Abstract
AIM
To investigate the clinical utility of serum annexin A2 (ANXA2) as a diagnostic marker for early hepatocellular carcinoma (HCC).
METHODS
This study was performed in HCC Clinic of Ain Shams University Hospitals, Cairo, Egypt and included: Group 1: Fifty patients with early stage HCC (Barcelona Clinic Liver Cancer stage A); Group 2: Twenty five patients with chronic liver disease; and Control Group: Fifteen healthy, age- and sex-matched subjects who were seronegative for viral hepatitis markers. The following laboratory investigations were done: Viral hepatitis markers [hepatitis B surface antigen and hepatitis C virus (HCV) antibodies], HCV RNA in HCV antibody-positive patients, serum alpha fetoprotein (AFP), and serum ANXA2 levels.
RESULTS
In this study, 88% of HCC patients (n = 44) were HCV-positive, while HBV infection represented only 8% of all HCC patients (n = 4); and two patients were negative for both viral markers. A highly significant difference was found between patients with HCC and chronic liver disease as well as controls with regard to serum ANXA2 levels (130, IQR 15-240; 15, IQR 15-17; and 17, IQR 15-30 ng/mL, respectively). The area under the curve of ANXA2 was 0.865; the cut-off value was established to be 18 ng/mL with a diagnostic sensitivity of 74% and a specificity of 88%, while the sensitivity and specificity of AFP at the cut-off value of 200 ng/dL were 20% and 100%, respectively.
CONCLUSION
Serum ANXA2 may serve as a biomarker for the early detection of HCC.
Keywords: Hepatocellular carcinoma, Hepatitis C virus, Annexin A2, Alpha-fetoprotein, Tumor markers
Core tip: Thirty percent of hepatocellular carcinoma (HCC) patients present with normal serum alpha fetoprotein, which highlights the need for new biomarkers for HCC. In the present study, a highly significant difference was observed among patients with HCC and chronic liver disease as well as controls with regard to serum annexin A2 (ANXA2) levels (130, IQR 15-240; 15, IQR 15-17; and 17, IQR 15-30 ng/mL, respectively). The area under the curve of ANXA2 was 0.865; the cut-off value was 18 ng/mL with a diagnostic sensitivity of 74% and specificity of 88%. Thus, ANXA2 may serve as a useful biomarker for the early detection of HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. According to the National Institute of Cancer in Egypt, HCC is considered one of the commonest malignancies in Egypt as a result of the high prevalence of hepatitis B and C infections, since these represent approximately 45.3% of all new cases of this type of cancer[1].
Because of the asymptomatic nature of early HCC as well as the lack of its effective screening strategies; most patients (> 80%) present with an overt advanced disease[2]. Approximately 30% of HCC cases with normal serum alpha fetoprotein (AFP) levels are diagnosed before the appearance of clinical manifestations, and this highlights the need for new and early reliable biomarkers for the detection of HCC[3].
Annexins are a family of proteins that bind anionic phospholipids in a calcium-dependent manner. Annexins were first discovered in animal cells and were named for their ability to “annex” or aggregate membranes. The annexins are expressed in vertebrates (ANXA), invertebrates (ANXB), fungi and protozoa (ANXC), plants (ANXD) and protists [e.g., algae (ANXE)][4,5].
Annexin A2 (ANXA2) is primarily expressed in human endothelial cells, mononuclear cells, macrophages, marrow cells and some tumor cells[6]. Moreover, ANXA2 is an inducible, calcium-dependent phospholipid-binding protein that is overexpressed in a variety of human malignancies and has emerged as an attractive candidate receptor for increased plasmin generation on the tumor cell surface[7]. It plays multiple roles in the regulation of cellular functions including angiogenesis, proliferation, apoptosis, cell migration, invasion and adhesion[8,9].
ANXA2 is almost undetectable in the normal liver and in chronic hepatitis tissues, while it is highly expressed in HCC[10]; moreover, serum levels of ANXA2 are elevated in patients with early stage HCC who are AFP-negative[11]. ANXA2 was reported to promote HCC metastasis and invasion through its interaction with HAb18G/CD147 (a member of the immunoglobulin family of proteins)[6]. Nevertheless, the importance of the change in serum levels of ANXA2 in the early stages of HCC has yet to be elucidated.
This study aimed to determine the clinical utility of the serum level of ANXA2 as a diagnostic biomarker of HCC and to correlate its level with that of AFP.
MATERIALS AND METHODS
This prospective case control study was conducted at the HCC clinic, Departments of Tropical Medicine and Clinical Pathology; Ain Shams University Hospitals (Cairo, Egypt), after approval from the Research and Ethics Committee of Ain Shams University was obtained in accordance with local research governance requirements. This study was performed in accordance with the 1964 Declaration of Helsinki and all subsequent revisions.
This study included two patient groups. Group 1: Fifty patients with early stage HCC on top of chronic liver disease (CLD) Child-Pugh class A and B. They were diagnosed according to the Barcelona Clinic Liver Cancer (BCLC) staging system (BCLC A)[12]; and Group 2: Twenty-five patients with CLD (without HCC), their diagnosis was based on clinical, laboratory, and ultrasonographic findings.
CLD in this study represented patients with: (1) persistent viral infection [hepatitis C virus (HCV) and/or hepatitis B virus (HBV)] or affected liver functions for more than 6 mo; and (2) ultrasound features suggestive of CLD: Coarse liver echo-texture, dilated portal vein, attenuated hepatic veins, splenomegaly and/or ascites.
Inclusion criteria for HCC group: (1) confirmed diagnosis of HCC according to the European Association for the Study of Liver Diseases[12]; (2) rarly stage HCC (Stage A) according to the BCLC staging system (single or 3 nodules < 3 cm; Performance Status 0)[12]; and (3) informed consent from all participants before enrollment in the study.
Exclusion criteria for HCC group: (1) intermediate or advanced stage HCC as defined by the BCLC staging system; (2) major vascular tumor invasion or metastasis as confirmed by radiological imaging studies; (3) patients with other suspected solid malignancies or liver metastasis; and (4) other types of CLD as autoimmune hepatitis.
In addition, fifteen age- and sex-matched healthy persons were enrolled, constituting the control group. The healthy controls were collected from the outpatient clinics among those coming for pre-employment screenings. Liver and systemic diseases were excluded by history, physical examination, laboratory and radiologic assessment.
All included patients and control subjects were subjected to the following: (1) full medical history and thorough clinical examination; and (2) laboratory investigations included: Liver function tests to determine the levels of aspartate aminotransferase, alanine aminotransferase, serum bilirubin (total and conjugated), serum albumin, and prothrombin time; viral markers hepatitis B surface antigen, and HCV by enzyme linked immunosorbent assay (ELISA) and detection of HCV RNA by real-time polymerase chain reaction; detection of the serum AFP level by electro-chemiluminescence; and determination of the serum concentration of ANXA2 by ELISA.
Samples
A total of 5 mL of venous blood was withdrawn from each subject under complete aseptic conditions. Then, 1.8 mL was placed into sodium citrate (3.2%) tubes in a ratio of 9:1 (blood: Citrate) for the PT assay. The remainder of the blood was placed in sterile vacutainers with a clot activator and was left to clot for 30 min. The serum was then separated by centrifugation at 1000 × g for 15 min and was divided into two aliquots: One aliquot was used for the immediate routine liver function tests and serum AFP detection, while the remaining portion of sera was stored as an aliquot at -20 °C until future use (i.e., the detection of the serum level of ANXA2). Frozen samples were allowed to thaw to room temperature just prior to the analysis. Hemolyzed samples were discarded, and repeated freezing and thawing was avoided.
Analytical methods
AFP: Quantitative determination of AFP was conducted in an Immulite immunoassay auto analyzer using an AFP kit supplied by DPC (DIAGNOSTICA Product Corporation, Los Angeles, CA, United States). This assay is based on an electro-chemiluminescence immunoassay technique. The antigen (sample), a biotinylated monoclonal AFP-specific antibody and a monoclonal AFP-specific antibody labeled with a ruthenium complex react to form a sandwich complex. Streptavidin-coated microparticles were added, and the complex was then bound to a solid phase via the interaction of biotin and streptavidin. The reaction mixture was aspirated into the measuring cell where the microparticles were magnetically captured onto the surface of the electrode. Unbound substances were then removed with ProCell. The application of a voltage to the electrode then induced chemiluminescent emission, which was measured by a photomultiplier. The results were determined via a calibration curve that was specifically generated by 2-point calibration and a master curve provided by the reagent barcode.
ANXA2 assay: This assay was performed with a commercially available ELISA kit supplied by Glory Science (Glory Science Co., Del Rio, TX, United States). This assay employs a quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for ANXA2 is pre-coated onto a microplate. Standards and samples are pipetted into the wells and any ANXA2 that is present becomes bound by the immobilized antibody. After any unbound substances are washed away, an enzyme-linked monoclonal antibody specific for ANXA2 is added to the wells. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of ANXA2 bound in the initial step. The color development is then stopped and the intensity of the color is measured at 450 nm[13].
Radiological investigations included abdominal ultrasound and triphasic spiral abdominal computed tomography (CT) to confirm the diagnosis and staging of HCC.
Statistical analysis
IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, United States) was used for the data analysis. The quantitative variables were presented as the mean and the standard deviation, while the qualitative variables were presented as frequencies and percentages. The values of skewed parameters were expressed as the median and IQR (25th-75th). An unpaired t test (t value) was used to compare a quantitative variable between two independent groups for parametric data, while a Mann-Whitney test (Z value) was used instead of the t test to compare a quantitative variable between two independent groups when the data were non parametric (SD > 25% of mean). A χ2 test (χ2 value) was used to compare a qualitative variable between two independent groups. The Spearman correlation test (rho value) was used to rank different non parametric variables against each other, either positively or inversely.
A P value < 0.05 was considered significant. The diagnostic accuracy of AFP and ANXA2 was determined by a receiver operating characteristic (ROC) curve analysis, the area under the curve (AUC) and its 95% confidence interval. The diagnostic cut-off, the related sensitivity and specificity, and the positive and negative predictive values (PPV, NPV) were also determined.
The statistical methods of this study were reviewed by Ahmed Mohamed Kamal, consultant in Biostatistics, Ain Shams University; Cairo, Egypt.
RESULTS
The demographic features of the included population were as follows: 33 males (66%) and 17 females (34%) with an age range from 28 to 62 years in Group 1 and 13 males (52%) and 12 (48%) females with an age range from 22 to 68 years in Group 2. The control group included 15 healthy subjects (11 males and 4 females) with an age range between 22 and 60 years.
Group 1 included 50 patients with early stage HCC on top of CLD. Among them, 44 patients (88%) were HCV-positive, 4 patients (8%) had HBV infection and two patients (4%) were negative for both viral markers and were diagnosed as cryptogenic cirrhosis.
Group 2 (CLD group) included 25 patients with CLD only (without HCC). All patients (100%) in this group were having HCV infection.
Descriptive statistics of the different laboratory parameters in all of the patient groups and the controls are shown in Table 1, Figures 1 and 2.
Table 1.
Descriptive statistical data of the various parameters in the three studied groups1
| Parameter | HCC (n = 50) | CLD (n = 25) | Control (n = 15) |
| ALT (U/L) | 43 (31-72.5)2 | 31 (22.5-41)2 | 25 (17-29)2 |
| AST (U/L) | 60 (42.25-97.25)2 | 45 (41-61.5)2 | 26 (21-35)2 |
| PT (s) | 13.8 ± 1.53 | 16 ± 3.37 | 12 ± 0.1 |
| Alb (g/dL) | 3.25 ± 0.53 | 2.75 ± 0.65 | 3.8 ± 0.28 |
| T.Bil (mg/dL) | 1.33 (1-2.2)2 | 2 (1.15-2.9)2 | 0.8 (0.6-0.9)2 |
| D.Bil (mg/dL) | 0.55 (0.29-0.9)2 | 0.6 (0.4-1.3)2 | 0.1 (0.1-0.2)2 |
| AFP (ng/mL) | 41.5 (8.4-191.25)2 | 8.5 (4.1-12.5)2 | 3.1 (2.3-4.6)2 |
| ANXA2 (ng/mL) | 130 (15-240)2 | 15 (15-17)2 | 17 (15-30)2 |
Values are given as the mean ± SD;
Values are given as the median (IQR). HCC: Hepatocellular carcinoma; CLD: Chronic liver disease; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; PT: Prothrombin time; INR: International normalized ratio; Alb: Albumin; T.Bil: Total bilirubin; D.Bil: Direct bilirubin; AFP: Alpha-fetoprotein; ANXA2: Annexin A2; IQR: Interquartile range.
Figure 1.
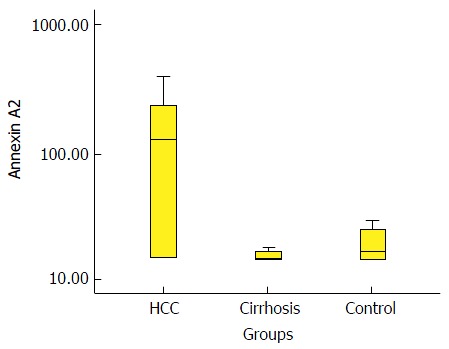
Box-plot diagram that shows the annexin A2 level in the three groups. HCC: Hepatocellular carcinoma.
Figure 2.
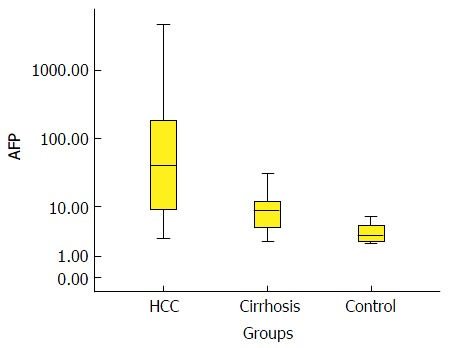
Box-plot diagram shows the alpha-fetoprotein level in the three groups. AFP: Alpha-fetoprotein; HCC: Hepatocellular carcinoma.
Comparative statistics between various groups in terms of AFP and ANXA2 using the Wilcoxon Rank Sum test (non-parametric data) are shown in Table 2. In regard to AFP, highly significant increases were observed in patients with HCC compared with patients with CLD (P < 0.01) and compared with controls (P < 0.01). In addition, the level of AFP was significantly higher in patients with CLD compared with the control group (P < 0.01). In regard to ANXA2, highly significant increases were observed in patients with HCC compared with patients with CLD (P < 0.01) and compared with controls (P < 0.01). However, a non-significant difference was observed between patients with CLD and controls with respect to ANXA2 (P > 0.05).
Table 2.
Comparison of the different studied groups with regard to alpha-fetoprotein and annexin A21
| Parameter |
HCC vs control |
CLD vs control |
HCC vs CLD |
|||
| Z value | P value | Z value | P value | Z value | P value | |
| AFP (ng/mL) | -5.006 | < 0.012 | -3.4 | < 0.012 | -4.17 | < 0.012 |
| ANXA2 (ng/mL) | -3.5 | < 0.012 | -1.6 | > 0.05 | -4.8 | < 0.012 |
Wilcoxon Rank Sum test (non-parametric data);
Statistically significant difference. AFP: Alpha-fetoprotein; ANXA2: Annexin A2; HCC: Hepatocellular carcinoma; CLD: Chronic liver disease.
The correlation analysis between ANXA2 and AFP in both HCC (r = 0.22; P = 0.124) and CLD (r = 0.28; P = 0.173) patients using Spearman’s rank correlation test revealed no statistically significant differences.
ROC curve analysis was performed to assess the diagnostic performance of AFP and ANXA2 in the discrimination of patients with HCC from those with CLD. This analysis revealed that the best cut-off value for AFP was 19.8 ng/mL, with a diagnostic sensitivity of 70%, a specificity of 96%, a PPV of 97.2%, a NPV of 61.5% and an efficacy of 78.7%. While the sensitivity and specificity of AFP at the cut-off value 200 ng/mL (the standard cut-off value used to diagnose HCC) was 20% and 100%, respectively, and the PPV was 100% and the NPV was 50%. The best cut-off value for ANXA2 was 18 ng/mL, the diagnostic sensitivity was 74%, the specificity was 88%, the PPV was 92.5%, the NPV was 62.9% and the efficacy was 78.7% (Figure 3 and Table 3).
Figure 3.
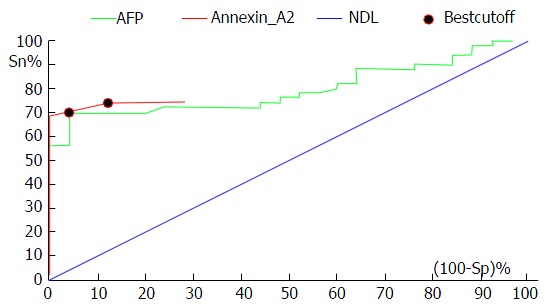
Receiver operating characteristic curve analysis shows the diagnostic performance of alpha-fetoprotein and annexin A2 in the discrimination of patients with hepatocellular carcinoma from those with chronic liver disease. AFP: Alpha-fetoprotein; NDL: Non-discriminating line (Diagonal line).
Table 3.
Diagnostic performance of serum alpha-fetoprotein and annexin A2 in the discrimination of patients with hepatocellular carcinoma from those with chronic liver disease
| Variable | Cut-off | Sn (%) | Sp (%) | NPV (%) | PPV (%) | Efficacy |
| AFP (ng/mL) | 19.8 | 70 | 96 | 61.5 | 97.2 | 78.7 |
| ANXA2 (ng/mL) | 18 | 74 | 88 | 62.9 | 92.5 | 78.7 |
AFP: Alpha-fetoprotein; ANXA2: Annexin A2; Sn: Sensitivity; Sp: Specificity; NPV: Negative predictive value; PPV: Positive predictive value.
DISCUSSION
HCC is the fifth most common cancer and is the third leading cause of cancer death worldwide[14]. Unlike other solid malignancies, the coexistence of inflammation and cirrhosis makes an early diagnosis and prognostic assessment of HCC much more difficult[15]. In addition, the conventional tests of hepatic function do not distinguish HCC from cirrhosis, and thus they contribute little to the diagnosis of such tumor[16].
Detection of circulating markers is the most effective method because it is simple, accurate and cost-effective, but no ideal biomarker has been found thus far[7]. For this reason, early diagnosis of HCC is critical to ensure a good prognosis. Worldwide, ongoing and continuous studies will determine and evaluate sensitive and specific new diagnostic markers for HCC.
The imaging-based diagnosis of small tumors is relatively inaccurate, as cirrhotic and dysplastic nodules can resemble HCC, and hence, a given imaging modality cannot always differentiate between benign hepatic lesions and HCC; as a result, early and small lesions might be overlooked[17].
Since AFP was discovered in the serum of individuals with HCC in 1964, it has been regarded as the most useful serum protein marker for patients at risk for HCC. However, its sensitivity for the detection of HCC ranges between 25%-60%, and its specificity is also low since serum AFP can also be detected in patients with cirrhosis (11%-47%) and chronic hepatitis (15%-58%)[15]. In addition, highly and poorly differentiated HCC cells usually produce little AFP in contrast to the high levels that are synthesized by moderately differentiated HCC cells[18]. Therefore, the positive rate of AFP in the diagnosis of HCC is generally only 60%-70%. The availability of a more sensitive serological marker that distinguishes between HCC and benign hepatic lesions would therefore be very useful and essential for early and specific diagnosis[19]. Unfortunately, surveillance programs are hindered by the poor performance of the commonly used serum markers, namely AFP[20], even in combination with abdominal ultrasound. A great effort has been put forth and continues to be applied in the search for better HCC biomarkers.
ANXA2 is a 36-kDa calcium and phospholipid binding cytoskeletal protein of the Annexin superfamily that is localized to the extracellular surface of endothelial cells and various types of tumor cells[21]. Many reports have shown that ANXA2 is differentially expressed between normal and malignant tissues and is potentially involved in tumor progression[22]. The increased expression of ANXA2 was reported in cancers of the breast, liver, prostate and pancreas. ANXA2 has also been demonstrated to play a role in processes that are essential for cancer metastasis, such as tumor cell migration, invasion, and adhesion[5].
The purpose of this study was to determine the clinical utility of the serum level of ANXA2 as a diagnostic marker of HCC and to correlate its level with that of alpha fetoprotein, which is currently the most widely used marker for HCC.
Our study revealed that 88% of patients with HCC were HCV-positive, while only 8% of patients with HCC were HBV-positive. This was in agreement with the results of El-Serag[2], Zidan et al[23] and Zekri et al[24] and reflects the close relationship between HCV and HCC. The prevalence of HCV infection in Egypt is high and its percentage in patients who develop HCC is higher than that in patients in other countries[25,26].
Our results revealed highly significant increases in the levels of AFP in patients with HCC compared with patients with CLD and subjects in the control group; this result was in agreement with that of El-Tayeh et al[27] and Awadallah et al[28]. They explained their results by an increase in the selective transcriptional activation of the AFP gene in malignant hepatocytes, which resulted in the increased secretion of AFP during the development of HCC.
Additionally, a highly statistically significant difference was observed between patients with CLD and control subjects with respect to AFP; this was in agreement with the result of Page et al[14], who declared that one of the limitations in the use of AFP for the diagnosis of HCC is its increase in patients who have hepatitis and CLD but who do not have HCC. El-Serag[2], stated that hepatic injury and regeneration alone (such as during active hepatitis C virus infection) can increase the serum levels of AFP in patients who do not have HCC. In 2011, AASLD guidelines[29] omitted AFP from the algorithm for surveillance and diagnosis of HCC.
By contrast, we found that ANXA2 levels were highly and significantly increased in patients with HCC compared with the levels in patients with CLD and in controls; however, no statistical significance was found between patients with CLD and the controls with respect to ANXA2 expression. This was in agreement with that of Zhang et al[6], Liu et al[22], Ibrahim et al[30] and Wang and Lin[31].
This was explained by Zhang et al[6] who stated that the ANXA2 gene is up-regulated in HBV- and/or HCV-associated HCC. In addition, Mohammad et al[32], stated that ANXA2 is rarely detected in either normal or chronic hepatic tissues but is over expressed at both the mRNA and protein levels in tumor and non-tumor regions of HCC (primarily localized within cancer cells).
It has been shown that the increased ANXA2 in HCC featured phosphorylation of its tyrosine residues. Interestingly, the tyrosine phosphorylation of ANXA2 was detected in HCC but not in cirrhotic tissue. These data suggest that tyrosine phosphorylation is an important event in hepatocarcinogenesis. It has been reported that ANXA2 is an excellent substrate for Src kinase. Mohammad et al[32] reported that the level of Src kinase activity in HCC is higher than that in cirrhotic tissues. These data suggest that ANXA2 in HCC may be tyrosine-phosphorylated via the elevated tyrosine kinase activity of Src or other kinases, and that increased levels of ANXA2 and the phosphorylation of its tyrosine residues may be related to human hepatocarcinogenesis.
The present study revealed no significant correlation between ANXA2 and AFP in either the CLD or the HCC group; this agrees with the study by Sun et al[9].
The clinical utility of AFP and ANXA2 in the discrimination of patients with HCC from those with CLD was assessed by ROC curve analysis. This revealed that the best diagnostic cut-off value of AFP for the discrimination of patients with HCC from those with CLD was 19.8 ng/mL. This had a diagnostic sensitivity of 70%, a specificity of 96%, a PPV of 97.2%, a NPV of 61.5% and an efficacy of 78.7% (AUC = 0.822). In regards to ANXA2, the best cut-off value was 18 ng/mL. This had a diagnostic sensitivity of 74%, a specificity of 88%, a PPV of 92.5%, a NPV of 62.9% and an efficacy of 78.7% (AUC = 0.873). In accordance with our results, Ibrahim et al[30] found that the AUC for AFP was 0.84 and that for ANXA2 was 0.89. In another Egyptian study, AUC for ANXA2 was 0.910 (95%CI: 0.84-0.97). Combining both ANXA2 and AFP increased the diagnostic efficiency (98% specificity and 97.6% PPV)[33].
In conclusion, our data show that the serum level of ANXA2 might be a good biomarker for the early detection of HCC since it had a higher sensitivity, specificity, and positive and negative predictive values than AFP. ANXA2 could differentiate between HCC and CLD since we found that the levels of ANXA2 were significantly higher in patients with HCC than in CLD patients and in controls. An in-depth analysis of the dynamic changes in serum ANXA2 in both normal and disease conditions as well as a future trial that includes a larger number of patients are emphasized.
ACKNOWLEDGMENTS
The authors thank all of the staff members of the HCC clinic (Hepatoma group) at Ain Shams University Hospitals, Cairo, Egypt.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in the world. Approximately 30% of individuals with HCC present with normal levels of serum alpha fetoprotein (AFP), and therefore, this highlights the need for new biomarkers for HCC.
Research frontiers
This research study was conducted at the HCC clinic, Ain Shams University Hospitals, Faculty of Medicine, Cairo, Egypt to investigate the potential role of annexin A2 (ANXA2) as a new biomarker for HCC. Compared with AFP, the results were encouraging. A future trial that involves a larger number of patients and the combination of both markers to increase the diagnostic accuracy is strongly recommended.
Innovations and breakthroughs
The literature suggests a benefit for ANXA2 as a potential tumor marker for HCC. The current trial adds that the level of ANXA2 in patients with chronic liver disease and healthy controls was significantly lower than that in patients with HCC.
Applications
The authors’ data show that the serum level of ANXA2 might be a good marker for HCC because it has a higher sensitivity, specificity, and positive and negative predictive values than AFP. ANXA2 may serve as a marker for the early detection of HCC and for the differential diagnosis between HCC and CLD because they found that the levels of ANXA2 were significantly higher in patients with HCC than in patients with CLD and in controls. An in-depth analysis of the dynamic changes in serum ANXA2 in both normal and disease conditions is therefore warranted.
Terminology
ANXA2 is an inducible, calcium-dependent phospholipid-binding protein that is overexpressed in a variety of human malignancies and has emerged as an attractive candidate receptor for increased plasmin generation on the tumor cell surface. It plays multiple roles in the cellular angiogenesis, proliferation, apoptosis, cell migration, invasion and adhesion.
Peer-review
The authors prospective case control study investigated 50 early stage HCC, 25 CLD and 15 healthy age-, sex- matched subjects with seronegative viral markers of hepatitis and normal liver function. AFP and ANXA2 were measured from each subject. Authors concluded that ANXA2 at cut-off value of 18 ng/mL was a good diagnostic marker for early HCC.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Research Ethics Committee of the Faculty of Medicine, Ain Shams University.
Clinical trial registration statement: This study is registered at (https://clinicaltrials.gov/ct2/show/NCT02541149), the registration identification number is (NCT02541149).
Informed consent statement: All study participants provided written informed consent prior to study enrollment.
Conflict-of-interest statement: None of the authors have any conflicts of interest or financial disclosures.
Data sharing statement: The technical appendix, statistical code, and dataset are available from the corresponding author at saratropical@yahoo.com. The participants gave informed consent for the data sharing.
Peer-review started: September 30, 2016
First decision: December 27, 2016
Article in press: March 13, 2017
P- Reviewer: Chuang WL, Ungtrakul T S- Editor: Gong ZM L- Editor: A E- Editor: Li D
References
- 1.Miller FD, Abu-Raddad LJ. Evidence of intense ongoing endemic transmission of hepatitis C virus in Egypt. Proc Natl Acad Sci USA. 2010;107:14757–14762. doi: 10.1073/pnas.1008877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Malaguarnera M, Vacante M, Fichera R, Cappellani A, Cristaldi E, Motta M. Chromogranin A (CgA) serum level as a marker of progression in hepatocellular carcinoma (HCC) of elderly patients. Arch Gerontol Geriatr. 2010;51:81–85. doi: 10.1016/j.archger.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Bharadwaj A, Bydoun M, Holloway R, Waisman D. Annexin A2 heterotetramer: structure and function. Int J Mol Sci. 2013;14:6259–6305. doi: 10.3390/ijms14036259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lokman NA, Ween MP, Oehler MK, Ricciardelli C. The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenviron. 2011;4:199–208. doi: 10.1007/s12307-011-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang HJ, Yao DF, Yao M, Huang H, Wu W, Yan MJ, Yan XD, Chen J. Expression characteristics and diagnostic value of annexin A2 in hepatocellular carcinoma. World J Gastroenterol. 2012;18:5897–5904. doi: 10.3748/wjg.v18.i41.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Ye Z, Yang Q, He X, Wang H, Zhao Z. Upregulated expression of annexin II is a prognostic marker for patients with gastric cancer. World J Surg Oncol. 2012;10:103. doi: 10.1186/1477-7819-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang SL, Chou YT, Wu CN, Ho MS. Annexin II binds to capsid protein VP1 of enterovirus 71 and enhances viral infectivity. J Virol. 2011;85:11809–11820. doi: 10.1128/JVI.00297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Gao G, Cai J, Wang Y, Qu X, He L, Liu F, Zhang Y, Lin K, Ma S, et al. Annexin A2 is a discriminative serological candidate in early hepatocellular carcinoma. Carcinogenesis. 2013;34:595–604. doi: 10.1093/carcin/bgs372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji NY, Park MY, Kang YH, Lee CI, Kim DG, Yeom YI, Jang YJ, Myung PK, Kim JW, Lee HG, et al. Evaluation of annexin II as a potential serum marker for hepatocellular carcinoma using a developed sandwich ELISA method. Int J Mol Med. 2009;24:765–771. doi: 10.3892/ijmm_00000290. [DOI] [PubMed] [Google Scholar]
- 11.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 12.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Riener MO, Stenner F, Liewen H, Soll C, Breitenstein S, Pestalozzi BC, Samaras P, Probst-Hensch N, Hellerbrand C, Müllhaupt B, et al. Golgi phosphoprotein 2 (GOLPH2) expression in liver tumors and its value as a serum marker in hepatocellular carcinomas. Hepatology. 2009;49:1602–1609. doi: 10.1002/hep.22843. [DOI] [PubMed] [Google Scholar]
- 14.Page AJ, Cosgrove DC, Philosophe B, Pawlik TM. Hepatocellular carcinoma: diagnosis, management, and prognosis. Surg Oncol Clin N Am. 2014;23:289–311. doi: 10.1016/j.soc.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Zhu K, Dai Z, Zhou J. Biomarkers for hepatocellular carcinoma: progression in early diagnosis, prognosis, and personalized therapy. Biomark Res. 2013;1:10. doi: 10.1186/2050-7771-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thapa BR, Walia A. Liver function tests and their interpretation. Indian J Pediatr. 2007;74:663–671. doi: 10.1007/s12098-007-0118-7. [DOI] [PubMed] [Google Scholar]
- 17.Bolog N, Andreisek G, Oancea I, Mangrau A. CT and MR imaging of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2011;20:181–189. [PubMed] [Google Scholar]
- 18.Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A, Kanke F, Satomura S. Clinical utility of highly sensitive Lens culinaris agglutinin-reactive alpha-fetoprotein in hepatocellular carcinoma patients with alpha-fetoprotein & lt; 20 ng/mL. Cancer Sci. 2011;102:1025–1031. doi: 10.1111/j.1349-7006.2011.01875.x. [DOI] [PubMed] [Google Scholar]
- 19.Cui Z, Yu X, Guo L, Wei Y, Zheng S, Li W, Chen P, Zhu J, Peng J. Combined analysis of serum alpha-fetoprotein and MAGE-A3-specific cytotoxic T lymphocytes in peripheral blood for diagnosis of hepatocellular carcinoma. Dis Markers. 2013;35:915–923. doi: 10.1155/2013/907394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 21.Gurluler E, Guner OS, Tumay LV, Turkel Kucukmetin N, Hizli B, Zorluoglu A. Serum annexin A2 levels in patients with colon cancer in comparison to healthy controls and in relation to tumor pathology. Med Sci Monit. 2014;20:1801–1807. doi: 10.12659/MSM.892319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Myrvang HK, Dekker LV. Annexin A2 complexes with S100 proteins: structure, function and pharmacological manipulation. Br J Pharmacol. 2015;172:1664–1676. doi: 10.1111/bph.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zidan A, Scheuerlein H, Schüle S, Settmacher U, Rauchfuss F. Epidemiological pattern of hepatitis B and hepatitis C as etiological agents for hepatocellular carcinoma in iran and worldwide. Hepat Mon. 2012;12:e6894. doi: 10.5812/hepatmon.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zekri AR, Hassan ZK, Bahnassy AA, Sherif GM, ELdahshan D, Abouelhoda M, Ali A, Hafez MM. Molecular prognostic profile of Egyptian HCC cases infected with hepatitis C virus. Asian Pac J Cancer Prev. 2012;13:5433–5438. doi: 10.7314/apjcp.2012.13.11.5433. [DOI] [PubMed] [Google Scholar]
- 25.Abdelgawad IA, Mossallam GI, Radwan NH, Elzawahry HM, Elhifnawy NM. Can Glypican3 be diagnostic for early hepatocellular carcinoma among Egyptian patients? Asian Pac J Cancer Prev. 2013;14:7345–7349. doi: 10.7314/apjcp.2013.14.12.7345. [DOI] [PubMed] [Google Scholar]
- 26.Shaker MK, Abdella HM, Khalifa MO, El Dorry AK. Epidemiological characteristics of hepatocellular carcinoma in Egypt: a retrospective analysis of 1313 cases. Liver Int. 2013;33:1601–1606. doi: 10.1111/liv.12209. [DOI] [PubMed] [Google Scholar]
- 27.El-Tayeh SF, Hussein TD, El-Houseini ME, Amer MA, El-Sherbini M, Elshemey WM. Serological biomarkers of hepatocellular carcinoma in Egyptian patients. Dis Markers. 2012;32:255–263. doi: 10.3233/DMA-2011-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awadallah AM, Issa HA, Soliman MS. Evaluation of Serum Chromogranin-A as a Useful Tumor Marker for Diagnosis of Hepatocellular Carcinoma. J Am Sci. 2011;7:999. [Google Scholar]
- 29.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim MA, Hashem ME, Mostafa EF, Refaey MM, Hamed EF, Islam Ibrahim I, Almahdy MA. Annexin A2 versus AFP as an efficient diagnostic serum marker for hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;2:780–785. [Google Scholar]
- 31.Wang CY, Lin CF. Annexin A2: its molecular regulation and cellular expression in cancer development. Dis Markers. 2014;2014:308976. doi: 10.1155/2014/308976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammad HS, Kurokohchi K, Yoneyama H, Tokuda M, Morishita A, Jian G, Shi L, Murota M, Tani J, Kato K, et al. Annexin A2 expression and phosphorylation are up-regulated in hepatocellular carcinoma. Int J Oncol. 2008;33:1157–1163. [PubMed] [Google Scholar]
- 33.El-Abd N, Fawzy A, Elbaz T, Hamdy S. Evaluation of annexin A2 and as potential biomarkers for hepatocellular carcinoma. Tumour Biol. 2016;37:211–216. doi: 10.1007/s13277-015-3524-x. [DOI] [PubMed] [Google Scholar]


