Abstract
A series of 2-hydroxypropyltrimethyl ammonium chloride chitosan (HACC) was prepared by the reaction of chitosan with glycidyl trimethyl ammonium chloride. Structure of HACC was characterized by FT IR and 1H NMR spectroscopies, and it was proved that substitution reaction mainly occurs on the N element. Antimicrobial activities of HACC was examined against S. aureus, E. coli, and A. niger. Results indicatd that the inhibitory effects of HACC solutions were varied with HACC concentration, quaternization degrees, pH values, metal ions, and heat treatment. The antimicrobial properties of handsheets prepared from HACC were studied by the inhibition zone method, and the sheets had good antimicrobial properties against S. aureus and E. coli, and low inhibition rate against A. niger.
Keywords: Chitosan, quaternary ammonium salt, antimicrobial property, paper
1 Introduction
Chitosan, with the structural formula poly-β-(1–4)-Dglucosamine, is the deacetylated derivative of chitin, and it is the second most abundant polysaccharide found on the earth, next to cellulose. As a natural renewable resource, it has a number of unique properties such as antimicrobial activity, nontoxicity, and biodegradability that attract scientific and industrial interest in many fields [1–4]. Nowadays, chitosan has be widely used as a flocculant [5,6], clarifier [7], thickener [8], fiber [9], film [10], affinity chromatography column matrix, gas-selective membrane, plant disease resistance-promotor, anti-cancer agent, wound healing promoting agent, and antimicrobial agent. However, these activities are limited to acidic conditions due to its poor solubility above pH~6.5. Because of its very stable crystalline structure with strong hydrogen bonds, researchers have focused on the preparation of chitosan derivatives soluble in water over a wide pH range; the solubility of chitosan can be improved by chemical modification. Chitosan has reactive amino and hydroxyl groups, both of which can be used to chemically alter chitosan’s properties under mild reaction conditions. Therefore, many water-soluble derivatives have been prepared by introducing hydrophilic groups such as carboxymethyl, dihydroxyethyl, sulfate, phosphate, hydroxyalkylamino, or by grafting a water-soluble polymer on the macromolecular chain of chitosan [11,12,13]. Polymeric quaternary ammonium compounds have received the most attention over the years [13–15].
Various kinds of paper have been used in the industry and daily activities, but bacteria and fungi will adhere to the surface of the paper through the making and using processes. Thus, it’s important to study ways to prevent the diffusion of illness by antimicrobial paper [16–18]. Antimicrobial packaging materials are interesting and promising applications of advanced active food packaging concepts. They can effectively control microbial contamination of various solid and semisolid foodstuffs by inhibiting the growth of microorganisms on the surface of the food that normally comes into direct contact with the packaging material. In recent years, research into environmentally friendly packaging materials and production methods has increased considerably. Current research focuses on antimicrobial reagents prepared from natural renewable materials and low toxicity toward mammalian cells [19–21]. Chitosan can disrupt the barrier properties of the outer membranes of gram-negative bacteria; this makes it a potentially useful indirect antimicrobial for food protection. Chitosan can also act as a chelating agent that selectively binds trace metals and thereby inhibits the production of toxins and microbial growth.
The notion of using chitosan combined with paper is not new [22–24]. It has been used as a papermaking additive and for the surface treatment of paper for decades. Chitosan graft copolymers have been exploited for making paper products of improved dry strength, and chitosan has been added to cellulose and unbleached sulfite to increase the burst, dry-tensile, and wet-tensile properties of handsheets [25–27]. Its use has been recommended for the manufacture of electric insulation papers and various types of technical papers, particularly wet-strength papers. Chitosan has been proved theoretically and practically to meet the criteria for wet-strength agents. One researcher has prepared chitosan-coated caper and studied the effects of nisin and different acids on the antimicrobial activity of that paper. Chitosan coatings obviously improved both the gloss and oxygen-barrier properties of the paper. Chitosan dissolved in 1.6, 3.2, and 6.4% lactic acid showed antimicrobial activity against Bacillus subtilis, whereas acetic and propionic acids (1.6, 3.2, and 6.4%) did not produce any notable activity.
In this paper, chitosan-N-2-hydroxypropyl trimethyl ammonium chloride (HACC) with different quaternization degrees was prepared. The antimicrobial activities of HACC against bacteria and fungi were systemically studied. The effects of some factors, e.g., HACC concentration, quaternization degrees, pH values, and incubation temperature of the medium on the antimicrobial activities of HACC were discussed. The antimicrobial properties of handsheets with HACC were also studied.
2 Material and method
2.1 Materials
Chitosan (CS) with Mw of 1.30×105 and deacetylation degree of 85.1% was obtained from Qingdao Lizhong Chitin Co. (Shandong, China) and was used as received. Other chemicals are analytical grade. The microorganisms tested were Escherichia coli, Staphylococcus aureus and Aspergillus niger ; they were provided by the College of Food Engineering and Biotechnology in Tianjin University of Science and Technology. Bacteria were incubated on nutrient agar at 37°C for 24 h; A. niger bacteria were incubated on potato dextrose agar (PDA) at 28°C for 72 h. A certain amount of HACC was added to the bleached softwood pulp to prepare handsheets on the Rapid Köthen handsheet making machine.
2.2 Preparation of HACC
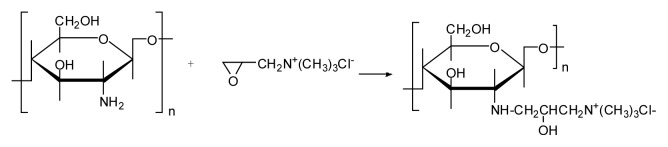
Chitosan was dispersed into the mixture of isopropanol and distilled water at 85°C. Glycidyl trimethyl ammonium chloride (GTMAC) was added in four portions at 2 h intervals. After stirring 8 h, the clear and yellowish reaction solution was poured into acetone. The reaction product was filtered, concentrated, and dried under vacuum at 40°C for 24 h to obtain the quaternized chitosan. The synthesis route is shown in Scheme 1. The degree of quaternization (DQ) was determined by conductometric titration of chloride ions using a standard AgNO3 solution in water. FT IR spectra were recorded by reflection method with a VECTOR 22 Fourier transform infrared spectrometer. 1H NMR spectra were obtained using a Varian UNITYplus-400 nuclear magnetic resonance.
Scheme 1.
The synthesis route of HTCC
2.3 Antimicrobial assays
A measured amount of HACC aqueous solutions, microorganism suspension with concentration of 106–107CFU/m, and culture medium were poured into autoclaved petri-dishes and were spread uniformly. A blank without HACC was prepared for comparison. Then the mixture were put in the incubator and incubated at standard conditions [19, 21]. We observed the visible colonies after regular incubation times; the inhibition rate η were calculated as η=(N1–N2)N1. Here N1 and N2 represent the number of colonies on the plates without and with HACC samples after inhibition, respectively. The antimicrobial properties of the handsheets were tested by the inhibition zone method [16, 18]. All sheets were cut into 10-mm diameter disks, placed on the petri-dishes with 0.2 ml of microorganism suspension and 16 ml of culture medium, and incubated at standard conditions. The antimicrobial effect of the sheets was determined by observing the existence of the clear zone at the contact area around the disks. All experiments were carried out in triplicate, and average values were reported.
3 Results and discussion
3.1 Preparation of HACC
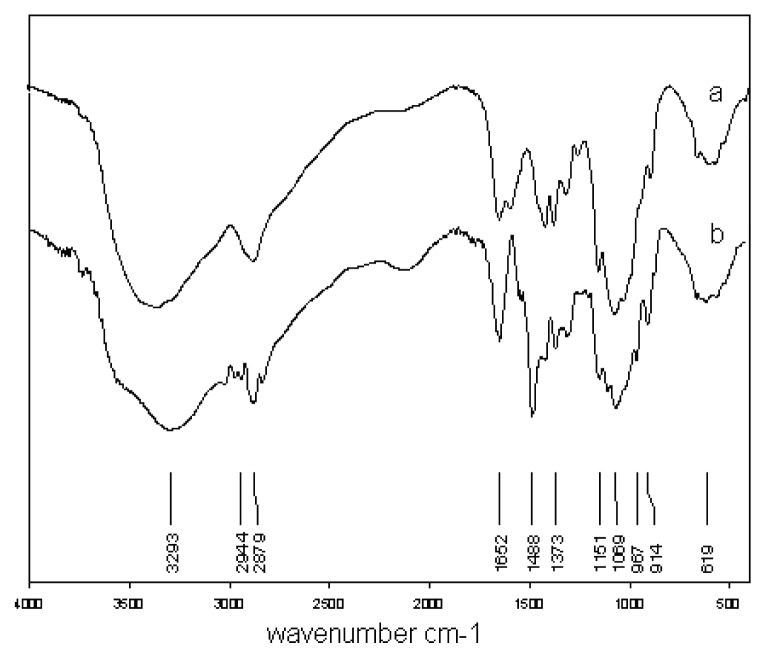
HACC was prepared by reacting chitosan with GTMAC under a neutral condition in which the hydroxyl groups of chitosan are not sufficiently nucleophilic to induce ring opening of GTMAC, whereas the amino group of chitosan is nucleophilic enough to do so. FT IR and 1H NMR analysis were carried out to identify the chitosan quaternary salt produced. Figure 1 shows the FT IR spectra of chitosan and HACC. The most striking difference between the two spectra is the band of the salt positioned at 1488 cm−1, which corresponds to an asymmetric angular bending of methyl groups of quaternary hydrogen. That peak was not detected in the infrared spectrum corresponding to the original chitosan. The N–H bending (1600 cm−1) of the primary amine was weak due to the change of the primary amine to the secondary amine (aliphatic) (Fig. 1).
Figure 1.
FTIR spectra of chitosan (a) and HTCC (b).
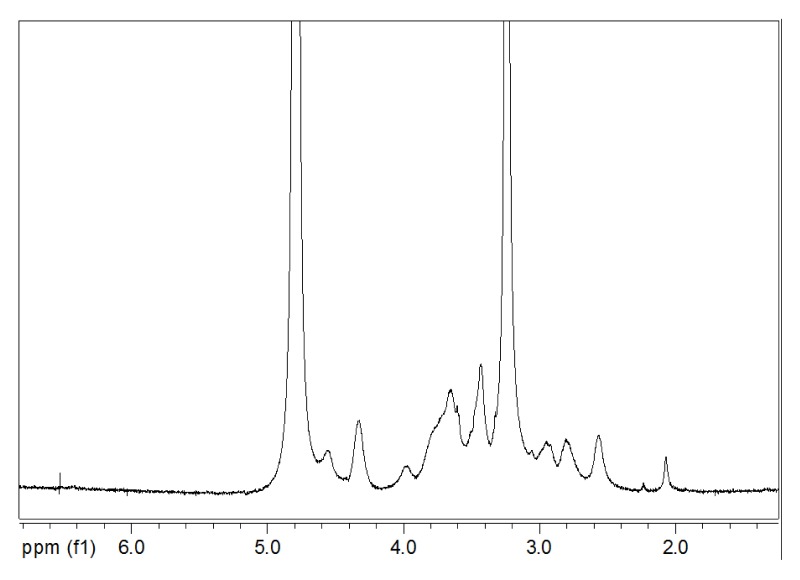
The 1H NMR spectrum of HACC is shown in Figure 2. As evidence of the reaction, the three methyl groups in the quaternary ammonium salt group were observed as a very strong peak at 3.2 ppm in 1H NMR spectrum of HACC.
Figure 2.
1H NMR spectrum of HTCC.
3.2 Antimicrobial Properties of HACC
The antimicrobial effect of HACC in different concentration is shown in Table 1. It was noted that the number of colonies of all test microorganisms formed on the dishes had decreased with increasing HACC concentration. As the concentration of HACC in the medium also indicates the concentration of cation, the inhibitory effect was strengthened with the concentration of quaternary ammonium groups in the experimental range.
Table 1.
Effect of factors on antimicrobial activities.
| Conditions | η (%) | |||
|---|---|---|---|---|
| S. aureus | E. coli | A. niger | ||
| Conc. of HACCa/% | 0.005 | 9.3 | 13.3 | 13.6 |
| 0.010 | 31.5 | 26.7 | 18.2 | |
| 0.025 | 88.1 | 80.7 | 20.5 | |
| 0.050 | 96.8 | 90.4 | 27.2 | |
| 0.100 | 100 | 95.6 | 54.5 | |
| Quaternization degrees of HACC | 1.00 | 90.4 | 88.2 | 47.7 |
| 1.06 | 92.8 | 90.7 | 50.0 | |
| 1.17 | 95.0 | 92.9 | 52.1 | |
| 1.23 | 96.8 | 95.6 | 54.5 | |
| 1.27 | 97.2 | 96.4 | 56.8 | |
| pH | 5.5 | 97.2 | 96.8 | 59.0 |
| 6.0 | 97.0 | 96.2 | 56.8 | |
| 6.5 | 96.8 | 95.8 | 56.8 | |
| 7.0 | 96.8 | 95.6 | 54.5 | |
| 7.5 | 96.1 | 94.2 | 50.0 | |
| 8.0 | 95.9 | 91.3 | 40.9 | |
| Metal ions | Controlb | 96.8 | 95.6 | 54.5 |
| Na+ | 77.8 | 78.2 | 26.9 | |
| Mg2+ | 82.6 | 81.8 | 33.9 | |
| Ca2+ | 86.4 | 88.6 | 40.8 | |
| Temperature of heat Treatment/°C | Controlc | 96.8 | 95.6 | 54.5 |
| 80 | 49.9 | 54.2 | 28.4 | |
| 100 | 25.8 | 28.9 | 19.9 | |
| 120 | 17.7 | 20.1 | 14.6 | |
HACC with quaternization degree of 1.23.
HACC solution without metal ions.
HACC solutions without heat treatment.
The samples demonstrate more effective antimicrobial ability against S. aureus than that of E. coli, as indicated by the lower colony unit. Thatmay be attributed to their different cell walls [28]. S. aureus, being a typical Grampositive bacterium, has a cell wall fully composed of peptide polyglycogen. The peptidoglycan layer is composed of networks with many pores that allow foreign molecules to enter the cell without difficulty. However, E. coli, a typical Gram-negative bacterium, has a cell wall made up of a thin membrane of peptide polyglycogen and an outer membrane constituted by lipopolysaccharides, lipoproteins, and phospholipids. Because of the bilayer structure, the outer membrane is a potential barrier against foreign molecules. 0.1% (w/v) HACC solution could inhibit the growth of S. aureus completely, whereas for E. coli, the inhibition rate was 95.6%. However, for A. niger, more HACC were needed to inhibit its growth. When 0.1% (w/v) HACC solution applied, the inhibition rate was only 54.5%. In the latter experiments, 0.05% (w/v) HACC solutions were used to study the effect of some factors on antimicrobial activity against S. aureus, whereas a concentration of 0.1% (w/v) was used against E. coli and A. niger.
HACC samples with five different degree of quaternization (DQ) were used to evaluate the effects of DQ on antimicrobial activity. As seen in Table 1, the inhibitory effects strengthened as the DQ of HACC increased. It was understandable that the concentration of positive charge increased with the increasing of DQ, thus the antimicrobial activity improved. HACC with a DQ of 1.23 was used in the latter experiments.
The effect of medium pH values on antimicrobial activity of HACC solvents was evaluated with six different pH values (adjusted with HCl and NaOH). As shown in Table 1, HACC had high antimicrobial activity against S. aureus and E. coli at different pH values, but the weakly basic condition slightly decreased the antimicrobial activity. HACC is a polyelectrolyte; therefore, increasing pH value, quaternary ammonium groups acted with hydroxy and the amount of quaternary ammonium structure decreased, thus antimicrobial activity decreased in under basic conditions.
Most of the water used in the papermaking industry comes from rivers or lakes, which contain abundant of metal ions, such as Ca2+, Mg2+, and Na+, and other metal ions. Therefore, it is important to estimate the effect of metal ions on antimicrobial activity. NaCl, MgCl2, and CaCl2 powder was added to HACC solutions, and the final concentration of metal ions was 0.2 mol/L. After stirred for about 1 h, the solutions were used in antimicrobial assays. Table 1 shows that antimicrobial activity decreased with the increasing concentrations of metal ions.
Chitosan is a powerful chelating agent that easily forms complexes with transition metals and heavy metals; chitosan-metal complexes can be used for sequestration or removal of metal ions, dyeing, catalysis, and water treatment, and also other purposes. Some of the complexes, such as chitosan-Ag, chitosan-Cu, chitosan-Zn are important as antimicrobial agents, for Ag+, Cu2+, and Zn2+ themselves have good antimicrobial properties; that is different from Ca2+, Mg2+, and Na+ [29]. The metal ion is like a bridge connecting one or more chains of HACC through interacting with –OH and quaternary ammonium groups, thus the antimicrobial activity decreased by different degrees for the differences of charge density, ions radius, and chelating ability.
To detect the effect of heat treatment on antimicrobial activity, HACC solutions were placed in different temperatures for 30 min and then cooled to the room temperature for the antimicrobial assays. For a control sample, HACC solutions without heat treatment were used as a contrast. Table 1 shows that heat treatment had a great effect on the antimicrobial activity. It could be postulated that HACC hydrolysis quickened in the solutions with increasing temperature and the concentration of positive charge decreased, thus the antimicrobial activity decreased.
3.3 Antimicrobial Properties of HACC for paper
In the inhibition zone test, an antimicrobial sample was placed on a solid agar medium containing the test strain. A clear zone surrounding the sample indicated the diffusion of antimicrobial substances from the sample material generating growth inhibition. The capability of the prepared sheets to inhibit the growth of the tested microorganisms is shown in Table 2. It was found that the diameter of the inhibition zone varied according to the test microorganisms. When the concentration of HACC was 0.5%, the width of inhibition area was 2.8 mm, 2.7 mm and 2.0 mm for S. aureus, E. coli, and A. niger, respectively, and no shrinkage was seen after 2 days, indicating that HACC is suitable for use as a antimicrobial for paper.
Table 2.
The antimicrobial activity of handsheets.
| Conc. of HACC a/% | Diameters of inhibition zones/mm | ||
|---|---|---|---|
| S. aureus | E. coli | A. niger | |
| 0.1% | 14.0 | 14.2 | 11.6 |
| 0.3% | 15.0 | 14.8 | 12.4 |
| 0.5% | 15.6 | 15.4 | 14.0 |
| 0.7% | 16.1 | 16.0 | 14.2 |
| 0.9% | 17.2 | 17.0 | 14.6 |
The weight ratio of HACC to the pulp without water.
4 Conclusions
2-hydroxypropyltrimethyl ammonium chloride chitosan (HACC) with quaternization degrees of 1.00 to 1.27 was prepared. HACC showed high antimicrobial activity against S. aureus and E. coli, and low antimicrobial activity against A. niger. The antimicrobial activity increased with increasing DQ of HACC and HACC solution concentrations and was slightly stronger in weakly acidic conditions than in basic conditions. The antimicrobial activity decreased in the presence of large amounts of Ca2+, Mg2+, and Na+, or under heat treatment of HACC solutions. When the ratio of HACC to dried pulp was 0.5%, the width of the inhibition area was greater than 2.0 mm, and no shrinkage was seen after 2 days. All these results show that chitosan quaternary ammonium salt derivatives are good candidates for antimicrobial agents that can be used in paper.
Acknowledgment
The financial support for this project was from the Tianjin Municipal Science and Technology Commission (Grant No.12ZCZDGX01100), the Scientific Research Foundation of the State Key Laboratory of Pulp and Paper Engineering (No.201119), and the Foundation of Tianjin Key Laboratory of Pulp & Paper (Tianjin University of Science & Technology, No.201313), P. R. China.
Footnotes
Conflict of interest statement: Authors state no conflict of interest.
References
- 1.Alireza A, Mohammed A. Applications of Chitosan in the Seafood Industry and Aquaculture, A Review. Food Bioprocess Technol. 2012;5:817–830. [Google Scholar]
- 2.Luca C, Driton V, Lam Jenny KW, Mahmoud S, Lisbeth I. Biomedical applications of amino acid-modified chitosans, A review. Biomater. 2012;33:7565–7583. doi: 10.1016/j.biomaterials.2012.06.104. [DOI] [PubMed] [Google Scholar]
- 3.Elsabee MZ, Naguib HF, Elsayed MR. Chitosan based nanofibers, review. Mater Sci Eng C. 2012;32:1711–1726. doi: 10.1016/j.msec.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Jayakumar R, Deepthy M, Manzoor K, Nair SV, Tamura H. Biomedical applications of chitin and chitosan based nanomaterials - A short review. Carbohy Polym. 2010;82:227–232. [Google Scholar]
- 5.Dong CL, Chen W, Liu C. Flocculation of algal cells by amphoteric chitosan-based flocculant. Biorecource Technol. 2014;170:239–247. doi: 10.1016/j.biortech.2014.07.108. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H, Sun HY, Wang FH, Zou J, Fan JT. Preparation of chitosan-based flocculant for high density waste drilling mud solid-liquid separation. J Appl Polym Sci. 2012;125(4):2646–2651. [Google Scholar]
- 7.Ghorbel-Bellaaj O, Jridi M, Ben Khaled H, Jellouli K, Nasri M. Bioconversion of shrimp shell waste for the production of antioxidant and chitosan used as fruit juice clarifier. Int J Food Sci Tech. 2012;47(9):1835–1841. [Google Scholar]
- 8.Sanchez R, Stringari GB, Franco JM, Valencia C, Gallegos C. Use of chitin, chitosan and acylated derivatives as thickener agents of vegetable oils for bio-lubricant applications. Carbohyd Polym. 2011;85(3):705–714. [Google Scholar]
- 9.Zhao XG, Lia J, Jin WP, Geng XP, Xua W, Ye T, Lei JQ, Li B, Wang L. Preparation and characterization of a novel pH-response dietary fiber: Chitosan-coated konjac glucomannan. Carbohyd Polym. 2015;117:1–10. doi: 10.1016/j.carbpol.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 10.El Miri N, Abdelouahdi K, Zahouily M, Fihri A, Barakat A, Solhy A, El Achaby M. Bio-nanocomposite films based on cellulose nanocrystals filled polyvinyl alcohol/chitosan polymer blend. J Appl Polym Sci. 2015;132(22):420–431. [Google Scholar]
- 11.Facin Bruno R, Bruna M, Dilmar B, Belfiore LA, Paulino AT. Immobilization and controlled release of beta-galactosidase from chitosan-grafted hydrogels. Food Chem. 2015;179:44–51. doi: 10.1016/j.foodchem.2015.01.088. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Valdez O, Ramirez-Wong DG, Saldivar-Guerra E, Luna-Barcenas G. Grafting of chitosan with styrene and maleic anhydride via nitroxide-mediated radical polymerization in supercritical carbon dioxide. Macromol Chem Phys. 2013;214(12):1396–1404. [Google Scholar]
- 13.Yang Z, Degorce-Dumas JR, Yang H, Guibal E, Li AM, Cheng RS. Flocculation of Escherichia coli using a quaternary ammonium salt grafted carboxymethyl chitosan flocculant. Environ Sci Technol. 2014;48(12):6867–6873. doi: 10.1021/es500415v. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Wang FJ, Yun DR, Guo YW, Ye YC, Wang YX, Tan HM. Preparation of a C6 quaternary ammonium chitosan derivative through a chitosan schiff base with click chemistry. J Appl Polym Sci. 2013;129:3185–3191. [Google Scholar]
- 15.Zhang WW, Xiao HN, Qian LY. Beeswax-chitosan emulsion coated paper with enhanced water vapor barrier efficiency. Appl Surf Sci. 2014;300:80–85. [Google Scholar]
- 16.Deokar AR, Lin LY, Chang CC, Ling YC. Single-walled carbon nanotube coated antibacterial paper, Preparation and mechanistic study. J Mater Chem B. 2013;1:2639–2646. doi: 10.1039/c3tb20188k. [DOI] [PubMed] [Google Scholar]
- 17.Soares NFF, Moreira FKV, Fialho TL, Melo NR. Triclosan-based antibacterial paper reinforced with nano-montmorillonite: A model nanocomposite for the development of new active packaging. Polym Adv Technol. 2012;23:901–908. [Google Scholar]
- 18.Yoosefi BA, Wang R, Xu R. Simple method of deposition of CuO nanoparticles on a cellulose paper and its antibacterial activity. Chem Eng J. 2015;262:999–1008. [Google Scholar]
- 19.Ben AA, Preziosi-Belloy L, Chalier P, Gontard N. Antimicrobial paper based on a soy protein isolate or modified starch coating including carvacrol and cinnamaldehyde. J Agric Food Chem. 2007;55:2155–2162. doi: 10.1021/jf0626009. [DOI] [PubMed] [Google Scholar]
- 20.Chen JL, Zhao YY. Effect of molecular weight, acid and plasticizer on the physicochemical and antibacterial properties of beta-chitosan based films. J Food Sci. 2012;77(5):E127–E136. doi: 10.1111/j.1750-3841.2012.02686.x. [DOI] [PubMed] [Google Scholar]
- 21.Cheng F, Betts JW, Kelly SM, Wareham DW, Kornherr A, Dumestre F, Schaller J, Heinze T. Whiter brighter and more stable cellulose paper coated with antibacterial carboxymethyl starch stabilized ZnO nanoparticles. J Mater Chem B. 2014;2:3057–3064. doi: 10.1039/c3tb21734e. [DOI] [PubMed] [Google Scholar]
- 22.Lv Y, Song C, Long Z, Dai L, He H, Yu LN, Zhang D. Preparation of cationic chitosan-g-polyacrylamide and its performance on strengthening paper and antibacterial activities. Asian J Chem. 2014;26(14):4235–4242. [Google Scholar]
- 23.Qu YJ, Han HZ, Zheng XW, Guo ZH, Yuhu L. Detection of surface pH of paper using a chitosan-modified silica fluorescent nanosensor. Sensor Actuat B-Chem. 2014;195:252–258. [Google Scholar]
- 24.Zhang AD, Ding DR, Ren JC, Zhu XL, Yao YH. Synthesis characterization and drug-release behavior of amphiphilic quaternary ammonium chitosan derivatives. J Appl Polym Sci. 2014;131:4–13. [Google Scholar]
- 25.Chen ZC, Zhang H, Song ZQ, Qian XR. Combination of glyoxal and chitosan as the crosslinking system to improve paper wet strength. Bioresources. 2013;8(4):6087–6096. [Google Scholar]
- 26.Reis AB, Yoshida CMP, Reis APC, Franco TT. Application of chitosan emulsion as a coating on Kraft paper. Polym Int. 2011;60(6):963–969. [Google Scholar]
- 27.Wang SM, Ge L, Song XR, Yu JH, Ge SG, Huang JD, Zeng F. Paper-based chemiluminescence ELISA: Lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens Bioelectron. 2012;31(1):212–218. doi: 10.1016/j.bios.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Qi LF, Xu ZR, Jiang X, Hu CH, Zou XF. Preparation and antibacterial activity of chitosan nanoparticles. Carbohyd Res. 2004;339:2693–2700. doi: 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Wang XH, Du YM, Fan LH, Liu H, Hu Y. Chitosan-metal complexes as antimicrobial agent: synthesis characterization and structure-activity study. Polym Bull. 2005;55:105–113. [Google Scholar]