a −80°C freezer for batch analysis. Fibrosis-4 (FIB-4), a noninvasive measure of liver fibrosis, was calculated with the use of a formula that included participant age, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) concentrations, and platelet counts, as follows: [age (years) × AST (U/L)]/[platelet counts (109 cells/L) × ALT1/2 (U/L)]. FIB-4, at a cutoff of >1.45, has a negative predictive value to exclude advanced fibrosis (stages 4–6 of the Ishak scale) of 90% with a sensitivity of 70%. A cutoff of >3.25 has a positive predictive value of 65% and a specificity of 97% to predict advanced disease (36). CD4 cell counts and HIV viral load were obtained through medical records with the participant’s permission.
Laboratory assays
Plasma zinc concentrations.
Determination of plasma zinc concentrations was obtained through venipuncture with the use of a BD trace-element evacuated tube. The plasma sample was first digested with the use of 0.2 mL + 0.2 mL HNO3 + 0.1 mL H2O2 in digestion tubes. The solution was transferred to an inductively coupled plasma MS tube, to which 20 μL yttrium and 9480 μL H2O was added, and it was then analyzed via inductively coupled plasma MS. The cutoff for zinc deficiency that was used for the analysis was <0.75 mg/L. Each analytical batch included duplicate samples, ≥1 method blank, a continuing calibration check sample, and a quality control sample. All method blanks during analysis were below the method detection limits. The readings of the continuing calibration check samples were always within acceptable range (85–115% of initial calibration). A certified reference material was used as the quality control sample throughout the analysis, and the recoveries for these samples were always within acceptable range (70–130%).
mtDNA 8-oxo-dG.
Blood drawn into BD Vacutainer cell preparation tubes was used to obtain PBMCs. This blood collection tube contains sodium citrate anticoagulant and Ficoll-Paque density medium separated by a gel barrier (37). Total DNA from the PBMCs was obtained with the use of a QIAamp DNA blood mini kit (Qiagen). The degree of oxidative damage was determined with the use of the previously described quantitative real-time PCR method (38, 39). Briefly, an iQ5 qPCR system (BioRad) was applied to detect the amount of mtDNA damage caused by mutagenic 8-oxo-dG with the use of 80 ng total genomic DNA extracted from PBMCs, and 1 of 2 samples was treated with human 8-oxoguanine glycosylase enzyme, which acts as both an N-glycosylase and an apurinic lyase (40, 41). Threshold cycle (Ct) values were obtained for both enzyme-treated and enzyme-nontreated samples (Ct2 and Ct1, respectively). The mean difference of Ct (Ct2−Ct1 = ΔCt) was representative of the amount of 8-oxo-dG and presented as ΔCt. Each analytical batch included duplicate samples and a no-template control to detect contaminating DNA. No significant amplifications were detected in the no-template controls.
Statistical analysis
Baseline demographics and characteristics of the cohort were reported with the use of frequencies and means ± SDs. Student’s t tests were used to compare the HIV and HIV/HCV groups at baseline. A chi-square test was used to compare the prevalence of zinc deficiency between the HIV and HIV/HCV groups. Multivariable linear and logistic regression analyses were used at baseline to examine the relations and dose-response effects between plasma zinc status, FIB-4, and mtDNA 8-oxo-dG in the entire cohort and separately by HIV/HCV and HIV groups, while controlling for age, sex, markers of HIV disease progression (HIV viral load and CD4 cell count), substance abuse, and energy and zinc intake. In addition, separate models were analyzed by sex. Mixed models were used to account for repeated measures in order to assess zinc status, FIB-4, and mtDNA 8-oxo-dG over time in the entire cohort and separately by HIV/HCV coinfection status, while adjusting for the variables of interest. The mixed-models method is a flexible approach that allows a wide variety of correlation patterns to be modeled. In addition, the model uses a random intercept to account for correlation between study visits per person and random slope based on time since baseline. The term “mixed” refers to both fixed and random effects in the same model. Separate mixed models were also analyzed by sex. Zinc status was analyzed as a continuous variable and a categorical variable for zinc deficiency (1 = yes and 0 = no). The P values reported are 2 sided, and P values of <0.05 were used to indicate statistical significance. SAS version 9.3 was used for all statistical analysis.
Results
Characteristics of the study population.
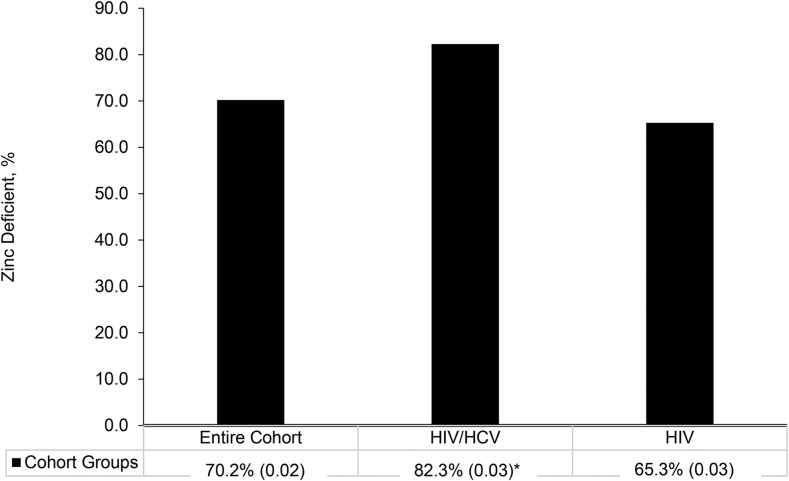
A total of 487 participants from the Miami Adult Studies in HIV cohort were included in the analyses, and their characteristics are displayed in Table 1. The majority of the participants were male (65.1%), African American (68.1%), and taking antiretroviral therapy (80.1%). Approximately 29% of the participants were coinfected with HIV and HCV. The prevalence of zinc deficiency was high in the entire cohort (70.16%) and significantly higher in the HIV/HCV group (82.3%) than it was in the HIV group (65.3%) (P < 0.001) (Figure 1). The mean plasma zinc concentration was also significantly lower in the HIV/HCV group (0.62 ± 0.17 mg/L) than in the HIV group (0.73 ± 0.28 mg/L; P = 0.001).
TABLE 1.
Baseline characteristics of the cohort1
| Variable | Value |
| Sex | |
| F | 34.9 (170) |
| M | 65.1 (317) |
| Age, y | 47.1 ± 7.67 |
| Race/ethnicity | |
| White non-Hispanic | 6.79 (33) |
| White Hispanic | 17.9 (87) |
| Black non-Hispanic | 68.1 (331) |
| Black Hispanic | 4.32 (21) |
| Other | 2.80 (14) |
| HIV/HCV coinfected | 28.8 (140) |
| CD4 cell count, cells/μL | 502 ± 347 |
| HIV viral load, log10 copies/mL | 2.75 ± 1.34 |
| BMI, kg/m2 | 27.4 ± 5.18 |
| ART | 81.1 (391) |
| Plasma zinc, mg/L | 0.70 ± 0.26 |
| Zinc deficiency2 | 70.2 (301) |
| FIB-4 score3 | 1.59 ± 1.38 |
| mtDNA 8-oxo-dG, ΔCt | 0.36 ± 0.31 |
Values are means ± SDs or % (n), n = 487. ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; Ct, threshold cycle; FIB-4, fibrosis-4; HCV, hepatitis C virus; mtDNA, mitochondrial DNA; PLT, platelet count; 8-oxo-dG, 8-hydroxyguanosine.
Defined as plasma zinc concentration <0.75 mg/L.
Calculated with the following formula: [age (years) × AST (U/L)]/[PLT (109 cells/L) × ALT1/2(U/L)].
FIGURE 1.
Prevalence of zinc deficiency among the entire cohort (n = 487) and HIV-monoinfected (347) and HIV/HCV-coinfected groups (n = 140). Values are percentages (SDs) of those with zinc deficiency (plasma zinc concentrations <0.75 mg/L). *Different from HIV, P < 0.05; chi-square test used in the analysis. HCV, hepatitis C virus.
Relation between zinc status and liver fibrosis.
Plasma zinc concentrations were significantly related to FIB-4 (P = 0.011) in the entire cohort at baseline (Table 2). For every unit decrease in plasma zinc, there was a 0.63-unit increase in the FIB-4 score. Those with zinc deficiency had a 0.44-unit increase in their FIB-4 scores compared with those without zinc deficiency in the entire cohort (P = 0.002). When we looked at the HIV group separately, there were no significant associations between plasma zinc or zinc deficiency and FIB-4 in the monoinfected group at baseline. However, significant associations were observed in the HIV/HCV participants between plasma zinc and FIB-4 at baseline. Higher FIB-4 scores were associated with lower plasma zinc as a continuous variable (P = 0.001) and with zinc deficiency (P = 0.031) in this group. We observed similar findings in the coinfected group in models analyzed by sex.
TABLE 2.
Associations between plasma zinc and FIB-4 at baseline and longitudinally in the entire cohort and separated by sex among HIV-monoinfected and HIV/HCV-coinfected groups1
| Entire cohort |
Women |
Men |
||||||||||
| Baseline multivariate analysis2 |
Longitudinal multivariate3 analysis |
Baseline multivariate analysis4 |
Longitudinal multivariate analysis5 |
Baseline multivariate analysis4 |
Longitudinal multivariate analysis5 |
|||||||
| β | P | β | P | β | P | β | P | β | P | β | P | |
| Cohort | ||||||||||||
| Zinc, mg/L | −0.63 ± 0.25 | 0.011 | −0.37 ± 0.17 | 0.033 | −0.53 ± 0.27 | 0.054 | −0.24 ± 0.16 | 0.126 | −0.76 ± 0.35 | 0.033 | −0.43 ± 0.24 | 0.073 |
| Zinc deficiency6 | −0.44 ± 0.14 | 0.002 | 0.38 ± 0.12 | 0.001 | −0.34 ± 0.16 | 0.043 | 0.08 ± 0.10 | 0.002 | −0.53 ± 0.20 | 0.008 | 0.52 ± 0.17 | 0.002 |
| HIV monoinfected | ||||||||||||
| Zinc, mg/L | −0.01 ± 0.202 | 0.98 | −0.06 ± 0.16 | 0.70 | −0.06 ± 0.17 | 0.72 | −0.11 ± 0.10 | 0.29 | 0.05 ± 0.29 | 0.87 | −0.07 ± 0.22 | 0.76 |
| Zinc deficiency6 | −0.01 ± 0.125 | 0.97 | 0.27 ± 0.13 | 0.038 | −0.01 ± 0.11 | 0.96 | 0.07 ± 0.07 | 0.34 | −0.02 ± 0.18 | 0.93 | 0.33 ± 0.18 | 0.066 |
| HIV/HCV coinfected | ||||||||||||
| Zinc, mg/L | −2.38 ± 0.72 | 0.001 | −1.79 ± 0.58 | 0.003 | −1.17 ± 0.82 | 0.16 | −0.36 ± 0.56 | 0.53 | −3.62 ± 1.17 | 0.003 | −3.23 ± 0.94 | 0.001 |
| Zinc deficiency6 | −0.70 ± 0.32 | 0.031 | 0.53 ± 0.26 | 0.045 | −0.44 ± 0.38 | 0.25 | −0.03 ± 0.25 | 0.92 | −0.84 ± 0.48 | 0.086 | 0.90 ± 0.42 | 0.036 |
Values are means ± SEs, n = 487. FIB-4 was calculated with the use of the following formula: [age (years) × AST (U/L)]/[PLT (109 cells/L) × ALT1/2(U/L)]. ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUDIT, Adult Use Disorders Identification Test; FIB-4, fibrosis-4; HCV, hepatitis C virus; PLT, platelet count.
Analyses conducted with the use of linear and logistic regression. All models were adjusted for sex, race, BMI, CD4 cell count, HIV viral load, AUDIT, and tobacco use.
Analysis conducted with the use of mixed models. All models were adjusted for sex, race, BMI, CD4 cell count, HIV viral load, AUDIT, and tobacco use.
Analyses conducted with the use of linear and logistic regression. All models were adjusted for race, BMI, CD4 cell count, HIV viral load, AUDIT, and tobacco use.
Analysis conducted with the use of mixed models. All models were adjusted for race, BMI, CD4 cell count, HIV viral load, AUDIT, and tobacco use.
Defined as plasma zinc concentration <0.75 mg/L.
Participants with zinc deficiency compared with those without zinc deficiency in the entire cohort were at a greater risk of having an FIB-4 score of >1.45, indicative of mild liver disease (OR: 1.91; 95% CI: 1.15, 3.16; P = 0.012). The HIV/HCV group had a 3 times greater risk of having an FIB-4 score >1.45 (OR: 3.05; 95% CI: 1.05, 8.87; P = 0.041) after adjusting for race, BMI, CD4 cell count, HIV viral load, and alcohol and tobacco use (results not shown in the tables).
Longitudinal analyses that used mixed models examining the relation between plasma zinc status and FIB-4 over time while adjusting for the variables of interest are also displayed in Table 2. In the entire cohort, plasma zinc concentrations (P = 0.033) and zinc deficiency (P = 0.001) were associated with lower FIB-4 scores over time. Thus, over a median of 24 mo, for every unit decrease in plasma zinc, there was an increase of 0.37 units in FIB-4, which shows increasing liver fibrosis occurring in conjunction with zinc deficiency over time. Similarly, those with zinc deficiency over time had an increase of 0.38 units of FIB-4 compared with those who were not zinc deficient. Longitudinal analyses indicated that in those who were HIV positive, there was a significant association over time between zinc deficiency and FIB-4 (P = 0.038); however, continuous zinc concentrations were not significantly associated with FIB-4. Both continuous lower plasma zinc concentrations (P = 0.003) and zinc deficiency were related to higher FIB-4 concentrations in the HIV/HCV group (P = 0.045). When models were analyzed separately by sex, only men in the HIV/HCV group were found to have plasma zinc concentrations and zinc deficiency significantly associated with FIB-4 over time (P = 0.0001 and P = 0.036, respectively).
Relation between zinc status and 8-oxo-dG.
A dose-response effect was also observed between 8-oxo-dG and plasma zinc status at baseline in the entire cohort (Table 3). For every unit decrease in plasma zinc there was an increase of 0.196 units in the 8-oxo-dG for the overall cohort (P < 0.001). Those with zinc deficiency had higher 8-oxo-dG by 0.088 units than did those who were not zinc deficient. Plasma zinc was associated with 8-oxo-dG (Table 3) in the HIV group (P = 0.009), and there was a trend toward significance between zinc deficiency and 8-oxo-dG (P = 0.08). Similar findings were observed in the regression models among men living with HIV, with plasma zinc being associated with 8-oxo-dG (P = 0.037); however, this relation with 8-oxo-dG as a marker of oxidative stress was not observed when analyzed with the use of zinc deficiency as a categorical variable. There were no significant findings between plasma zinc and 8-oxo-dG at baseline in those in the HIV/HCV group, in the entire cohort or models of coinfected women; however, in men in the HIV/HCV group, there was a significant relation between plasma zinc concentrations and 8-oxo-dG at baseline (P = 0.037).
TABLE 3.
Associations between plasma zinc and mtDNA 8-oxo-dG at baseline and longitudinally in the entire cohort and separated by sex among HIV-monoinfected and HIV/HCV-coinfected groups1
| Entire cohort |
Women |
Men |
||||||||||
| Baseline multivariate analysis2 |
Longitudinal multivariate analysis3 |
Baseline multivariate analysis4 |
Longitudinal multivariate analysis5,6 |
Baseline multivariate analysis4 |
Longitudinal multivariate analysis5,7 |
|||||||
| β | P | β | P | β | P | β | P | β | P | β | P | |
| Cohort | ||||||||||||
| Zinc, mg/L | −0.20 ± 0.05 | <0.001 | −0.04 ± 0.03 | 0.12 | −0.14 ± 0.09 | 0.11 | −0.06 ± 0.06 | 0.37 | −0.21 ± 0.07 | 0.003 | −0.03 ± 0.03 | 0.22 |
| Zinc deficiency8 | −0.09 ± 0.03 | 0.011 | 0.05 ± 0.02 | 0.044 | −0.05 ± 0.06 | 0.34 | 0.05 ± 0.04 | 0.27 | −0.11 ± 0.04 | 0.017 | 0.04 ± 0.03 | 0.16 |
| HIV monoinfected | ||||||||||||
| Zinc, mg/L | −0.12 ± 0.05 | 0.009 | −0.03 ± 0.03 | 0.19 | −0.11 ± 0.08 | 0.15 | −0.05 ± 0.06 | 0.44 | −0.12 ± 0.06 | 0.037 | −0.03 ± 0.03 | 0.32 |
| Zinc deficiency8 | −0.05 ± 0.03 | 0.08 | 0.03 ± 0.02 | 0.20 | −0.03 ± 0.05 | 0.53 | 0.03 ± 0.04 | 0.47 | −0.06 ± 0.04 | 0.11 | 0.02 ± 0.03 | 0.34 |
| HIV/HCV coinfected | ||||||||||||
| Zinc, mg/L | −0.53 ± 0.30 | 0.08 | −0.15 ± 0.11 | 0.16 | −0.07 ± 0.41 | 0.87 | — | — | −1.11 ± 0.49 | 0.037 | −0.47 ± 0.21 | 0.032 |
| Zinc deficiency8 | −0.13 ± 0.13 | 0.32 | 0.04 ± 0.05 | 0.44 | −0.18 ± 0.20 | 0.37 | — | — | −0.18 ± 0.20 | 0.37 | — | — |
Values are means ± SEs, n = 487. AUDIT, Adult Use Disorders Identification Test; HCV, hepatitis C virus; mtDNA, mitochondrial DNA; 8-oxo-dG, 8-hydroxyguanosine.
Analyses conducted with the use of linear and logistic regression. All models were adjusted for sex, race, BMI, CD4 cell count, HIV viral load, AUDIT, and tobacco use.
Analysis conducted with the use of mixed models. All models were adjusted for sex, race, BMI, CD4 cell count, HIV viral load, AUDIT, and tobacco use.
Analyses conducted with the use of linear and logistic regression. All models were adjusted for race, BMI, CD4 cell count, HIV viral load, AUDIT, and tobacco use.
Analysis conducted with the use of mixed models. All models were adjusted for race, BMI, CD4 cell count, HIV viral load, AUDIT, and tobacco use.
Because of small sample size, longitudinal models in women in the HIV/HCV group did not converge.
Because of small sample size, longitudinal models in men in the HIV/HCV group with zinc deficiency did not converge.
Defined as plasma zinc concentration <0.75 mg/L.
The relation between plasma zinc status and mtDNA 8-oxo-dG when using mixed models longitudinally and controlling for variables of interest is displayed in Table 3. Regardless of HCV serostatus, zinc deficiency was associated with higher 8-oxo-dG concentrations (P = 0.044), showing increasing oxidative stress with increasing 8-oxo-dG concentrations over time. When the analyses were conducted separately for the HIV and HIV/HCV groups, there were no significant findings with plasma zinc and 8-oxo-dG over time; however, in men in the HIV/HCV group, plasma zinc was significantly related to 8-oxo-dG over time (P = 0.032). Models that included only women were not significantly associated with 8-oxo-dG over time.
Relation between liver fibrosis and 8-oxo-dG.
The relation between FIB-4 scores and 8-oxo-dG at baseline and longitudinally is shown in Table 4. At baseline, there was a significant relation between FIB-4 and 8-oxo-dG in the entire cohort (P = 0.017) that was strengthened when only men were considered in the model (P = 0.004). Over time, FIB-4 scores were positively associated with 8-oxo-dG in the entire cohort (P < 0.001), showing that for every 1-unit increase of FIB-4 score, a 0.60-unit increase in 8-oxo-dG occurred. Similar findings were observed in the HIV/HCV group (P < 0.023). When only men were analyzed, FIB-4 was associated with 8-oxo-dG longitudinally in the combined cohort (P < 0.001) and in the HIV/HCV group (P = 0.023). When only the HIV group or only women were considered, the relations between FIB-4 and 8-oxo-dG were not significant.
TABLE 4.
Associations between FIB-4 and mtDNA 8-oxo-dG at baseline and longitudinally in the entire cohort and separated by sex among HIV-monoinfected and HIV/HCV-coinfected groups1
| Entire cohort |
Women |
Men |
||||||||||
| Baseline multivariate analysis3 |
Longitudinal multivariate analysis4 |
Baseline multivariate analysis5 |
Longitudinal multivariate analysis6 |
Baseline multivariate analysis5 |
Longitudinal multivariate analysis6 |
|||||||
| FIB-4 score2 | β | P | β | P | β | P | β | P | β | P | β | P |
| Cohort | 0.42 ± 0.17 | 0.017 | 0.60 ± 0.17 | <0.001 | 0.20 ± 0.19 | 0.29 | 0.08 ± 0.12 | 0.51 | 0.76 ± 0.26 | 0.004 | 0.75 ± 0.23 | 0.001 |
| HIV monoinfected | 0.15 ± 0.20 | 0.47 | −0.14 ± 0.22 | 0.53 | 0.20 ± 0.20 | 0.33 | 0.11 ± 0.12 | 0.38 | −0.60 ± 0.36 | 0.094 | −0.32 ± 0.32 | 0.32 |
| HIV/HCV coinfected | 0.59 ± 0.35 | 0.10 | 0.61 ± 0.26 | 0.023 | −0.56 ± 0.45 | 0.23 | −0.52 ± 0.31 | 0.11 | 0.88 ± 0.36 | 0.022 | 0.83 ± 0.35 | 0.023 |
Values are means ± SEs, n = 487. ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUDIT, Adult Use Disorders Identification Test; FIB-4, fibrosis-4; HCV, hepatitis C virus; mtDNA, mitochondrial DNA; PLT, platelet count; 8-oxo-dG, 8-hydroxyguanosine.
Calculated with the use of the following formula: [age (years) × AST (U/L)]/[PLT (109 cells/L) × ALT1/2(U/L)].
Analyses conducted with the use of linear and logistic regression. All models were adjusted for sex, race, BMI, CD4 cell count, HIV viral load, AUDIT, and tobacco use.
Analysis conducted with the use of mixed models. All models were adjusted for sex, race, BMI, CD4 cell count, HIV viral load, AUDIT, and tobacco use.
Analyses conducted with the use of linear and logistic regression. All models were adjusted for race, BMI, CD4 cell count, HIV viral load, AUDIT, and tobacco use.
Analysis conducted with the use of mixed models. All models were adjusted for race, BMI, CD4 cell count, HIV viral load, AUDIT, and tobacco use.
Discussion
The results of this study indicate that lower plasma concentrations of zinc (an antioxidant) are associated with higher FIB-4 scores, a marker of liver fibrosis, and that, over time, zinc concentrations deteriorate as liver fibrosis increases in a cohort of adults with HIV and HIV/HCV coinfection. Moreover, zinc deficiency compared with no zinc deficiency increased the risk of having more progressed liver disease. Plasma zinc concentrations were also longitudinally associated with mtDNA 8-oxo-dG, a biomarker of oxidative stress in the mitochondria. These relations were also more prevalent in the models that included men only, confirming previous reports of more advanced fibrosis in men (42). To our knowledge, this is the first study that shows a significant association between plasma zinc concentrations and liver disease or FIB-4 index scores over time in adults with HIV or HIV/HCV coinfection. Previously, we showed a significant difference between zinc concentrations in people with HIV and HIV/HCV coinfection; however, to our knowledge, a relation between zinc status and a measure of liver fibrosis has not been previously shown, which may have been due to a much smaller sample size (14). In this study, we also showed that zinc concentrations were lower in the HIV/HCV group and were associated with faster liver disease progression than in the HIV group.
Our data, consistent with our previous studies and those of others in people living with HIV (11–13), demonstrated a high prevalence of zinc deficiency (70.2%) or plasma zinc <0.75 mg/L in HIV-infected adults (Table 1). Those who were in the HIV/HCV coinfection group had an even higher prevalence of zinc deficiency, 82.3%. We also demonstrated that there were lower plasma zinc concentrations in HIV/HCV coinfection than in HIV monoinfection (14). Lower plasma zinc concentrations were also found in HCV-infected participants compared with healthy controls in a study by Guo et al. (43). Moreover, low plasma zinc concentrations were found in liver biopsy specimens from patients with alcoholic liver disease and those with chronic active hepatitis (44). In addition, a relation was previously found between lower blood zinc concentrations and an increased severity of liver fibrosis determined with liver biopsy in a cross-sectional study (45).
HCV infection in an HIV-infected patient is associated with accelerated progression to hepatic fibrosis compared with in individuals with HCV infection alone (46). ROSs are key contributors to hepatic fibrosis in HCV and HIV/HCV coinfection (47). We previously showed that HIV/HCV-coinfected adults have higher levels of oxidative stress, including mtDNA 8-oxo-dG, than do those who are HIV monoinfected (14, 39). In this study, 8-oxo-dG was measured in the mitochondria as a measure of oxidative stress because mitochondrial damage is increased during HIV replication (48), and the mtDNA-to–nuclear DNA ratio is significantly lower in patients with selective antiretroviral combinations, suggesting a more rapid decrease of mtDNA than nuclear DNA. This condition is supported by the lack of protected introns in mtDNA compared with nuclear DNA (49).
Several studies have provided evidence that plasma concentrations of zinc are correlated with copper and zinc superoxide dismutase (SOD) activity (50–52). A study in transgenic mice suggested that the antioxidant enzyme mitochondrial manganese SOD and the cytosolic isoenzyme copper and zinc SOD work in combination to decrease and block the release of cytochrome c into the cytosol, which is a product of mitochondrial destruction, and thereby reduce cellular apoptosis (53). This relation may explain the damaging effect of zinc deficiency on the progression of liver fibrosis and increased hepatocellular apoptosis.
In this study, we examined mitochondrial-specific oxidative stress and found higher levels of oxidative stress to be associated with liver disease progression as measured by liver fibrosis. It has been observed that altered mitochondria are present in HCV-infected individuals and in animal models. Specifically, the electron transport chain has been shown to be a major producer of ROSs in HCV-infected hepatocytes, leading to liver injury (54). Mechanisms for the increased pathogenesis seen in HIV/HCV coinfection include ROS generation from both HIV and HCV in hepatic stellate cells and hepatocytes, which stimulate phosphorylation of proteins, leading to increased expression of fibrogenic genes (47).
The regulation of zinc homeostasis occurs in the liver (55), and if zinc concentrations are deficient, the function of the liver may be compromised (44). In fact, an improvement in ALT and AST among participants who were supplemented with zinc was demonstrated (25, 56). In another study, Kawaguchi et al. (57) showed that zinc-supplemented HCV-infected patients decreased concentrations of α-fetoprotein, which is considered to be a predictor of hepatocellular carcinoma (58), indicating that there is a role for zinc in liver disease. Himoto et al. (59) suggested that zinc deficiency may escalate hepatic steatosis by encouraging iron accumulation and lipid peroxidation in the liver of individuals with HCV-related liver disease. Although the mechanisms of lipid peroxidation (59) may be independent of events occurring in the mitochondria (60), zinc deficiency may increase their synergism, particularly when single and multiple infections coincide.
Studies in animal models have demonstrated the mechanisms for the increased DNA damage in the liver seen during zinc deficiency. Kawasaki et al. (60), conducted a study in rats that revealed an inverse relation between zinc deficiency and total 8-oxo-dG from the rat bone marrow. The investigators hypothesized that the generation of 8-oxo-dG may be due to an increase in superoxide radicals because of lower concentrations of zinc and copper SOD. DNA damage in the cell, however, may be prevented or reduced by zinc because of its role as an antioxidant.
HIV/HCV coinfection is associated with greater oxidative stress and lower antioxidant concentrations (14). In our previous study that supplemented zinc to zinc-deficient HIV-positive adults in Miami, we showed that zinc delayed immunologic failure, decreased diarrhea, and was safe as an adjunct therapy in HIV disease (24). Others have shown that zinc supplementation may be advantageous in both HIV and HCV infections alone; however, the effects of antioxidant supplementation on liver disease in HIV/HCV have yet to be fully determined. Zinc is essential for liver function and recuperation and, in this study, seemed to influence liver disease outcomes. Future studies on zinc supplementation in this population may elucidate the impact of these relations (55).
In conclusion, lower plasma zinc concentrations were associated with faster progression of liver fibrosis and mitochondrial oxidative stress, confirming the association between lower antioxidant defenses, increased oxidative stress, and a faster rate of liver fibrosis. These findings support future studies of zinc supplementation as a potential antifibrotic adjuvant therapy to improve liver disease outcomes in people living with HIV and at risk of liver diseases.
Acknowledgments
We thank Jag Khalsa, chief of the Division of Pharmacotherapies and Medical Consequences of Drug Abuse at the National Institutes on Drug Abuse, NIH, for his leadership and advice. AC and MKB designed the study; SSM, CF, TS, and VR conducted the research; SSM and YL analyzed the data; SSM wrote the manuscript; and AC and MKB edited the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ALT, alanine aminotransferase; AST, aspartate aminotransferase; Ct, threshold cycle; FIB-4, fibrosis-4; HCV, hepatitis C virus; mtDNA, mitochondrial DNA; PBMC, peripheral blood mononuclear cell; ROS, reactive oxygen species; SOD, superoxide dismutase; 8-oxo-dG, 8-hydroxyguanosine.
References
- 1.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, Kowalska JD, de Wit S, Law M, el Sadr W, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014;384:241–8. [DOI] [PubMed] [Google Scholar]
- 2.Puoti M, Manno D, Nasta P, Carosi G. The burden of HIV and hepatitis C virus coinfection. Curr Opin HIV AIDS 2007;2:460–5. [DOI] [PubMed] [Google Scholar]
- 3.Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, Koziel MJ. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 2001;33:562–9. [DOI] [PubMed] [Google Scholar]
- 4.de Lédinghen V, Barreiro P, Foucher J, Labarga P, Castéra L, Vispo ME, Bernard PH, Martin-Carbonero L, Neau D, García-Gascó P, et al. Liver fibrosis on account of chronic hepatitis C is more severe in HIV-positive than HIV-negative patients despite antiretroviral therapy. J Viral Hepat 2008;15:427–33. [DOI] [PubMed] [Google Scholar]
- 5.Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology 2002;36:S47–56. [DOI] [PubMed] [Google Scholar]
- 6.Novo E, Cannito S, Paternostro C, Bocca C, Miglietta A, Parola M. Cellular and molecular mechanisms in liver fibrogenesis. Arch Biochem Biophys 2014;548:20–37. [DOI] [PubMed] [Google Scholar]
- 7.Lin W, Wu G, Li S, Weinberg EM, Kumthip K, Peng LF, Méndez-Navarro J, Chen WC, Jilg N, Zhao H, et al. HIV and HCV cooperatively promote hepatic fibrogenesis via induction of reactive oxygen species and NFkappaB. J Biol Chem 2011;286:2665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mastroianni CM, Lichtner M, Mascia C, Zuccalà P, Vullo V. Molecular mechanisms of liver fibrosis in HIV/HCV coinfection. Int J Mol Sci 2014;15:9184–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad AS. Impact of the discovery of human zinc deficiency on health. J Trace Elem Med Biol 2014;28:357–63. [DOI] [PubMed] [Google Scholar]
- 10.Dreosti IE. Zinc and the gene. Mutat Res 2001;475:161–7. [DOI] [PubMed] [Google Scholar]
- 11.Baum MK, Shor-Posner G, Zhang G, Lai H, Quesada JA, Campa A, Jose-Burbano M, Fletcher MA, Sauberlich H, Page JB. HIV-1 infection in women is associated with severe nutritional deficiencies. J Acquir Immune Defic Syndr Hum Retrovirol 1997;16:272–8. [DOI] [PubMed] [Google Scholar]
- 12.Beach RS, Mantero-Atienza E, Shor-Posner G, Javier JJ, Szapocznik J, Morgan R, Sauberlich HE, Cornwell PE, Eisdorfer C, Baum MK. Specific nutrient abnormalities in asymptomatic HIV-1 infection. AIDS 1992;6:701–8. [DOI] [PubMed] [Google Scholar]
- 13.Jones CY, Tang AM, Forrester JE, Huang J, Hendricks KM, Knox TA, Spiegelman D, Semba RD, Woods MN. Micronutrient levels and HIV disease status in HIV-infected patients on highly active antiretroviral therapy in the Nutrition for Healthy Living cohort. J Acquir Immune Defic Syndr 2006;43:475–82. [DOI] [PubMed] [Google Scholar]
- 14.Baum MK, Sales S, Jayaweera DT, Lai S, Bradwin G, Rafie C, Page JB, Campa A. Co-infection with hepatitis C virus (HCV), oxidative stress and antioxidant status in HIV-positive drug users in Miami. HIV Med 2011;12:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum MK, Campa A, Lais S, Lai H, Page JB. Zinc status in HIV-1 infection and drug abuse. Clin Infect Dis 2003;37 Suppl 2:S117–23. [DOI] [PubMed] [Google Scholar]
- 16.Kalkan A, Bulut V, Avci S, Celik I, Bingol NK. Trace elements in viral hepatitis. J Trace Elem Med Biol 2002;16:227–30. [DOI] [PubMed] [Google Scholar]
- 17.Camps J, Bargallo T, Gimenez A, Alie S, Caballeria J, Pares A, Joven J, Masana L, Rodes J. Relationship between hepatic lipid peroxidation and fibrogenesis in carbon tetrachloride-treated rats: effect of zinc administration. Clin Sci (Lond) 1992;83:695–700. [DOI] [PubMed] [Google Scholar]
- 18.Takahara T, Furui K, Funaki J, Nakayama Y, Itoh H, Miyabayashi C, Sato H, Seiki M, Ooshima A, Watanabe A. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology 1995;21:787–95. [PubMed] [Google Scholar]
- 19.Dashti HM, Mathew TC, Jadaon MM, Ashkanani E. Zinc and liver cirrhosis: biochemical and histopathologic assessment. Nutrition 1997;13:206–12. [DOI] [PubMed] [Google Scholar]
- 20.Kojima-Yuasa A, Ohkita T, Yukami K, Ichikawa H, Takami N, Nakatani T, Opare Kennedy D, Nishiguchi S, Matsui-Yuasa I. Involvement of intracellular glutathione in zinc deficiency induced activation of hepatic stellate cells. Chem Biol Interact 2003;146:89–99. [DOI] [PubMed] [Google Scholar]
- 21.Giménez A, Pares A, Alie S, Camps J, Deulofeu R, Caballeria J, Rodes J. Fibrogenic and collagenolytic activity in carbon-tetrachloride-injured rats: beneficial effects of zinc administration. J Hepatol 1994;21:292–8. [DOI] [PubMed] [Google Scholar]
- 22.Stamoulis I, Kouraklis G, Theocharis S. Zinc and the liver: an active interaction. Dig Dis Sci 2007;52:1595–612. [DOI] [PubMed] [Google Scholar]
- 23.DiSilvestro RA, Carlson GP. Effects of mild zinc deficiency, plus or minus acute phase response, on CCl4 hepatotoxicity. Free Radic Biol Med 1994;16:57–61. [DOI] [PubMed] [Google Scholar]
- 24.Baum MK, Lai S, Sales S, Page JB, Campa A. Randomized controlled clinical trial of zinc supplementation to prevent immunological failure in HIV-positive adults. Clin Infect Dis 2010;50:1653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuoka S, Matsumura H, Nakamura H, Oshiro S, Arakawa Y, Hayashi J, Sekine N, Nirei K, Yamagami H, Ogawa M, et al. Zinc supplementation improves the outcome of chronic hepatitis C and liver cirrhosis. Clin Biochem Nutr 2009;45:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci USA 1988;85:6465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muftuoglu M, Mori M, de Souza-Pinto NC. Formation and repair of oxidative damage in the mitochondrial DNA. Mitochondrion 2014;17:164–81. [DOI] [PubMed] [Google Scholar]
- 28.Gredilla R, Bohr VA, Stevnsner T. Mitochondrial DNA repair and association with aging—an update. Exp Gerontol 2010;45:478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klungland A, Bjelland S. Oxidative damage to purines in DNA: role of mammalian Ogg1. DNA Repair (Amst) 2007;6:481–8. [DOI] [PubMed] [Google Scholar]
- 30.Lu AL, Li X, Gu Y, Wright PM, Chang DY. Repair of oxidative DNA damage: mechanisms and functions. Cell Biochem Biophys 2001;35:141–70. [DOI] [PubMed] [Google Scholar]
- 31.Wei YH, Wu SB, Ma YS, Lee HC. Respiratory function decline and DNA mutation in mitochondria, oxidative stress and altered gene expression during aging. Chang Gung Med J 2009;32:113–32. [PubMed] [Google Scholar]
- 32.de Souza-Pinto NC, Eide L, Hogue BA, Thybo T, Stevnsner T, Seeberg E, Klungland A, Bohr VA. Repair of 8-oxodeoxyguanosine lesions in mitochondrial DNA depends on the oxoguanine DNA glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial DNA of OGG1-defective mice. Cancer Res 2001;61:5378–81. [PubMed] [Google Scholar]
- 33.Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci USA 2001;98:10469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez-Matute P, Pérez-Martínez L, Blanco JR, Oteo JA. Role of mitochondria in HIV infection and associated metabolic disorders: focus on nonalcoholic fatty liver disease and lipodystrophy syndrome. Oxid Med Cell Longev 2013;2013:493413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. II. Addiction 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 36.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, et al. ; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 37.Corkum CP, Ings DP, Burgess C, Karwowska S, Kroll W, Michalak TI. Immune cell subsets and their gene expression profiles from human PBMC isolated by vacutainer cell preparation tube (CPT™) and standard density gradient. BMC Immunol 2015;16:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin CS, Wang LS, Tsai CM, Wei YH. Low copy number and low oxidative damage of mitochondrial DNA are associated with tumor progression in lung cancer tissues after neoadjuvant chemotherapy. Interact Cardiovasc Thorac Surg 2008;7:954–8. [DOI] [PubMed] [Google Scholar]
- 39.Shin D-H, Martinez SS, Parsons M, Jayaweera DT, Campa A, Baum MK. Relationship of oxidative stress with HIV Disease Progression in HIV/HCV co-infected and HIV mono-infected adults in Miami. Int J Biosci Biochem Bioinforma 2012;2:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Kemp PA, Thomas D, Barbey R, de Oliveira R, Boiteux S. Cloning and expression in Escherichia coli of the OGG1 gene of Saccharomyces cerevisiae, which codes for a DNA glycosylase that excises 7,8-dihydro-8-oxoguanine and 2,6-diamino-4-hydroxy-5-N-methylformamidopyrimidine. Proc Natl Acad Sci USA 1996;93:5197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nash HM, Bruner SD, Schärer OD, Kawate T, Addona TA, Spooner E, Lane WS, Verdine GL. Cloning of a yeast 8-oxoguanine DNA glycosylase reveals the existence of a base-excision DNA-repair protein superfamily. Curr Biol 1996;6:968–80. [DOI] [PubMed] [Google Scholar]
- 42.Collazos J, Cartón JA, Asensi V. Gender differences in liver fibrosis and hepatitis C virus-related parameters in patients coinfected with human immunodeficiency virus. Curr HIV Res 2011;9:339–45. [DOI] [PubMed] [Google Scholar]
- 43.Guo CH, Chen PC, Ko WS. Status of essential trace minerals and oxidative stress in viral hepatitis C patients with nonalcoholic fatty liver disease. Int J Med Sci 2013;10:730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bode JC, Hanisch P, Henning H, Koenig W, Richter FW, Bode C. Hepatic zinc content in patients with various stages of alcoholic liver disease and in patients with chronic active and chronic persistent hepatitis. Hepatology 1988;8:1605–9. [DOI] [PubMed] [Google Scholar]
- 45.Iwata K, Enomoto H, Nishiguchi S, Aizawa N, Sakai Y, Iwata Y, Tanaka H, Ikeda N, Takashima T, Saito M, et al. Serum zinc value in patients with hepatitis virus-related chronic liver disease: association with the histological degree of liver fibrosis and with the severity of varices in compensated cirrhosis. J Clin Biochem Nutr 2014;55:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soto B, Sánchez-Quijano A, Rodrigo L, del Olmo JA, García-Bengoechea M, Hernández-Quero J, Rey C, Abad MA, Rodríguez M, Sales Gilabert M, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol 1997;26:1–5. [DOI] [PubMed] [Google Scholar]
- 47.Lin W, Weinberg EM, Chung RT. Pathogenesis of accelerated fibrosis in HIV/HCV co-infection. J Infect Dis 2013;207 Suppl 1:S13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodríguez-Mora S, Mateos E, Moran M, Martín MÁ, López JA, Calvo E, Terrón MC, Luque D, Muriaux D, Alcamí J, et al. Intracellular expression of Tat alters mitochondrial functions in T cells: a potential mechanism to understand mitochondrial damage during HIV-1 replication. Retrovirology 2015;12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montaner JS, Côté HC, Harris M, Hogg RS, Yip B, Chan JW, Harrigan PR, O’Shaughnessy MV. Mitochondrial toxicity in the era of HAART: evaluating venous lactate and peripheral blood mitochondrial DNA in HIV-infected patients taking antiretroviral therapy. J Acquir Immune Defic Syndr 2003;34 Suppl 1:S85–90. [DOI] [PubMed] [Google Scholar]
- 50.Sharif R, Thomas P, Zalewsky P, Fenech M. Zinc supplementation influences genomic stability biomarkers, antioxidant activity, and zinc transporter genes in an elderly Australian population with low zinc status. Mol Nutr Food Res 2015;59:1200–12. [DOI] [PubMed] [Google Scholar]
- 51.Song Y, Leonard SW, Traber MG, Ho E. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J Nutr 2009;139:1626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song Y, Elias V, Loban A, Scrimgeour AG, Ho E. Marginal zinc deficiency increases oxidative DNA damage in the prostate after chronic exercise. Free Radic Biol Med 2010;48:82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujimura M, Morita-Fujimura Y, Noshita N, Sugawara T, Kawase M, Chan PH. The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J Neurosci 2000;20:2817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi J. Oxidative stress, endogenous antioxidants, alcohol, and hepatitis C: pathogenic interactions and therapeutic considerations. Free Radic Biol Med 2012;52:1135–50. [DOI] [PubMed] [Google Scholar]
- 55.Mohammad MK, Zou Z, Cave M, Barve A, McClain CJ. Zinc and liver disease. Nutr Clin Pract 2012;27:8–20. Erratum in: Nutr Clin Pract 2012;27(2):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Himoto T, Hosomi N, Nakai S, Deguchi A, Kinekawa F, Matsuki M, Yachida M, Masaki T, Kurokochi K, Watanabe S, et al. Efficacy of zinc administration in patients with hepatitis C virus-related chronic liver disease. Scand J Gastroenterol 2007;42:1078–87. [DOI] [PubMed] [Google Scholar]
- 57.Kawaguchi T, Nagao Y, Abe K, Imazeki F, Honda K, Yamasaki K, Miyanishi K, Taniguchi E, Kakuma T, Kato J, et al. Effects of branched-chain amino acids and zinc-enriched nutrients on prognosticators in HCV-infected patients: a multicenter randomized controlled trial. Mol Med Rep 2015;11:2159–66. [DOI] [PubMed] [Google Scholar]
- 58.Richardson P, Duan Z, Kramer J, Davila JA, Tyson GL, El-Serag HB. Determinant of serum alpha-fetoprotein levels in hepatitis C infected patients. Clin Gastroenterol Hepatol 2012;10:428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Himoto T, Nomura T, Tani J, Miyoshi H, Morishita A, Yoneyama H, Haba R, Masugata H, Masaki T. Exacerbation of insulin resistance and hepatic steatosis deriving from zinc deficiency in patients with HCV-related chronic liver disease. Biol Trace Elem Res 2015;163:81–8. [DOI] [PubMed] [Google Scholar]
- 60.Kawasaki I, Suzuki Y, Yanagisawa H. Zinc deficiency enhances the induction of micronuclei and 8-hydroxy-2′-deoxyguanosine via superoxide radical in bone marrow of zinc-deficient rats. Biol Trace Elem Res 2013;154:120–6. [DOI] [PubMed] [Google Scholar]