Abstract
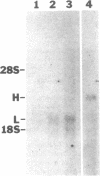
We have explored the cellular loci of endothelin (ET) actions and formation in the brain, using cerebellar mutant mice as well as primary and continuous cell cultures. A glial role is favored by several observations: (i) mutant mice lacking neuronal Purkinje cells display normal ET receptor binding and enhanced stimulation by ET of inositolphospholipid turnover; (ii) in weaver mice lacking neuronal granule cells, ET stimulation of inositolphospholipid turnover is not significantly diminished; (iii) C6 glioma cells and primary cultures of cerebellar astroglia exhibit substantial ET receptor binding and ET-induced stimulation of inositolphospholipid turnover; (iv) ET promotes mitogenesis of C6 glioma cells and primary cerebellar astroglia; and (v) primary cultures of cerebellar astroglia contain ET mRNA. ET also appears to have a neuronal role, since it stimulates inositolphospholipid turnover in primary cultures of cerebellar granule cells, and ET binding declines in granule cell-deficient mice. Thus, ET can be produced by glia and act upon both glia and neurons in a paracrine fashion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambar I., Kloog Y., Kochva E., Wollberg Z., Bdolah A., Oron U., Sokolovsky M. Characterization and localization of a novel neuroreceptor for the peptide sarafotoxin. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1104–1110. doi: 10.1016/s0006-291x(88)80987-2. [DOI] [PubMed] [Google Scholar]
- Ambar I., Kloog Y., Schvartz I., Hazum E., Sokolovsky M. Competitive interaction between endothelin and sarafotoxin: Binding and phosphoinositides hydrolysis in rat atria and brain. Biochem Biophys Res Commun. 1989 Jan 16;158(1):195–201. doi: 10.1016/s0006-291x(89)80197-4. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Growth factors, oncogenes and inositol lipids. Cancer Surv. 1986;5(2):413–430. [PubMed] [Google Scholar]
- Blackstone C. D., Supattapone S., Snyder S. H. Inositolphospholipid-linked glutamate receptors mediate cerebellar parallel-fiber-Purkinje-cell synaptic transmission. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4316–4320. doi: 10.1073/pnas.86.11.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Giaid A., Gibson S. J., Ibrahim B. N., Legon S., Bloom S. R., Yanagisawa M., Masaki T., Varndell I. M., Polak J. M. Endothelin 1, an endothelium-derived peptide, is expressed in neurons of the human spinal cord and dorsal root ganglia. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7634–7638. doi: 10.1073/pnas.86.19.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D., Sokolovsky M. Dual pathways in muscarinic receptor stimulation of phosphoinositide hydrolysis. Biochemistry. 1987 Jan 27;26(2):633–638. doi: 10.1021/bi00376a039. [DOI] [PubMed] [Google Scholar]
- Jones C. R., Hiley C. R., Pelton J. T., Mohr M. Autoradiographic visualization of the binding sites for [125I]endothelin in rat and human brain. Neurosci Lett. 1989 Feb 27;97(3):276–279. doi: 10.1016/0304-3940(89)90610-1. [DOI] [PubMed] [Google Scholar]
- Kloog Y., Bousso-Mittler D., Bdolah A., Sokolovsky M. Three apparent receptor subtypes for the endothelin/sarafotoxin family. FEBS Lett. 1989 Aug 14;253(1-2):199–202. doi: 10.1016/0014-5793(89)80958-5. [DOI] [PubMed] [Google Scholar]
- Komuro I., Kurihara H., Sugiyama T., Yoshizumi M., Takaku F., Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett. 1988 Oct 10;238(2):249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- Lin W. W., Lee C. Y., Chuang D. M. Cross-desensitization of endothelin- and sarafotoxin-induced phosphoinositide turnover in neurons. Eur J Pharmacol. 1989 Aug 3;166(3):581–582. doi: 10.1016/0014-2999(89)90381-6. [DOI] [PubMed] [Google Scholar]
- MacCumber M. W., Ross C. A., Glaser B. M., Snyder S. H. Endothelin: visualization of mRNAs by in situ hybridization provides evidence for local action. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7285–7289. doi: 10.1073/pnas.86.18.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh P. W., Johnson D. J., Boehmer C. G., Catton M. D., Vinters H. V., Maggio J. E., Too H. P., Vigna S. R. Substance P receptor binding sites are expressed by glia in vivo after neuronal injury. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5193–5197. doi: 10.1073/pnas.86.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen R. J., Eicher E. M., Sidman R. L. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci U S A. 1976 Jan;73(1):208–212. doi: 10.1073/pnas.73.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Novelli A., Alho H., Guidotti A., Costa E. The activation of inositol phospholipid metabolism as a signal-transducing system for excitatory amino acids in primary cultures of cerebellar granule cells. J Neurosci. 1986 Jul;6(7):1905–1911. doi: 10.1523/JNEUROSCI.06-07-01905.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol. 1973 Nov 15;152(2):103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Simpson A. W., Ashley C. C. Free calcium rise and mitogenesis in glial cells caused by endothelin. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1115–1122. doi: 10.1016/0006-291x(89)92718-6. [DOI] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Yanagisawa M., Yamashita K., Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989 May 15;264(14):7856–7861. [PubMed] [Google Scholar]
- Watanabe H., Miyazaki H., Kondoh M., Masuda Y., Kimura S., Yanagisawa M., Masaki T., Murakami K. Two distinct types of endothelin receptors are present on chick cardiac membranes. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1252–1259. doi: 10.1016/0006-291x(89)91377-6. [DOI] [PubMed] [Google Scholar]
- Wilkin G. P., Balázs R., Wilson J. E., Cohen J., Dutton G. R. Preparation of cell bodies from the developing cerebellum: structural and metabolic integrity of the isolated cells. Brain Res. 1976 Oct 15;115(2):181–199. doi: 10.1016/0006-8993(76)90506-0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Inoue A., Ishikawa T., Kasuya Y., Kimura S., Kumagaye S., Nakajima K., Watanabe T. X., Sakakibara S., Goto K. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6964–6967. doi: 10.1073/pnas.85.18.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Masaki T. Molecular biology and biochemistry of the endothelins. Trends Pharmacol Sci. 1989 Sep;10(9):374–378. doi: 10.1016/0165-6147(89)90011-4. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Thomas R., D'Orleans-Juste P., Antunes E., Walder C., Warner T. D., Vane J. R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]