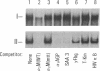Abstract
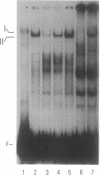
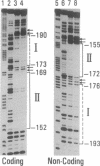
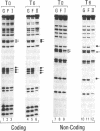
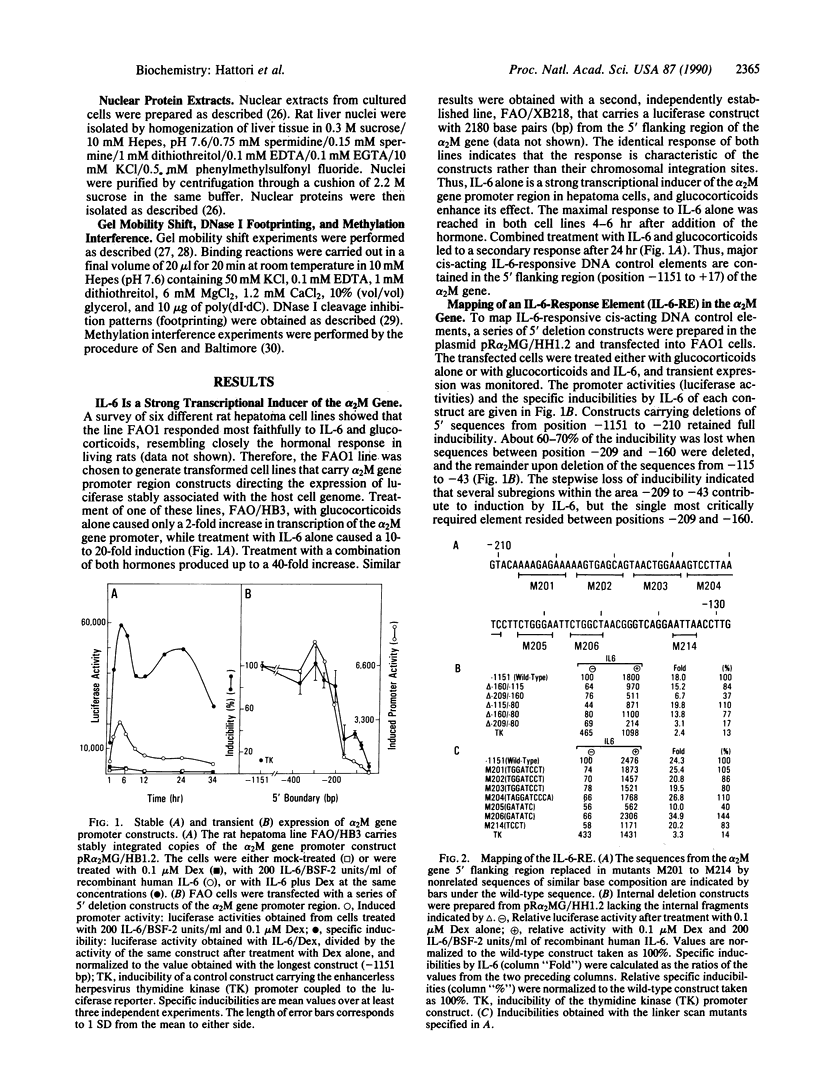
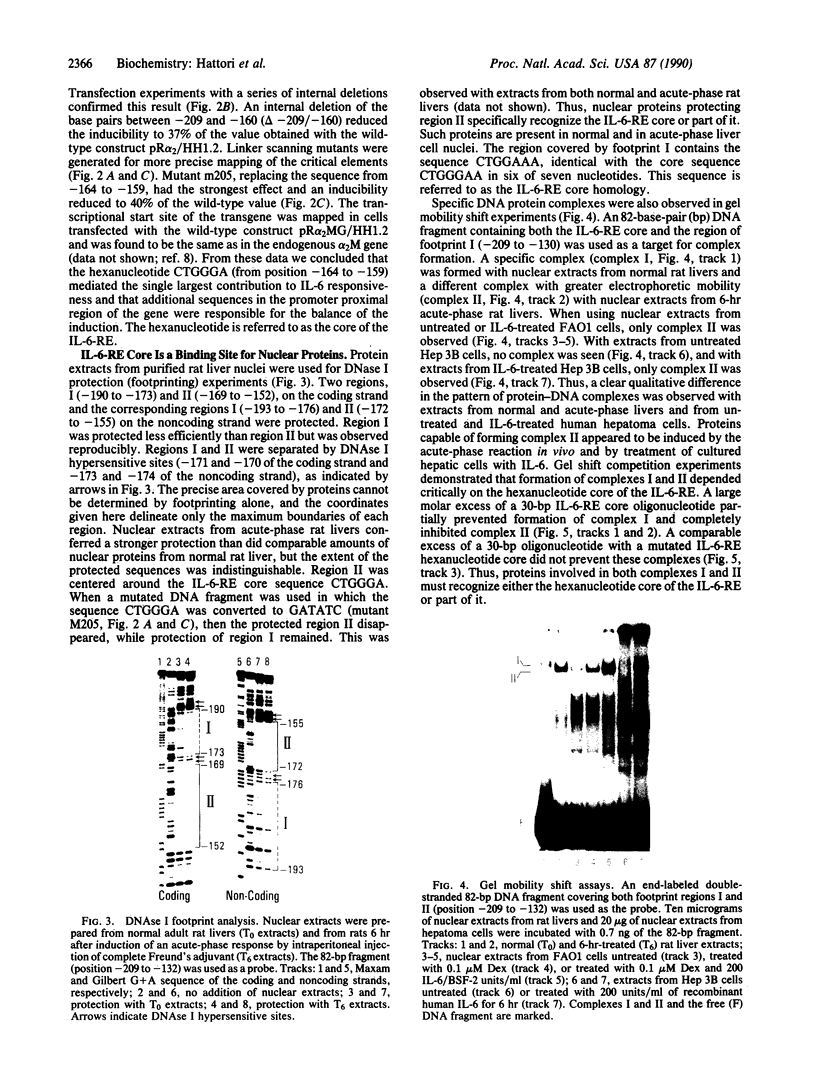
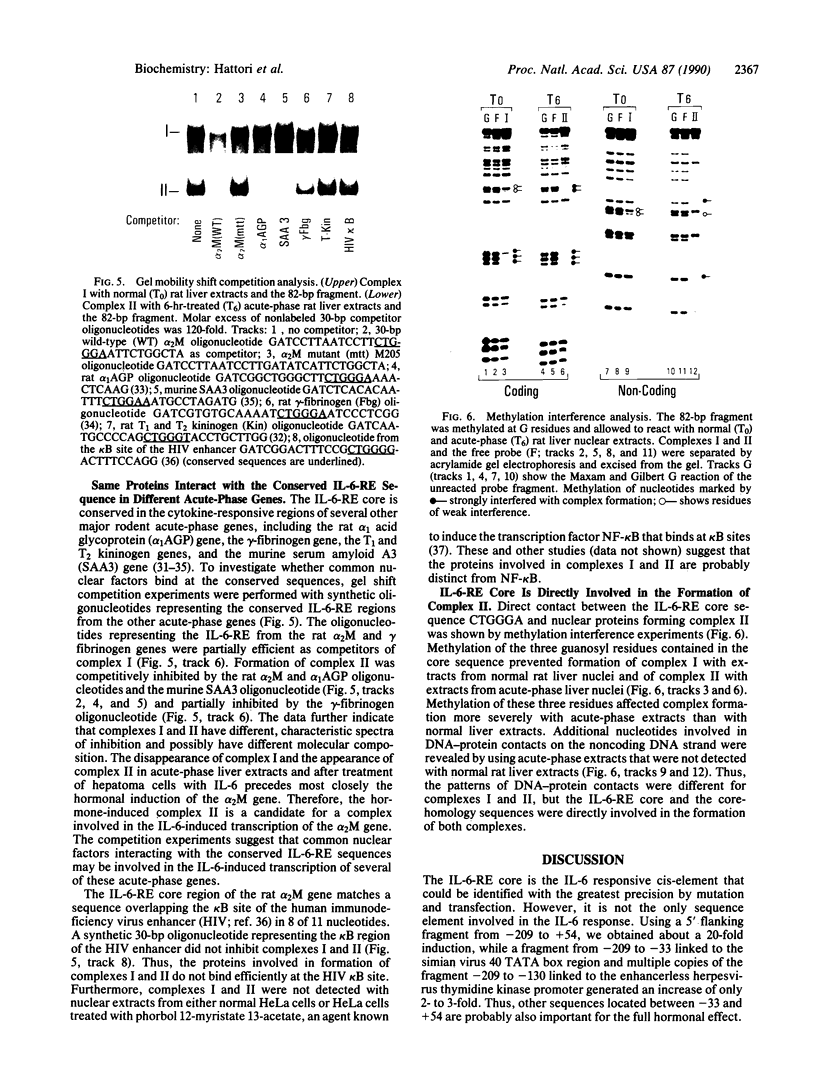
Interleukin 6 (IL-6) was established as a transcriptional inducer of the rat alpha 2-macroglobulin gene, a prototype liver acute-phase gene. Maximum induction occurred when the 5' flanking sequences of this gene (position -209 to -43) directed expression from the gene's own TATA box and transcription start site. Removal of the hexanucleotide CTGGGA (position -164 to -159) abolished 60-70% of the hormonal induction in FAO1 rat hepatoma cells. This hexanucleotide was defined as the IL-6 response element (IL-6-RE). The IL-6-RE is well conserved in the cytokine-responsive regions of other acute-phase genes and serves as a binding site for nuclear proteins. A characteristic DNA-protein complex (complex I) was formed with nuclear proteins from normal rat livers. A different, hormone-inducible complex (complex II) was assembled specifically with nuclear proteins from acute-phase rat livers or from IL-6-treated human Hep 3B hepatoma cells. Complex II was competitively inhibited by oligonucleotides representing the conserved IL-6-RE sequence from other acute-phase genes. Thus, the proteins building complex II likely participate in a general signal transduction mechanism mediating the transcriptional activation by IL-6 of several acute-phase genes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcone R., Gualandi G., Ciliberto G. Identification of sequences responsible for acute-phase induction of human C-reactive protein. Nucleic Acids Res. 1988 Apr 25;16(8):3195–3207. doi: 10.1093/nar/16.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J., Tran-Thi T. A., Northoff H., Hirsch F., Schlayer H. J., Gerok W., Heinrich P. C. The acute-phase induction of alpha 2-macroglobulin in rat hepatocyte primary cultures: action of a hepatocyte-stimulating factor, triiodothyronine and dexamethasone. Eur J Cell Biol. 1986 Mar;40(1):86–93. [PubMed] [Google Scholar]
- Baumann H. Hepatic acute phase reaction in vivo and in vitro. In Vitro Cell Dev Biol. 1989 Feb;25(2):115–126. doi: 10.1007/BF02626167. [DOI] [PubMed] [Google Scholar]
- Baumann H., Prowse K. R., Marinković S., Won K. A., Jahreis G. P. Stimulation of hepatic acute phase response by cytokines and glucocorticoids. Ann N Y Acad Sci. 1989;557:280-95, discussion 295-6. doi: 10.1111/j.1749-6632.1989.tb24021.x. [DOI] [PubMed] [Google Scholar]
- Birch H. E., Schreiber G. Transcriptional regulation of plasma protein synthesis during inflammation. J Biol Chem. 1986 Jun 25;261(18):8077–8080. [PubMed] [Google Scholar]
- Borth W., Luger T. A. Identification of alpha 2-macroglobulin as a cytokine binding plasma protein. Binding of interleukin-1 beta to "F" alpha 2-macroglobulin. J Biol Chem. 1989 Apr 5;264(10):5818–5825. [PubMed] [Google Scholar]
- Dennis P. A., Saksela O., Harpel P., Rifkin D. B. Alpha 2-macroglobulin is a binding protein for basic fibroblast growth factor. J Biol Chem. 1989 May 5;264(13):7210–7216. [PubMed] [Google Scholar]
- Deschatrette J., Weiss M. C. Characterization of differentiated and dedifferentiated clones from a rat hepatoma. Biochimie. 1974;56(11-12):1603–1611. doi: 10.1016/s0300-9084(75)80286-0. [DOI] [PubMed] [Google Scholar]
- Fey G. H., Hattori M., Northemann W., Abraham L. J., Baumann M., Braciak T. A., Fletcher R. G., Gauldie J., Lee F., Reymond M. F. Regulation of rat liver acute phase genes by interleukin-6 and production of hepatocyte stimulating factors by rat hepatoma cells. Ann N Y Acad Sci. 1989;557:317–331. doi: 10.1111/j.1749-6632.1989.tb24024.x. [DOI] [PubMed] [Google Scholar]
- Fordis C. M., Howard B. H. Use of the CAT reporter gene for optimization of gene transfer into eukaryotic cells. Methods Enzymol. 1987;151:382–397. doi: 10.1016/s0076-6879(87)51030-8. [DOI] [PubMed] [Google Scholar]
- Fowlkes D. M., Mullis N. T., Comeau C. M., Crabtree G. R. Potential basis for regulation of the coordinately expressed fibrinogen genes: homology in the 5' flanking regions. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2313–2316. doi: 10.1073/pnas.81.8.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M. R., Shiels B. R., Northemann W., de Bruijn M. H., Kan C. C., Chain A. C., Noonan D. J., Fey G. H. Sequence of rat liver alpha 2-macroglobulin and acute phase control of its messenger RNA. J Biol Chem. 1987 Jan 5;262(1):446–454. [PubMed] [Google Scholar]
- Hayashida K., Okubo H., Noguchi M., Yoshida H., Kangawa K., Matsuo H., Sakaki Y. Molecular cloning of DNA complementary to rat alpha 2-macroglobulin mRNA. J Biol Chem. 1985 Nov 15;260(26):14224–14229. [PubMed] [Google Scholar]
- Helle M., Brakenhoff J. P., De Groot E. R., Aarden L. A. Interleukin 6 is involved in interleukin 1-induced activities. Eur J Immunol. 1988 Jun;18(6):957–959. doi: 10.1002/eji.1830180619. [DOI] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Specific covalent binding of platelet-derived growth factor to human plasma alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1984 Jan;81(2):342–346. doi: 10.1073/pnas.81.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R., Kitamura N., Ohkubo H., Nakanishi S. Differing utilization of homologous transcription initiation sites of rat K and T kininogen genes under inflammation condition. J Biol Chem. 1987 Feb 15;262(5):2345–2351. [PubMed] [Google Scholar]
- Kishimoto T., Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1988;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Kunz D., Zimmermann R., Heisig M., Heinrich P. C. Identification of the promoter sequences involved in the interleukin-6 dependent expression of the rat alpha 2-macroglobulin gene. Nucleic Acids Res. 1989 Feb 11;17(3):1121–1138. doi: 10.1093/nar/17.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Lowell C. A., Potter D. A., Stearman R. S., Morrow J. F. Structure of the murine serum amyloid A gene family. Gene conversion. J Biol Chem. 1986 Jun 25;261(18):8442–8452. [PubMed] [Google Scholar]
- Matsuda T., Hirano T., Nagasawa S., Kishimoto T. Identification of alpha 2-macroglobulin as a carrier protein for IL-6. J Immunol. 1989 Jan 1;142(1):148–152. [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Northemann W., Heisig M., Kunz D., Heinrich P. C. Molecular cloning of cDNA sequences for rat alpha 2-macroglobulin and measurement of its transcription during experimental inflammation. J Biol Chem. 1985 May 25;260(10):6200–6205. [PubMed] [Google Scholar]
- Northemann W., Shiels B. R., Braciak T. A., Fey G. H. Structure and negative transcriptional regulation by glucocorticoids of the acute-phase rat alpha 1-inhibitor III gene. Biochemistry. 1989 Jan 10;28(1):84–95. doi: 10.1021/bi00427a013. [DOI] [PubMed] [Google Scholar]
- Northemann W., Shiels B. R., Braciak T. A., Hanson R. W., Heinrich P. C., Fey G. H. Structure and acute-phase regulation of the rat alpha 2-macroglobulin gene. Biochemistry. 1988 Dec 27;27(26):9194–9203. doi: 10.1021/bi00426a018. [DOI] [PubMed] [Google Scholar]
- Oliviero S., Cortese R. The human haptoglobin gene promoter: interleukin-6-responsive elements interact with a DNA-binding protein induced by interleukin-6. EMBO J. 1989 Apr;8(4):1145–1151. doi: 10.1002/j.1460-2075.1989.tb03485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M. O., Sperling L., Herbomel P., Yaniv M., Weiss M. C. Tissue-specific expression is conferred by a sequence from the 5' end of the rat albumin gene. EMBO J. 1984 Nov;3(11):2505–2510. doi: 10.1002/j.1460-2075.1984.tb02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli V., Cortese R. Interleukin 6 induces a liver-specific nuclear protein that binds to the promoter of acute-phase genes. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8202–8206. doi: 10.1073/pnas.86.21.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowse K. R., Baumann H. Hepatocyte-stimulating factor, beta 2 interferon, and interleukin-1 enhance expression of the rat alpha 1-acid glycoprotein gene via a distal upstream regulatory region. Mol Cell Biol. 1988 Jan;8(1):42–51. doi: 10.1128/mcb.8.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y., Hattori M., Hayashida K., Ishibashi H., Okubo H., Sakaki Y. Sequence analysis of the putative regulatory region of rat alpha 2-macroglobulin gene. Gene. 1987;57(1):73–80. doi: 10.1016/0378-1119(87)90178-8. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]