Abstract
AIM
To assess the arrhythmic determinants and prognosis of patients presenting with myocardial infarction and non-obstructive coronary arteries (MINOCA) with normal ejection fraction (EF).
METHODS
This is an observational analysis of 131 MINOCA patients with normal EF. Three cardiac magnetic resonance (CMR) diagnosis classes were recognized according to the late gadolinium enhancement (LGE) pattern: Myocardial infarction (MI) (n = 34), myocarditis (n = 47), and “no LGE” (n = 50). Ventricular events occurring during hospitalization were recorded and the entire population was followed-up at 1 year.
RESULTS
Ventricular arrhythmia was observed in 18 (13.8%) patients during hospitalization. The “no LGE” patients experienced fewer ventricular events than the MI and myocarditis patients [4.0% vs 26.5% and 14.9%, respectively (P = 0.013)]. There was no significant difference between the MI and myocarditis groups. On multivariate analysis, LGE transmural extent [OR = 1.52 (1.08-2.15), P = 0.017] and ST-segment elevation [OR = 4.65 (1.61-13.40), P = 0.004] were independent predictors of ventricular arrhythmic events, irrespective of the diagnosis class. Finally, no patient experienced sudden cardiac death or ventricular arrhythmia recurrence at 1-year.
CONCLUSION
MINOCA patients with normal EF presented no 1-year cardiovascular events, irrespective of the CMR diagnosis class. LGE transmural extent and ST segment elevation at admission are risk markers of ventricular arrhythmia during hospitalization.
Keywords: Ventricular tachycardia, Myocarditis, Myocardial infarction, Late gadolinium enhancement, Cardiac magnetic resonance, Myocardial infarction and non-obstructive coronary arteries
Core tip: Out of 131 myocardial infarction and non-obstructive coronary arteries patients, 18 experienced a ventricular arrhythmic event during hospitalization, consisting of 17 ventricular tachycardia and one ventricular fibrillation. No patient died during the 1-year follow-up. Cardiac magnetic resonance classified the underlying diagnosis in 61.8% of the cases, as a myocarditis or a myocardial infarction. Rather than the diagnosis itself, late gadolinium enhancement and ST-segment elevation were found as valuable tools to stratify the risk for arrhythmia of these patients. These findings may be useful to select patients who might be eligible for either arrhythmia prevention or secondary prevention therapy.
INTRODUCTION
Sudden cardiac death is still the most common cause of death worldwide, accounting for over 50% of all deaths from cardiovascular disease. Coronary artery disease represents approximately 80% of all cases[1]. However, 1%-12% of patients with chest pain and cardiac troponin elevation present with normal coronary arteries on angiography analysis[2]. This pathological entity is called myocardial infarction and non-obstructive coronary arteries (MINOCA) and encompasses myocarditis, transient apical ballooning syndrome, and authentic ischemic injuries[2]. Cardiac magnetic resonance (CMR) imaging is helpful for providing detailed information on myocardial tissue characteristics, and has become the gold standard for in vivo detection of necrosis, notably in acute myocardial infarction (MI) and myocarditis[3].
Early-sustained ventricular arrhythmias complicate 2%-20% of acute MIs and are associated with increased hospital mortality rates[4,5]. While the arrhythmic prognosis of MI with abnormal coronary angiography is well known, there is little data concerning MINOCA, even when the arrhythmic prognosis seems to be relatively good[6,7]. As a result of the lack of data concerning this entity, there are no specific guidelines concerning hospitalization duration, follow-up, or treatment for this specific setting.
Our study sought to evaluate the risk of ventricular arrhythmias of presumed low-risk MINOCA patients at both early-stage consultation and 1-year follow-up, based on the diagnosis class established by CMR imaging.
MATERIALS AND METHODS
One hundred and sixty-seven patients were retrospectively enrolled between 2007 and 2012 in the French university hospitals of Nantes and Angers. The inclusion criteria were: (1) hospitalization for acute anginal chest pain; (2) increase in troponin rates superior to the normal range; (3) left ventricular ejection fraction (LVEF) ≥ 45%; and (4) absence of coronary artery stenosis or thrombosis (stenosis < 50% of the diameter of the epicardial vessel).
All patients underwent CMR and the arrhythmic evaluation included at least 48 h of electrocardiography (ECG) monitoring after admission.
In order to avoid overestimating the arrhythmic risk in this population, patients hospitalized for sustained ventricular tachycardia (n = 8) or cardiopulmonary arrest were not included in the study. Patients presenting with Tako-Tsubo (n = 21) were therefore also from the study due to this syndrome’s specific pathophysiology. Seven more patients could not be included due to poor CMR quality so that the study was performed on 131 patients (Figure 1). The study complies with the Declaration of Helsinki and local ethics committee has approved the research protocol.
Figure 1.
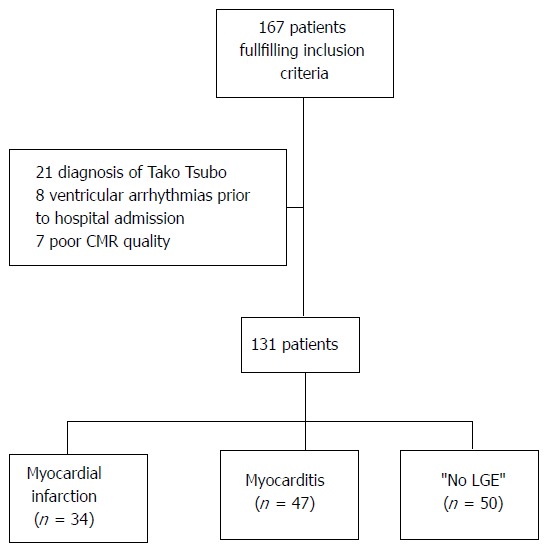
Flow chart of the study population. CMR: Cardiac magnetic resonance; LGE: Late gadolinium enhancement.
Ventricular tachycardia (VT) was defined as at least three consecutive ventricular beats with a rate > 100 bpm[8]. Prolonged VT was defined as at least eight consecutive ventricular beats[9]. Ventricular fibrillation was defined as an irregular ventricular rhythm with marked variability in the QRS cycle length, morphology, and rapid amplitude, usually over 300 bpm/200 ms (cycle length: 180 ms or less)[8].
All data concerning the initial hospitalization were recorded from the patient medical files. Repolarization abnormalities were defined as ST-segment depression ≥ 0.1 mV at 0.08 s from the junction (J point) (STD), asymmetrical T wave inversion ≥ 0.1 mV deep in two or more leads except lead aVR, and ST-segment elevation ≥ 0.2 mV (STE). Q waves > 0.3 mV in depth or > 0.04 s in duration in at least two leads, except lead aVR, were considered abnormal.
At the 1-year follow-up, the following outcomes were collected: Ventricular arrhythmia, death from any cause, cardiovascular (CV) death, and particularly sudden cardiac death. The chosen treatments were evaluated on hospital discharge and at 1 year. If necessary, the referring cardiologists or general practitioners were contacted to obtain information concerning the patients at 1-year follow-up. Data on 1-year survival was completed for every patient.
CMR was performed using 1.5T scanners (Avanto, Siemens, Erlangen, Germany) with 8-element phased-array cardiac receiver coils. The median time from presentation of MINOCA to CMR was 7 d (interquartile range: 4; 13).
LV function was determined by means of cine imaging, in multiple short-axis views covering the entire LV. The typical in-plane resolution was 1.6 mm × 1.9 mm and 7.0-mm thickness sections were used. Temporal resolution was around 35-45 ms.
Late gadolinium enhancement (LGE) was performed 10 min after administering the gadolinium-based contrast agent, at a cumulative dose of 0.2 mmol/kg, by means of a two-dimensional segmented inversion recovery gradient-echo pulse sequence. The inversion time was set to null the signal of the viable myocardium, typically ranging from 240 to 300 ms.
A post-hoc core analysis was specifically performed for this study, using a dedicated software package (Medis 7.1, Mass, Leiden, The Netherlands). For all qualitative assessments, the recommended 17-segment system was applied, requiring the consensus of two blinded observers.
On all the short-axis cine slices, the endocardial and epicardial borders were outlined manually on the end-diastolic and end-systolic images, with the exclusion of the trabeculae and papillary muscles. The reproducibility of the LVEF and LV volumes assessments was good, the details of which are published elsewhere[10].
The cine imaging was assessed visually by analyzing the LV wall thickening using a three-grade scale: 0 = normal, 1 = hypokinesia, 2 = akinesia. We counted the number of segments affected in order to determine the extent of hypokinesis. Furthermore, pericardial effusion was noted when considered exceeding trivial effusion[3].
LGE was evaluated by means of visual analysis. We estimated the maximal transmural extent of LGE within each segment as a percentage of the LV using a five-grade scale: 0% = 0, 0%-25% = 1, 25%-50% = 2, 50%-75% = 3, and > 75% = 4 (Figure 2). The number of segments with LGE defined the LGE transversal extent.
Figure 2.
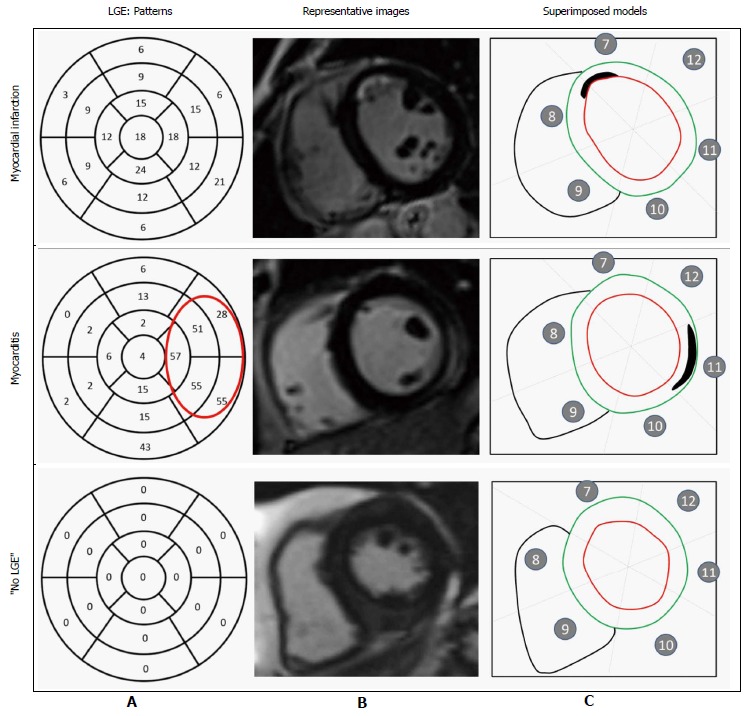
Pattern of late gadolinium enhancement. A: Incidence of late gadolinium enhancement (LGE) within each segment (percentage) of all patients, including those with and without LGE. In myocarditis cases, LGE was predominantly on the lateral wall. In myocardial infarction, no specific pattern of LGE could be identified; B: Representative short-axis slice images showing LGE location; C: Superimposed segmental models showing location and spatial extent of LGE (outlined).
Following analysis by means of CMR, the patients were classified according to LGE pattern, as described: Subendocardial LGE was revealed in the MI group, subepicardial or medioventricular LGE in the myocarditis group, and absence of LGE in the “no LGE” group[11].
When the extent of LGE was transmural (> 75%), the border zones were analyzed to more precisely determine the CMR diagnosis, for example, if the signal of LGE borders was subendocardial, myocardial infarction was diagnosed.
Statistical analysis
Statistical analyses were performed using SPSS Version 15.0 software for Windows (SPSS Inc., Chicago, Illinois, United States). The data was presented as mean ± standard deviation (SD) or median (25th; 75th percentiles) in cases of non-normal distribution, with categorical data expressed as frequencies and percentages. Continuous variables were compared by means of the unpaired t test or Wilcoxon rank-sum test, when necessary. Non-continuous variables were compared using the χ2 test. Differences were considered significant with a P < 0.05. For the multivariate analysis of ventricular events during hospitalization, clinical and CMR data were tested by means of an ascending step-by-step binomial logistic regression analysis, including variables with P values < 0.05 in univariate analysis. The Hosmer-Lemeshow goodness-of-fit test was used to assess the applied models.
RESULTS
A total of 131 patients (87 men, median age: 48.5 ± 16 years) fulfilled our criteria. LGE was present in 81 of the 131 patients (61.8%). There were 34 (25.9%) patients classified in the MI group, 47 (35.9%) in the myocarditis group, and 50 (38.2%) in the “no LGE” group (Table 1). The myocarditis group exhibited a specific pattern of myocarditic lesions, located predominantly in the free lateral wall (42/47 cases) and in the subepicardial layers (45/47 cases). The ischemic lesions were distributed homogeneously (Figure 2).
Table 1.
Patient characteristics
| All patients (n = 131) | MI (n = 34) | Myocarditis (n = 47) | “No LGE” (n = 50) | P | |
| Age, yr | 48.5 ± 16.1 | 52.4 ± 14.2 | 40.5 ± 13.51 | 53.4 ± 16.93 | < 0.001 |
| Risk factors, n (%) | |||||
| Male gender | 87 (66.4) | 22 (64.7) | 39 (83.0) | 26 (52.0)23 | 0.005 |
| Hypertension | 39 (29.8) | 13 (38.2) | 8 (17.0) | 18 (36.0) | 0.06 |
| Diabetes | 11 (8.4) | 4 (11.8) | 2 (4.3) | 5 (10.0) | 0.42 |
| Dyslipidemia | 38 (29.0) | 9 (26.5) | 10 (21.3) | 19 (38.0) | 0.18 |
| Current smoker | 45 (34.4) | 14 (41.4) | 12 (25.5) | 19 (38.0) | 0.27 |
| Family history of premature CHD | 25 (19.1) | 11 (34.2) | 5 (10.6) | 9 (18.0) | 0.05 |
| Time from symptom onset to admission, h | 9 [2; 24] | 3.5 [2; 13.5] | 13 [4; 48]1 | 9.5 [2; 24] | 0.01 |
| Hospitalization duration, d | 6 [4; 7] | 5.5 [4; 7] | 6 [5; 7] | 5 [4; 7] | 0.42 |
| ECG monitoring duration, d | 5 [4; 6] | 5 [4; 6] | 5 [4; 7] | 4 [3; 6] | 0.12 |
| Primary ECG abnormality, n (%) | 91 (69.5) | 26 (76.5) | 34 (72.3) | 31 (62.0) | 0.32 |
| ST-segment elevation | 46 (35.1) | 9 (26.5) | 22 (46.8) | 15 (30.0) | 0.10 |
| ST-segment depression | 8 (6.1) | 2 (5.9) | 4 (8.5) | 2 (4.0) | 0.65 |
| T-wave inversion | 31 (23.7) | 11 (32.4) | 8 (17.0) | 12 (24.0) | 0.27 |
| Q wave | 2 (1.5) | 2 (5.9) | 0 | 0 | 0.06 |
| Atrioventricular block | 1 (0.8) | 0 | 0 | 1 (2.0) | 0.28 |
| Laboratory measurements | |||||
| Peak troponin, μg/L | 2.1 ± 5 | 3.6 ± 6.1 | 2.9 ± 6.3 | 0.5 ± 0.623 | 0.013 |
| Leucocytes on admission, G/L | 9.3 ± 3.6 | 9.3 ± 3.9 | 9.2 ± 3.5 | 9.2 ± 3.4 | 0.99 |
| CRP on admission, mg/L | 28.2 ± 41.1 | 8.8 ± 18.9 | 39.5 ± 38.41 | 31.0 ± 50.42 | 0.004 |
| Coronary atheroma, n (%) | 45 (34.6) | 15 (45.5) | 14 (29.8) | 16 (32.0) | 0.31 |
| β-blocker use, n (%) | |||||
| During hospitalization | 91 (69.5) | 27 (79.4) | 31 (66.0) | 33 (66.0) | 0.34 |
| At 1 yr | 16 (12.2) | 10 (29.4) | 1 (2.1) | 5 (10.0) | 0.05 |
| ACEI use, n (%) | |||||
| During hospitalization | 56 (43.1) | 16 (48.5) | 16 (34.0) | 24 (48.0) | 0.29 |
| At 1 yr | 17 (13.0) | 10 (29.4) | 1 (2.1)1 | 6 (12.0) | 0.042 |
P significant between MI and myocarditis groups;
P significant between MI and “no LGE” groups;
P significant between myocarditis and “no LGE” groups. MI: Myocardial infarction; LGE: Late gadolinium enhancement; ACEI: Angiotensin converting enzyme inhibitor; CHD: Coronary heart disease; CRP: C-reactive protein.
Patients classed in the myocarditis group were younger (P < 0.001) and more frequently male (P < 0.005) than the others. No differences in cardiovascular risk factor were noted.
The median time between symptom onset and hospital admission was 9 h (2; 24). Patients from the MI group were admitted to the hospital more quickly than those in the myocarditis group (P = 0.002).
The prevalence of ECG abnormalities was 69.5%, predominantly consisting of STE (35.7%) and T-wave inversion (23.1%). There were no differences in the frequency of ECG abnormalities between the groups.
Troponin rates were lower in the “no LGE” group compared to those of the MI and myocarditis groups (P = 0.001 and P = 0.013, respectively).
The MI patients presented with lower C-reactive protein (CRP) rates than the myocarditis and “no LGE” patients (P < 0.001 and P = 0.020, respectively). Elevated CRP was observed in 42 (93.3%), 22 (66.7%), and 37 (82.2%) patients from the myocarditis, MI, and “no LGE” groups, respectively.
During hospitalization, 69% of patients were treated with β-blockers and 43% received angiotensin-converting enzyme inhibitors (ACEIs), with no difference observed between the different groups. At 1 year, only 13% of the patients were receiving either β-blockers or ACEIs, with a trend for higher rates of treatment seen in the MI group, though this difference was not statistically significant. Notably, no other antiarrhythmic drug than β-blockers was given at any time of the study.
The mean LVEF was 58.6% ± 8.2%. No significant differences were observed in either LVEF or LV end-diastolic volume between the groups. The “no LGE” group exhibited smaller LV end-systolic volumes than those of the MI and myocarditis patients. LV wall thickening was more often altered in the MI group. Moreover, the MI patients presented with higher rates of akinesia compared to the myocarditis and “no LGE” patients (58.8% vs 4.3% and 2.0%, P < 0.001). Pericardial effusion was detected nonspecifically in 20 patients (15.4%) (Table 2).
Table 2.
Cardiac magnetic resonance parameters
| Total (n = 131) | MI (n = 34) | Myocarditis (n = 47) | “no LGE” (n = 50) | P | |
| LVEF (%) | 58.6 ± 8.2 | 57.1 ± 7.8 | 57.9 ± 8.6 | 60.2 ± 7.8 | 0.18 |
| LVEDV (mL) | 154.7 ± 37.7 | 158.8 ± 41 | 159.6 ± 28.6 | 147.3 ± 42.1 | 0.21 |
| LVESV (mL) | 64.5 ± 22.1 | 69.5 ± 25.5 | 67.1 ± 16.9 | 58.6 ± 22.923 | 0.048 |
| LV wall thickening abnormality, n (%) | 62 (47.3) | 30 (88.2) | 22 (46.8)1 | 10 (20.0)23 | < 0.001 |
| Hypokinetic extent, segments | 1.1 ± 1.5 | 2 ± 1.6 | 1.2 ± 1.61 | 0.4 ± 1.123 | < 0.001 |
| 0 segment, n (%) | 69 (52.7) | 4 (11.8) | 25 (53.2) | 40 (80.0) | |
| 1-2 segments, n (%) | 43 (32.9) | 20 (58.8) | 14 (29.8) | 9 (18.0) | |
| > 2 segments, n (%) | 19 (14.5) | 10 (29.4) | 8 (17.0) | 1 (2.0) | |
| LGE transmural extent, segments | 0.9 ± 0.8 | 3.6 ± 0.6 | 2.1 ± 0.91 | 0 | < 0.001 |
| < 50%, n (%) | 83 (73.4) | 2 (5.9) | 31 (66.0) | 0 | |
| > 50%, n (%) | 48 (36.6) | 32 (94.1) | 16 (34.0) | 0 | |
| LGE transversal extent, segments | 1.9 ± 2.2 | 1.9 ± 1.3 | 3.5 ± 2.4 | 023 | < 0.001 |
| 0 segment, n (%) | 50 (38.2) | 0 | 0 | 50 (100.0) | |
| 1-2 segments, n (%) | 46 (35.1) | 26 (76.5) | 20 (42.6) | 0 | |
| > 2 segments, n (%) | 35 (26.7) | 8 (23.5) | 27 (57.4) | 0 | |
| LGE/CINE concordance, n (%) | 49 (37.4) | 30 (88.2) | 19 (41.3)1 | 0 | < 0.001 |
| Pericardial effusion, n (%) | 20 (15.3) | 4 (11.8) | 8 (17.4) | 8 (16) | 0.8 |
P significant between MI and myocarditis groups;
P significant between MI and “no LGE” groups;
P significant between myocarditis and “no LGE” groups. MI: Myocardial infarction; LGE: Late gadolinium enhancement; LV: Left ventricle; LVEDV: Left ventricular end diastolic volume; LVESV: Left ventricular end systolic volume; LVEF: Left ventricular ejection fraction.
During hospitalization, 18 patients experienced a ventricular arrhythmic event, consisting of 17 VTs and one ventricular fibrillation (Tables 3 and 4). No differences were observed between the MI and the myocarditis groups (n = 9, 26.5% vs n = 6, 12.8%, P = 0.10), whereas the MI patients exhibited higher rates of VT than the “no LGE” group (n = 2, 4.0%, P = 0.001). All these events occurred in the early stages of hospitalization, with a median onset of 1 (0-1) d. One episode of ventricular fibrillation occurred in a myocarditis patient on day 3, who was successfully defibrillated. We found no differences in cardiovascular risk factor, CRP, coronary atheroma, or use of β-blockers and ACEIs in correlation with the ventricular events.
Table 3.
Acute event rates
| All patients (n = 131) | MI (n = 34) | Myocarditis (n = 47) | “No LGE” (n = 50) | P | |
| Ventricular event, n (%) | 18 (13.8) | 9 (26.5) | 7 (14.9) | 2 (4.0)1 | 0.013 |
| VT, n (%) | 17 (13.0) | 9 (26.5) | 6 (12.8) | 2 (4.0)1 | 0.011 |
| Prolonged VT or VF, n (%) | 8 (6.1) | 4 (11.8) | 4 (8.5) | 01 | 0.06 |
| VF, n (%) | 1 (0.8) | 0 | 1 (2.1) | 0 | 0.41 |
P significant between MI and “no LGE” groups. MI: Myocardial infarction; LGE: Late gadolinium enhancement; VF: Ventricular fibrillation; VT: Ventricular tachycardia.
Table 4.
Acute event characteristics
| Patient | Gender | Age (yr) | ECG | Troponin (μg/L) | LVEF (%) | Diagnosis | LGE transmural extent1 | β-blocker use | ACEI use | VT | VF | CMR delay (d) | Ventricular arrhythmia length |
| 13 | Male | 39 | STE | 0.3 | 51.7 | MI | 4 | 1 | 0 | 1 | 0 | 1 | 10 VPBs |
| 52 | Male | 45 | Normal | 0.4 | 57.7 | MI | 3 | 0 | 0 | 1 | 0 | 1 | 10 VPBs |
| 53 | Female | 30 | STE | 17.3 | 45.5 | MI | 4 | 1 | 1 | 1 | 0 | 0 | 30 VPBs |
| 63 | Male | 51 | Normal | 0.3 | 57.8 | MI | 3 | 1 | 1 | 1 | 0 | 1 | 6 VPBs |
| 64 | Male | 56 | STE | 1.6 | 61.2 | MI | 4 | 1 | 1 | 1 | 0 | 1 | 15 VPBs |
| 75 | Male | 32 | STE | 0.8 | 63.2 | MI | 4 | 1 | 0 | 1 | 0 | 0 | 7 VPBs |
| 92 | Male | 57 | STD | 0.4 | 48.1 | MI | 3 | 1 | 0 | 1 | 0 | 0 | 6 VPBs |
| 128 | Female | 55 | STD | 0.6 | 49.8 | MI | 4 | 1 | 1 | 1 | 0 | 1 | 5 VPBs |
| 97 | Male | 83 | STD | 0.2 | 46.5 | MI | 2 | 1 | 1 | 1 | 0 | 0 | 6 VPBs |
| 3 | Male | 45 | STE | 0.1 | 66.9 | Myocarditis | 3 | 1 | 0 | 1 | 0 | 2 | 9 VPBs |
| 37 | Male | 40 | STE | 4.8 | 56.1 | Myocarditis | 2 | 0 | 1 | 1 | 0 | 2 | 5 VPBs |
| 44 | Male | 25 | STE | 36.6 | 65.7 | Myocarditis | 3 | 0 | 0 | 0 | 1 | 3 | VF |
| 94 | Male | 28 | STE | 1.4 | 57.1 | Myocarditis | 2 | 1 | 0 | 1 | 0 | 0 | 4 VPBs |
| 76 | Male | 53 | STE | 1.4 | 69.6 | Myocarditis | 2 | 1 | 1 | 1 | 0 | 0 | 7 VPBs |
| 104 | Female | 42 | STE | 21.2 | 57.1 | Myocarditis | 4 | 1 | 0 | 1 | 0 | 1 | 13 VPBs |
| 131 | Female | 31 | STD | 0.6 | 51.7 | Myocarditis | 1 | 1 | 0 | 1 | 0 | 1 | 8 VPBs |
| 80 | Male | 34 | STE | 0.4 | 53.1 | No LGE | 0 | 0 | 1 | 1 | 0 | 0 | 7 VPBs |
| 125 | Male | 56 | Normal | 0.1 | 62.4 | No LGE | 0 | 1 | 1 | 1 | 0 | 4 | 3 VPBs |
LGE transmural extent: 0 = 0%, 1 = 0%-25%, 2 = 25%-50%, 3 = 50%-75%, and 4 > 75%. LGE: Late gadolinium enhancement; ECG: Electrocardiography; LVEF: Left ventricular ejection fraction; STD: ST-segment depression; STE: ST-segment elevation; VT: Ventricular tachycardia; VF: Ventricular fibrillation; VPBs: Ventricular premature beats; MI: Myocardial infarction; ACEI: Angiotensin-converting enzyme inhibitor.
The transmural extent of LGE was more marked in the MI group (P < 0.001). An LGE transmural extent > 50% was observed in 32 patients (94.1%) in the MI group, in contrast to 16 patients (34.0%) in the myocarditis group (P < 0.001). LGE was more contained in the MI group, exhibiting lower transversal extents than the myocarditis group. Only eight patients (23.5%) were found to have more than two segments with LGE presence in the MI group, vs 27 (57.4%) in the myocarditis group (P = 0.002).
Of note, the concordance among Cine and LGE data was higher in the MI group compared to the myocarditis group (88.2% vs 41.3%, P < 0.001).
The multivariate analysis demonstrated that STE [OR = 5.72 (1.77-18.46), P = 0.004] and LGE transmural extent [OR = 1.50 (1.02-2.20), P = 0.039] were both independently related to ventricular events during hospitalization (Table 5). STE presented high sensitivity for the diagnosis of prolonged VT (83%), achieving a specificity of 77% and negative predictive value of 92%.
Table 5.
Univariate and multivariate analysis for ventricular arrhythmia
|
Univariate analysis |
Multivariate analysis |
|||
| Odds ratio (95%CI) | P | Odds ratio (95%CI) | P | |
| STE | 4.65 (1.61-13.40) | 0.004 | 5.72 (1.77-18.46) | 0.004 |
| STD | - | 0.06 | ||
| T-wave inversion | - | 0.99 | ||
| Troponin | 1.10 (1.02-1.20) | 0.02 | - | 0.27 |
| Hypokinetic extent | - | 0.09 | ||
| LGE transmural extent | 1.52 (1.08-2.15) | 0.017 | 1.50 (1.02-2.20) | 0.039 |
| LVEF | - | 0.31 | ||
| LVEDV | - | 0.3 | ||
| LVESV | - | 0.17 | ||
| Pericardial effusion | - | 0.24 | ||
| Coronary atheroma | - | 0.68 | ||
| CMR diagnosis | - | 0.12 | ||
| MI or myocarditis | 0.17 (0.04-0.77) | 0.022 | ||
LVEDV: Left ventricular end-diastolic volume; LVESV: Left ventricular end-systolic volume; LVEF: Left ventricular ejection fraction; STD: ST-segment depression; STE: ST-segment elevation; MI: Myocardial infarction; CMR: Cardiac magnetic resonance.
At 1 year, no patients presented with sudden cardiac death or recurrence of symptomatic ventricular event. One patient died of cancer.
DISCUSSION
The key point of the study is to demonstrate that none of the patients presenting with MINOCA and normal LVEF died of cardiovascular death or sudden cardiac death following the initial phase of hospitalization. ST segment elevation and LGE extent were the best predictors of in-hospital ventricular arrhythmic events, irrespective of the etiology.
During hospitalization, 13.8% of the patients experienced a ventricular arrhythmic event, primarily consisting of VT, with one case of ventricular fibrillation. Most of these events occurred during the first 48 h following symptom onset. These results are consistent with the literature, with previous reports of similar rates of early arrhythmic outcomes among patients with MINOCA[7,12-14] or in cases of myocarditis[15]. Our study demonstrated that, in the absence of decreased LVEF, MI and myocarditis patients are affected by the same arrhythmic risks. Following the initial phase of the disease, where the risk of ventricular arrhythmia is present, the arrhythmic risk appears very low.
The initial arrhythmic risk of myocarditis is well known and studies have shown that in patients < 35 years, myocarditis was the second most common identified cause of sudden cardiac death after coronary artery disease[16].
Our study demonstrated that STE is an independent predictor of ventricular arrhythmia at early-stage disease, achieving a good negative predictive value of 92% for prolonged VT. Data on the prognostic role of ECG abnormalities are currently scarce. This finding was assumed to be in relation to the transient character of the ST changes in myocarditis[17], as well as the weak relationship between STE and LGE localization[17,18].
The adverse prognostic value of LGE-CMR in ischemic and non-ischemic cardiomyopathy has been proven in patients with reduced LVEF[19]. Conversely, Grün et al[20] demonstrated a relevant cardiac mortality of 15% in 203 patients with myocarditis, which was primarily driven by the presence of LGE. Nevertheless, a large proportion of their patients presented with heart failure and decreased LVEF. In another report of fewer selected patients with suspected myocarditis, LGE was found in only 28% of cases, and LGE and LVEF were defined as predictors for a composite of cardiac death and heart failure[15]. In our study, considering the excellent prognosis of the population, we have found that LGE is not useful for evaluating cardiac outcomes.
Despite this, however, the transmural extent of LGE was identified as an independent predictor for ventricular arrhythmia during the acute phase of the disease. This could therefore be of great value, if performed very early after the hospitalization, to identify at-risk patients requiring special attention and perhaps more prolonged rhythmic monitoring.
In our study, CMR imaging provided etiological diagnosis in 81 patients (61.8%), which is consistent with previous reports[21,22]. Clinical evaluations including biopsy have demonstrated that myocarditis could be present in patients with normal CMR results in 32% to 47% of cases[20,23]. It is likely that the frequency of myocarditis was underestimated in our study, especially in the “no LGE” group, as suggested by the CRP levels that were similar to those with myocarditis. It has been suggested that global LV involvement in myocarditis may be the cause of LGE absence[24].
The choice of medical management strategy for these patients remains controversial. There is little data, which is also conflicted, on the use of a secondary prevention treatment involving at least β-blockers in patients affected by myocarditis mimicking acute MI[25]. In our cohort, 69.5% of the patients received β-blockers during hospitalization, reflecting the management of a recent MI. Regarding the relatively-high percentage of patients who experienced ventricular arrhythmia, there is no doubt that this treatment is of interest during this period of time.
In our study, the absence of sudden cardiac death at 1 year suggests that β-blockers should not be continued over the long term, even if the β-blocker treatment had already been stopped in most of the population, with only one myocarditis patient still undergoing treatment. The need to prolong the treatment immediately after the hospital discharge is, however, a more controversial topic. LGE and ST segment elevation may represent valuable tools to stratify the risk of these patients and select those eligible for secondary prevention therapy.
The first limitation of our study was the sample size, which was too small to detect any statistical difference between the myocarditis group and the MI group. Secondly, the retrospective study design also, evidently, posed a limit. Finally, no systematic rhythm monitoring was organized to screen the last recurrence of ventricular events after discharge. Similarly, the therapeutic management that may be chosen based on such preclinical events was left to the discretion of the referring cardiologist, and the use of β-blockers or ACEIs was extremely low at follow-up (12.2% and 13.0%, respectively). Nevertheless, none of our patients presented with sudden cardiac death or severe arrhythmia during follow-up. We must also admit that prognosis may differ by ethnicity but are not stressed in our study.
In conclusion, our study indicated that patients with MINOCA and normal LVEF did not present any 1-year CV events, particularly no sudden cardiac death, after hospital discharge. Most of them had even stopped treatment at 1-year follow-up.
STE on admission and LGE transmural extent appear to be good markers for identifying patients at risk of ventricular events in the early stages of disease.
COMMENTS
Background
About 1%-12% of patients with chest pain and cardiac troponin elevation present with normal coronary arteries on angiography analysis. While the arrhythmic prognosis of myocardial infarction with abnormal coronary angiography is well known, there is little data concerning myocardial infarction with non-obstructive coronary arteries (MINOCA). This study sought to evaluate the risk of ventricular arrhythmias of presumed low-risk MINOCA patients, based on the diagnosis class established by cardiac magnetic resonance (CMR) imaging.
Research frontiers
Numerous patients are affected by MINOCA, and yet prognosis is expected to be favorable as soon as left-ventricular ejection fraction (LVEF) is good. Nevertheless, the mere presence of a myocardial injury/scar/fibrosis, let us empirically dare for the occurrence of ventricular arrhythmia.
Innovations and breakthroughs
CMR was used systematically to identify MINOCA’s aetiology, but also to assess myocardial injury and its extent. Continuous electrocardiography was also systematically performed to solve the question of arrhythmic risk during the first days after symptoms onset. It was continued by a one-year clinical follow-up. Nevertheless, this study does not focus on medical therapy, including the effect of β-blockers on ventricular arrhythmia.
Applications
This study provides evidence on the good prognosis (including arrhythmic events) presented by patients with MINOCA and preserved LVEF. When ventricular arrhythmias occurred, they correlated with myocardial injury, as assessed by transmural late gadolinium enhancement (LGE) by CMR. Therefore, this study provides reassuring data about survival but also point out the need to stress the effect of antiarrhythmic therapies on the newly-identified risk markers that are LGE and ST segment elevation.
Terminology
MINOCA is a recent terminology that relates to ischemic injuries, myocarditis, tako-tsubo cardiomyopathy, hypertrophic cardiomyopathy, dilated cardiomyopathy, and other causes such as pericarditis and amyloidosis.
Peer-review
This paper is interesting, novel, and an overall well-conducted study. It provides a timely study on this field.
Footnotes
Supported by The French Federation of Cardiology (Fédération française de Cardiologie).
Institutional review board statement: The study was reviewed and approved by the ethics committee of the University Hospital of Angers.
Informed consent statement: All patients gave their informed consent for the completion of the study.
Conflict-of-interest statement: The authors have no conflict of interest to disclose.
Data sharing statement: Technical details and statistical methods are available with the corresponding author at lobiere@chu-anger.fr.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: France
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: July 13, 2016
First decision: September 2, 2016
Article in press: December 19, 2016
P- Reviewer: Lee TM, Lymperopoulos A S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
References
- 1.Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, Mariani R, Gunson K, Jui J. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kardasz I, De Caterina R. Myocardial infarction with normal coronary arteries: a conundrum with multiple aetiologies and variable prognosis: an update. J Intern Med. 2007;261:330–348. doi: 10.1111/j.1365-2796.2007.01788.x. [DOI] [PubMed] [Google Scholar]
- 3.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccini JP, Berger JS, Brown DL. Early sustained ventricular arrhythmias complicating acute myocardial infarction. Am J Med. 2008;121:797–804. doi: 10.1016/j.amjmed.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khatib SM, Stebbins AL, Califf RM, Lee KL, Granger CB, White HD, Armstrong PW, Topol EJ, Ohman EM. Sustained ventricular arrhythmias and mortality among patients with acute myocardial infarction: results from the GUSTO-III trial. Am Heart J. 2003;145:515–521. doi: 10.1067/mhj.2003.170. [DOI] [PubMed] [Google Scholar]
- 6.Chopard R, Jehl J, Dutheil J, Genon VD, Seronde MF, Kastler B, Schiele F, Meneveau N. Evolution of acute coronary syndrome with normal coronary arteries and normal cardiac magnetic resonance imaging. Arch Cardiovasc Dis. 2011;104:509–517. doi: 10.1016/j.acvd.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Raymond R, Lynch J, Underwood D, Leatherman J, Razavi M. Myocardial infarction and normal coronary arteriography: a 10 year clinical and risk analysis of 74 patients. J Am Coll Cardiol. 1988;11:471–477. doi: 10.1016/0735-1097(88)91519-7. [DOI] [PubMed] [Google Scholar]
- 8.Katritsis DG, Zareba W, Camm AJ. Nonsustained ventricular tachycardia. J Am Coll Cardiol. 2012;60:1993–2004. doi: 10.1016/j.jacc.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 9.Scirica BM, Braunwald E, Belardinelli L, Hedgepeth CM, Spinar J, Wang W, Qin J, Karwatowska-Prokopczuk E, Verheugt FW, Morrow DA. Relationship between nonsustained ventricular tachycardia after non-ST-elevation acute coronary syndrome and sudden cardiac death: observations from the metabolic efficiency with ranolazine for less ischemia in non-ST-elevation acute coronary syndrome-thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2010;122:455–462. doi: 10.1161/CIRCULATIONAHA.110.937136. [DOI] [PubMed] [Google Scholar]
- 10.Bière L, Donal E, Terrien G, Kervio G, Willoteaux S, Furber A, Prunier F. Longitudinal strain is a marker of microvascular obstruction and infarct size in patients with acute ST-segment elevation myocardial infarction. PLoS One. 2014;9:e86959. doi: 10.1371/journal.pone.0086959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 12.Da Costa A, Isaaz K, Faure E, Mourot S, Cerisier A, Lamaud M. Clinical characteristics, aetiological factors and long-term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3-year follow-up study of 91 patients. Eur Heart J. 2001;22:1459–1465. doi: 10.1053/euhj.2000.2553. [DOI] [PubMed] [Google Scholar]
- 13.Kang WY, Jeong MH, Ahn YK, Kim JH, Chae SC, Kim YJ, Hur SH, Seong IW, Hong TJ, Choi DH, et al. Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe? Int J Cardiol. 2011;146:207–212. doi: 10.1016/j.ijcard.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Larsen AI, Galbraith PD, Ghali WA, Norris CM, Graham MM, Knudtson ML. Characteristics and outcomes of patients with acute myocardial infarction and angiographically normal coronary arteries. Am J Cardiol. 2005;95:261–263. doi: 10.1016/j.amjcard.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Schumm J, Greulich S, Wagner A, Grün S, Ong P, Bentz K, Klingel K, Kandolf R, Bruder O, Schneider S, et al. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson. 2014;16:14. doi: 10.1186/1532-429X-16-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkel BG, Holst AG, Theilade J, Kristensen IB, Thomsen JL, Ottesen GL, Bundgaard H, Svendsen JH, Haunsø S, Tfelt-Hansen J. Nationwide study of sudden cardiac death in persons aged 1-35 years. Eur Heart J. 2011;32:983–990. doi: 10.1093/eurheartj/ehq428. [DOI] [PubMed] [Google Scholar]
- 17.Di Bella G, Florian A, Oreto L, Napolitano C, Todaro MC, Donato R, Calamelli S, Camastra GS, Zito C, Carerj S, et al. Electrocardiographic findings and myocardial damage in acute myocarditis detected by cardiac magnetic resonance. Clin Res Cardiol. 2012;101:617–624. doi: 10.1007/s00392-012-0433-5. [DOI] [PubMed] [Google Scholar]
- 18.Meléndez-Ramírez G, de Micheli A, Soto ME, Meave-González A, Kimura-Hayama E, Alcántara M, González-Pacheco H. Agreement between ST elevation and late enhancement evaluated by MRI in patients with acute myocarditis. J Electrocardiol. 2014;47:212–218. doi: 10.1016/j.jelectrocard.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marbán E, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert EM, Hill S, Ong P, Klingel K, et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–1615. doi: 10.1016/j.jacc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, Pennell DJ, Prasad SK. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J. 2007;28:1242–1249. doi: 10.1093/eurheartj/ehm113. [DOI] [PubMed] [Google Scholar]
- 22.Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–870. doi: 10.1161/CIRCULATIONAHA.114.011201. [DOI] [PubMed] [Google Scholar]
- 23.Francone M, Chimenti C, Galea N, Scopelliti F, Verardo R, Galea R, Carbone I, Catalano C, Fedele F, Frustaci A. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC Cardiovasc Imaging. 2014;7:254–263. doi: 10.1016/j.jcmg.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, et al. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson. 2014;16:36. doi: 10.1186/1532-429X-16-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kindermann I, Kindermann M, Kandolf R, Klingel K, Bültmann B, Müller T, Lindinger A, Böhm M. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–648. doi: 10.1161/CIRCULATIONAHA.108.769489. [DOI] [PubMed] [Google Scholar]


