Abstract
To analyze the predictive effect of thrombelastogram (TEG) in the changes of coagulation functions of patients with traumatic brain hemorrhage, as well as to provide a practice basis for clinical guidance. 54 cases were observed from Aug. 2013–Oct. 2014. All patients received a TEG test 1d, 3d and 7d after traumatic injury. According to the statistical analysis, the comparison among the aforementioned coagulation function parameters in each group of patients, K, α and Ma all had significant differences. In the comparison between different time points in the same group, there was still a significant difference. Compared to the patients, the changes of R and K reached the lowest at 1d and the highest at 3d, but there was no significant difference between two groups at 7d. The changes of α and Ma reached its highest at 1d and the lowest at 3d after traumatic injury, but there was no significant difference at 7d. There was some difference in changes of coagulation functions between all groups. The former was more serious and the changes of coagulation functions had certain regularity, i.e., after traumatic injury, 1d showed a hypercoagulable state; 3d showed a hypocoagulable state; the coagulation functions of 7d returned to normal.
Keywords: TEG, traumatic brain hemorrhage, coagulation functions, predictive effect
1 Introduction
After traumatic brain hemorrhage, the coagulation functions of patients may be dysfunctional, i.e., the coagulation system shows a hypercoagulable state due to being activated abnormally [1], thereby causing fibrinolysis. Being in a hypercoagulable state for a long term, blood may become viscous, and the blood flow may slow down, easily resulting in thrombus [2]. Clinically, a fast and efficient test method with strong specificity is needed to predict the changes of coagulation functions of patients with traumatic hemorrhage, and to provide guidance for clinical treatment [3]. Thus, as a technology for recording the blood coagulation process, TEG emerged at the right moment. It’s not only mainly applied to the research on coagulation and fibrinolysis process [4], but also applied to determining platelet functions [5]. Through analyzing the predictive effect of thrombelastogram (TEG) in the changes of coagulation functions of patients with traumatic brain hemorrhage, this research provides a practice basis for clinical guidance. The research results are hereby reported as follows.
2 Data and method
2.1 General data
54 patients with traumatic hemorrhage were admitted to our hospital from Aug. 2013 to Oct. 2014 were selected as research subjects, including 42 cases of patients with traumatic brain hemorrhage and 12 cases of patients with other traumatic hemorrhage, wherein, 40 cases were males while 14 cases were females, with an age range from 23 to 62, average age 35.6±5.8. The patients with traumatic brain hemorrhage were classified as Group A, including 29 cases of males and 13 cases of females, with an age range from 24 to 61, average age 36.8±5.3. The GCS score of all patients were not higher than 8, wherein, 15 cases got 3~5 points; 27 cases got 6~8 points. The causes of injuries were as shown as follows: 21 cases of car accidents, 7 cases of crushing injuries, 7 cases of falling injuries, and 5 cases of other injuries. The CT examination showed that all were simple brain traumas, comprising 7 cases of simple brain contusion & laceration, 17 cases of brain contusion & laceration combined with diffuse axonal injury, 11 cases of brain contusion & laceration combined with primary brain stem injury, 3 cases of brain contusion & laceration combined with right frontal lobe hematoma and 4 cases of epidural hematoma. Prothrombin time (PT), activated partial thromboplastin time (APTT) and fibrinogen coagulation time (CT) were all normal. The patients with other traumatic hemorrhage were classified as Group B, including 8 cases of males and 4 cases of females, with an age range from 23 to 62, average age 36.6±5.6. The causes of injuries were as shown as follows: 6 cases of car accidents, 3 cases of crushing injuries, 2 cases of falling injuries and 1 case of other injury. The CT examination showed that there were 6 cases of limb fracture, 4 cases of rib fracture combined with pulmonary contusion and 2 cases of crushing injury of bilateral lower extremities. Prothrombin time (PT), activated partial thromboplastin time (APTT) and fibrinogen coagulation time (CT) were all normal. 15 cases of patients with neither coagulation disorder nor blood disease (5 cases) admitted to our hospital and healthy volunteers (10 cases) were selected in the control group, including 8 cases of males and 2 cases of females, with an age range from 23 to 72, average age 31.7±6.14. According to statistical analysis, the general information of the three groups of subjects had no significant difference (P > 0.05). Thus, this research is comparable and maneuverable.
Ethical approval
The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Informed consent
Informed consent has been obtained from all individuals included in this study.
2.2 Inclusion criteria
The post-traumatic patients less than 24h, i.e., the patients admitted to hospital, with an age range from 18 to 75, who were diagnosed with simple brain trauma after receiving cranial CT or spinal MRI examination, were included in the research. All patients suffered from closed brain trauma, and their GCS score was not higher than 8, without liver dysfunction or coagulation disorder [6].
2.3 Exclusion criteria
The patients with congenital coagulation disorders and liver dysfunction were excluded in this research. In addition, the patients who took any anticoagulant 6 months prior to the onset or had taken aspirin for a long term, and the patients with antithrombin should also be excluded in the research [7].
2.4 Method
All patients in Group A and Group B underwent the collection of venous blood samples on the morning of 1d, 3d and 7d with an empty belly after getting injured, 2ml each time. For the subjects in Group C, their blood samples only needed to be collected once[8]. For all blood samples, whole-blood recalcification method was adopted to test the changes of their coagulation functions [9], specifically as follows: blood samples are directly injected into a cylinder; as the liquid blood cannot vibrate the cylinder to drive the movement of the cylinder axis, the result recorded is a straight line; when the blood gradually starts to coagulate, the cylinder vibrates; then driven by the viscous fibrous protein in the blood, it can be transmitted to the cylinder axis; accordingly, the cylinder axis and alloy wires both move [10]. The electrical signal with a changed action signal can be recorded as a swinging curve after being amplified, i.e., TEG.
2.5 Monitoring indicators
R means the response time, which is the period from the starting time when the blood is injected to the cylinder to the time when it starts to coagulate, equal to the generation time of fibrous protein at the initial stage. Normally, its reference range is from 2 to 8 min. K means the coagulation time, which is the period from the end point of R to the time when the curve amplitude reaches 20min, equal to the generation time of thrombin. Normally, its reference range is from 1 to 3min. At this moment, the elasticity of sludged blood is 25. α means the coagulation angle, which can represent the generation speed of fibrous protein. Normally, its reference range is from 55° to 78°. Ma means the maximum amplitude of thrombus, which represents the maximum solidness of fibrous protein. Normally, its reference range is from 51mm to 69 mm [11]. The TEG (5000 Series) used in this research was provided by Haemoscope Corporation, the US.
2.6 Statistical method
The statistical software SPSS 17.0 was adopted to conduct statistical analysis on all data in the research. The measurement data were represented by (±SE). One-way ANOVA was adopted for the age comparison among groups. For the comparison of coagulation functions among all groups of subjects at various time points, two-way multilevel ANOVA was adopted. Rank sum test was adopted for enumeration data. Therefore, taking P < 0.05 as data has a significant difference.
3 Result
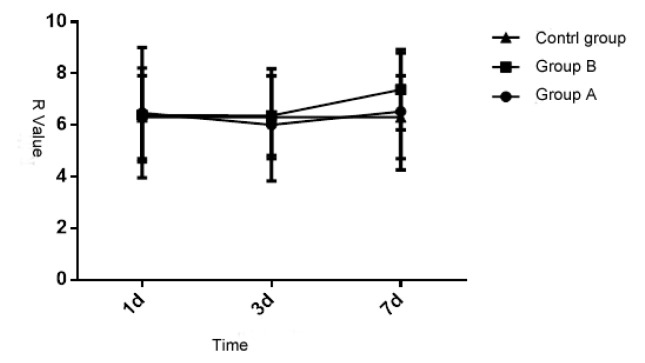
3.1 Comparison of R value of the patients in the three groups
Upon statistical analysis, the R value of the patients in three groups at different times is of significant difference (P < 0.05), but the inter-group comparison is of no significant difference (P > 0.05). Each group and measurement time have interaction (P < 0.05). See details in Table 1 and Figure 1. Thus it can be seen that the impact of different injuries on the starting process of intrinsic coagulation is of no significant difference.
Table 1.
Variance analysis of r value of the patients in three groups at different times
| Source of variation | SS | df | MS | F value | P value |
|---|---|---|---|---|---|
| Treatment | 31.420 | 2 | 15.154 | 2.194 | 0.124 |
| Measurement time | 16.007 | 2 | 7.403 | 33.620 | 0.000 |
| Treatment× Measurement time | 15.531 | 4 | 3.050 | 15.441 | 0.000 |
| Inter-group difference | 278.050 | 38 | 6.304 | ||
| Intra-group difference | 18.614 | 77 | 0.142 |
Figure 1.
Comparative analysis of r value of the patients in the three groups at different times
3.2 Comparison of K value of the patients in three groups
Upon statistical analysis, the K value of the patients in three groups at different times is of significant difference (P < 0.05), but the inter-group comparison is of no significant difference (P > 0.05). Each group and measurement time have interaction (P < 0.05). See details in Table 2 and Figure 2. Thus it can be seen that the impact of different injuries on coagulation function is of significant difference. However, with time extension, the impact of different injuries on each time point during the coagulation process is different.
Table 2.
Variance analysis of m value of the patients in the three groups at different times
| Source of variation | SS | df | MS | F value | P value |
|---|---|---|---|---|---|
| Treatment | 19.234 | 2 | 15.061 | 272.101 | 0.000 |
| Measurement time | 25.055 | 2 | 12.072 | 48.352 | 0.000 |
| Treatment×Measurement time | 68.153 | 4 | 16.205 | 64.356 | 0.000 |
| Inter-group difference | 343.743 | 38 | 8.088 | ||
| Intra-group difference | 19.520 | 77 | 0.154 |
Figure 2.
Comparative analysis of m value of the patients in the three groups at different times
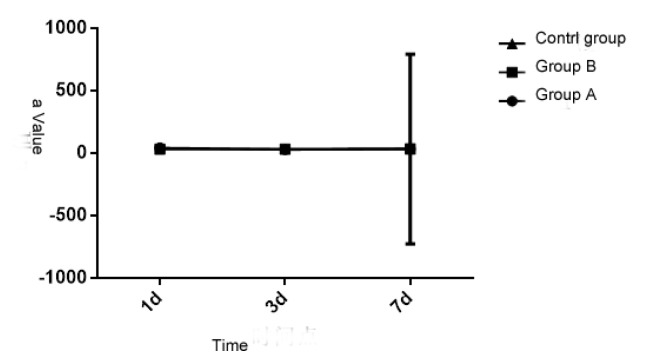
3.3 Comparison of α value of the patients in the three groups
Upon statistical analysis, the K value of the patients in three groups at different times is of significant difference (P < 0.05), but the inter-group comparison is also of significant difference (P < 0.05). Each group and measurement time have interaction (P < 0.05). See details in Table 2 and Figure 2. Thus it can be seen that the generating speed of sludged blood of brain trauma and other injuries will change and will get right after 7 days. Compared with the control group, the R and K value of patients in Group A and B reduces after getting injury for 1 day (P < 0.05), but the Ma and α value increases (P < 0.05), indicating that the blood is in high aggregation state; the R and K value rise significantly after 3 days (P < 0.05), and the Ma and α value decreases (P < 0.05), indicating that the blood is in low aggregation state; all parameters get right after 7 days (P > 0.05).
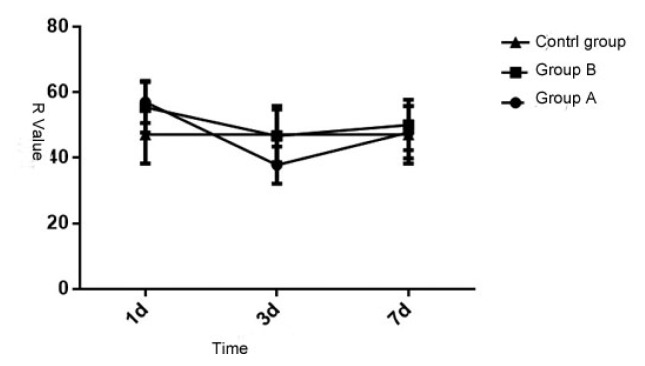
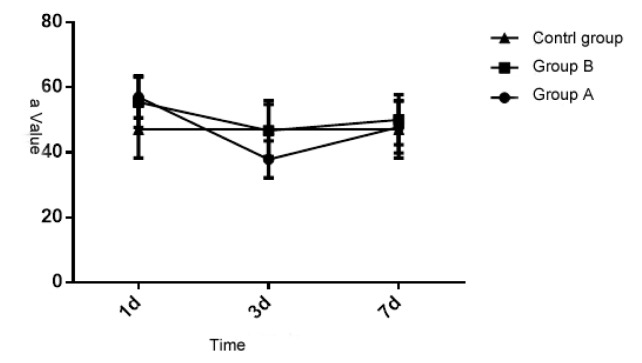
3.4 Comparison of Ma value of the patients in three groups
Upon statistical analysis, the Ma value of the patients in three groups at different times is of significant difference (P < 0.05), but the inter-group comparison is also of significant difference (P < 0.05). Each group and measurement time have interaction (P < 0.05). See details in Table 4 and Figure 4. Thus it can be seen that different injuries can cause coagulation disorder dysfunction, and the blood coagulation will get right after 7 days.
Table 4.
Variance analysis of ma value of the patients in the three groups at different times
| source of variation | SS | df | MS | F value | P value |
|---|---|---|---|---|---|
| Treatment | 443.544 | 2 | 216.217 | 1.314 | 0.000 |
| Measurement time | 1202.231 | 2 | 645.560 | 160.001 | 0.000 |
| Treatment×Measurement time | 1451.173 | 4 | 280.460 | 100.067 | 0.000 |
| Inter-group difference | 6108.603 | 38 | 148.370 | ||
| Intra-group difference | 300.090 | 77 | 3.750 |
Figure 4.
Comparative analysis of ma value of the patients in the three groups at different times
4 Discussion
After brain trauma, brain tissue will release plenty of thrombocinase and accordingly generate extrinsic coagulation, and the coagulation disorder dysfunction of the injured area is more obvious [12]. As for the patients with the traumatic brain hemorrhage, monitor and analyze the coagulation function at different time points with various parameters of thrombelastogram, indicating that the injuries at any parts of the patients may cause coagulation disorder dysfunction, and the coagulation function disorder of the patients with the traumatic brain hemorrhage will be more durable and obvious [13]. Maybe, compared with other injuries, the reduction degree of the arachidonic acid content in the brain tissue reduces more significantly, which causes the cyclooxygenase and function of platelet reduce significantly, accordingly prolongs the time of blood coagulation of the patients with the traumatic brain hemorrhage and finally causes coagulation disorder dysfunction [14]. In addition to this, compared the brain trauma with other injuries, the content of adenosine diphosphate is different and will get right after 3 days. This study shows that the reason why the parameters of thrombelastogram of the patients with the traumatic brain hemorrhage shows abnormality after 6–8 hours getting injuries and is in high aggregation state is that the injured brain tissue or vascular endothelium tissue is injured, blood and brain barrier are injured, and then brain tissue secrete and release plenty of tissue factors, activating the coagulation system [15].
The study results show that: the blood of the patients with the traumatic brain hemorrhage is in low aggregation state in 3 days after injuries, the Ma and α value in thrombelastogram are lower than normal reference range, indicating that the function and quantity of platelet are less and the generation and coagulation speed of fibrin are obviously lower than that patients in the control group. When the patients get injuries, massive bleeding will need plenty of platelet. Massive blood transfusion will dilute blood, so the rate of all-body infection will increase, resulting in that the megakaryocyte in the marrow can get mature normally, and moreover, the drug effect will also suppress the normal function of platelet [16]. It is indicated by the study results that the coagulation function of the patients with the traumatic brain hemorrhage changes from high aggregation state to low aggregation state, and gradually get right; the significance of thrombelastogram in clinical test is to monitor the coagulation function of patients, know about the condition of patients and make a better preparation after prognosis.
In this study, there are 26 patients with blood in high aggregation state at early stage of treatment; 12 patients with blood changing to be in low aggregation state; and the function and quantity of their platelet are less. Therefore, fibrinolytic hemostatic shall be used with caution [17] for the patients with the traumatic brain hemorrhage at early treatment stage, and conduct real-time monitoring on each coagulation function, and adjust dosage and time according to the index changes. In general, it will be of great benefit to observe the coagulation disorder dysfunction or fibrinolytic system malfunction caused by traumatic brain hemorrhage, better take advantages of diagnosis with thrombelastogram, early treatment and improving survival rate, and shorten the time appearing coagulation disorder dysfunction.
Figure 3.
Comparative analysis of α value of the patients in the three groups at different times
Table 3.
Variance analysis of α value of the patients in the three groups at different times
| source of variation | SS | df | MS | F value | P value |
|---|---|---|---|---|---|
| Treatment | 58.627 | 2 | 28.758 | 0.100 | 0.000 |
| Measurement time | 424.857 | 2 | 206.873 | 81.268 | 0.000 |
| Treatment×Measurement time | 805.007 | 4 | 203.004 | 76.085 | 0.000 |
| Inter-group difference | 5414.273 | 38 | 130.566 | ||
| Intra-group difference | 205.286 | 77 | 2.535 |
Footnotes
Conflict of interest statement: Authors state no conflict of interest
References
- 1.Haiying Cai. Study of the application value of thrombelastogram in multiple-trauma patients [D] Zhejiang Univertsity; 2010. [Google Scholar]
- 2.Bing Zhang. Relation between coagulation disorder dysfunction and thrombelastogram and severity and prognosis of septicopyemia [D] Shanxi Medical University; 2013. [Google Scholar]
- 3.Nekludov M, Bellander BM, Blombäck M, et al. Platelet dysfunction in patients with severe traumatic brain injury. J Journal of Neurotrauma. 2007;24(11):1699–1706. doi: 10.1089/neu.2007.0322. [DOI] [PubMed] [Google Scholar]
- 4.Yan Z. Application of thrombelastogram in severe thrombocytopenia patients. D Chongqing Medical University. 2011 [Google Scholar]
- 5.Finfer SR, Cohen J. severe traumatic brain injury. J Resuscitation. 2001;48(1):77–90. doi: 10.1016/s0300-9572(00)00321-x. [DOI] [PubMed] [Google Scholar]
- 6.Giacino JT, Whyte J, Bagiella E, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. J New England Journal of Medicine. 2012;366(9):819–826. doi: 10.1056/NEJMoa1102609. [DOI] [PubMed] [Google Scholar]
- 7.Xinghua P, Buzhen Z, Zhilong C, et al. Over-body inflammation and its prevention and cure measures. J Medical Journal of National Defending Forces in Southwest China. 2001;11(1):70–72. [Google Scholar]
- 8.Bulger EM, May S, Brasel KJ, et al. Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. J Jama. 2010;304(13):1455–1464. doi: 10.1001/jama.2010.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiaoqiang R. Comparative study on estimating plenty of blood transfusion for traumatic patients at early stage with four scoring standards. D Suzhou University. 2014 [Google Scholar]
- 10.Yong L. Early-stage coagulation function changes of craniocerebral trauma patients and its impact on prognosis. Suzhou University; 2008. [Google Scholar]
- 11.Mondello S, Papa L, Buki A, et al. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. J Crit Care. 2011;15(3):R156. doi: 10.1186/cc10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hongsheng L, Man W, Qin S, et al. Clinical significance of the coagulation disorder dysfunction of acute severe traumatic patients. J Traumatic surgery magazine. 2014;16(5):399–402. [Google Scholar]
- 13.Xueyan L, Chengxiao Z. Clinical application status and limitation of thrombelastogram. J Medical innovation of china. 2014;(8):127–129. [Google Scholar]
- 14.Spiotta AM, Stiefel MF, Gracias VH, et al. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury: Clinical Article. J Journal of Neurosurgery. 2010;113(3):571–580. doi: 10.3171/2010.1.JNS09506. [DOI] [PubMed] [Google Scholar]
- 15.Xuemei T, Zhou Z. Coagulation function changes of patients with traumatic spinal fracture in perioperative period. J Chongqing Medical Journal. 2007;36(16):1596–1597. [Google Scholar]
- 16.Andriessen TMJC, Horn J, Franschman G, et al. Epidemiology, severity classification, and outcome of moderate and severe traumatic brain injury: a prospective multicenter study. J Journal of Neurotrauma. 2011;28(10):2019–2031. doi: 10.1089/neu.2011.2034. [DOI] [PubMed] [Google Scholar]
- 17.Cuifen p. Study on the Clinical Application of Thrombelastogram in Pregnancy Combined with Thrombocytopenia. D Chongqing Medical University. 2010 [Google Scholar]