Abstract
Epithelioid hemangioma (EH) is an uncommon benign vascular lesion, also known as angioblastic lymphoid (or angiolymphoid) hyperplasia with eosinophilia, characterized by an unclear etiopathogenesis.
It usually affects young to middle-aged adults and develops in the head and neck region, as painless cutaneous or subcutaneous reddish papules or nodules.
Large vessels involvement is extremely rare, and to date only two cases affecting the brachial artery have been cited in literature.
In this report we present a further case of EH of the brachial artery and review the pertinent literature.
Keywords: Epithelioid Hemangioma, Brachial Artery
1 Introduction
The term epithelioid hemangioma (EH) was first introduced by Enzinger and Weiss in 1983 and it is used in the current WHO to designate this uncommon and distinct vascular formation [1, 2].
EH is now well accepted as a synonymous with angiolymphoid hyperplasia with eosinophilia, the more popular term first used by Wells and Whimster in 1969, to describe this entity occurring in the skin [3].
This lesion has been actually referred to with a plethora of different names (such as inflammatory angiomatous nodule, atypical granuloma, pseudopyogenic granuloma, and histiocytoid hemangioma) that reflect the different ways of presentation and the debate about the reactive rather than true neoplastic etiology of this condition [1].
Many reports sustain the reactive or hyperplastic nature of the vascular proliferation, based on the frequent history of local trauma, the frequent association with a damaged vessel and the almost invariable infiltrate of eosinophils [3, 4]. The presence of a lymphoid component that tends to become predominant in old lesions seems to support this hypothesis.
On the other hand Rosai proposed EH as part of a spectrum of “tumors of histiocytoid endothelial cells” encompassing emangioendothelioma and angiosarcoma [5].
EH usually affects young to middle-aged adults and develops in the head and neck region, as painless cutaneous or subcutaneous reddish papules or nodules. Preauricular area and scalp are the most affected sites, however occurrence in penis [6], oral cavity [7], orbits [8], and conjunctiva [9] has also been described. Although EH is usually superficial, bony and deep-soft tissue lesions are also on record [10].
Large vessels involvement is extremely rare and to date only two cases affecting the brachial artery have been reported in literature [11, 12].
In this report we present a further case of EH of the brachial artery and review the pertinent literature, focusing on clinical and histopathological features.
2 Case presentation
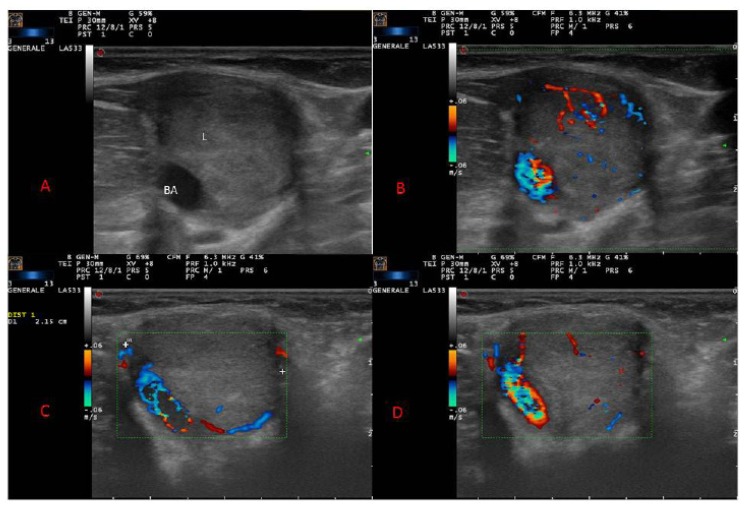
A 33-year-old Caucasian man presented with a painless nodule in his left upper arm. On physical examination, he had an oval, mobile, pulsatile mass of hard-elastic consistency of approximately 2 cm of maximal diameter localized to the medial aspect of his left arm, in the crook of the elbow. There were no associated skin papules or plaques. Past medical history was noncontributory. There was no history of trauma excepted for a blood test in the same site 5 months before. Laboratory tests did not reveal a peripheral eosinophilia. An IgE level was not assessed. Ultrasonography (US) showed a hypoechoic homogeneous well-defined solid nodule, measuring 2.1 cm in diameter, surrounding the brachial artery. At color and power Doppler some peripheral penetrating vessels were evident (Fig. 1 A–D).
Figure 1.
Ultrasound (US) of brachial artery showed a hypoechoic homogeneous well circumscribed solid lesion (L) arising from brachial artery (A); color Doppler showing peripheral and penetrating vascular network (B,C,D).
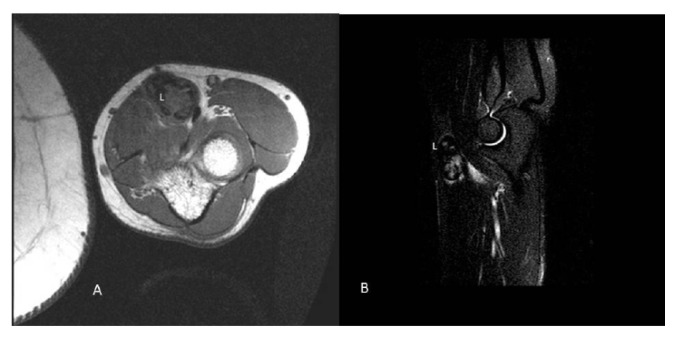
A core biopsy was performed. Histology showed fibrous tissue with lymphocytes, eosinophils and hemosiderin deposits, entrapping some proliferating small vessels with focally plump endothelium. A diagnosis of benign/reactive lesion suspicious for epithelioid hemangioma was made, and a wait-and-see approach was initially chosen. After 2 months the lesion enlarged and it become symptomatic, causing formication in the left hand. A magnetic resonance imaging (MRI) was performed for further evaluation. MRI showed an oval mass measuring 3.1 × 2.5 cm, dislocating the brachial artery and the median nerve, that was dishomogeneous hypointense relative to muscle tissues both on SPIR (Selective Partial Inversion Recovery) and STIR (Short Time Inversion Recovery) images (Fig. 2 A,B). The patient was scheduled for elective surgical resection. Intraoperatively the mass was not dissociable from the vessel wall so the patient underwent en bloc resection of the mass and brachial artery with interposition of a reversed saphenous vein graft taken from his left leg. The patient tolerated the procedure well with no perioperative complications.
Figure 2.
Magnetic Resonance Imaging (MRI) of the left upper extremity showed a lesion (L) localized in the septum between the brachioradial and the rotund pronator muscle. Both SPIR (A) and STIR (B) images revealed a dishomogeneous hypointense enhancement relative to muscle tissues.
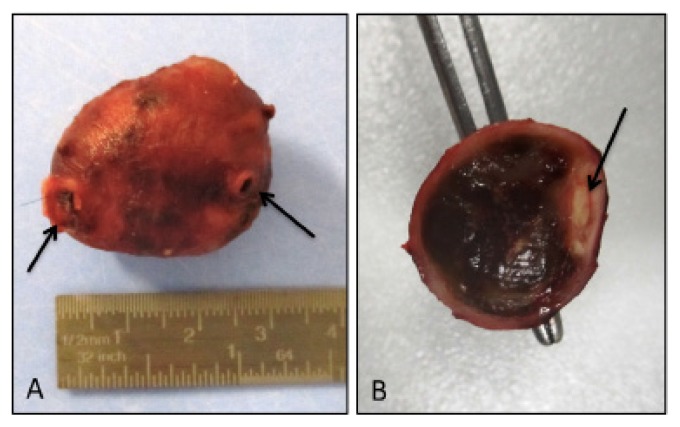
On macroscopic examination the resected specimen consisted of an encapsulated smooth mass, measuring 3 × 2 × 1.7 cm, surrounding a 3.5 cm long segment of vessel (Fig 3A). On cross-section the mass was made of brownish soft tissue with a hemorrhagic appearance, eccentrically encasing the lumen of the muscular artery (Fig. 3B).
Figure 3.
Macroscopic examination. A well-defined encapsulated mass encasing a segment of vessel was resected (arrows) (A); on cross-section the mass was made of brownish hemorrhagic appearing tissue, and the arterial lumen was evident at the periphery of the nodule (arrow) (B).
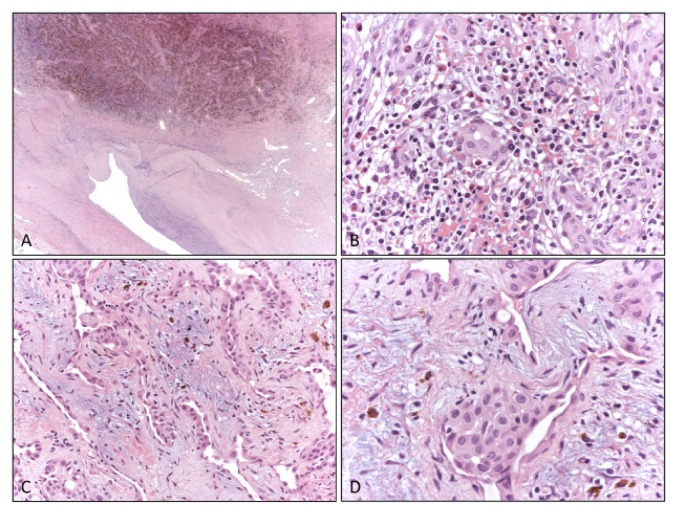
Microscopic examination showed a hemorrhagic mass inside the tunica media, dislocating the arterial lumen (Fig. 4A). The lesion was composed of fibrotic tissue with multiple foci of extravasated blood and hemosiderin deposits (Fig. 4A) associated with chronic inflammatory infiltrate including numerous eosinophils (Fig. 4B) and benign vascular proliferation consisting of small to medium, thin-wall vessels (Fig. 4C). Plump endothelial cells, with epithelioid appearance, protruding into the lumen, lined these vessels (Fig. 4D). These histological features were characteristic of epithelioid hemangioma. Margins were negative for tumor growth.
Figure 4.
Histologic appearance. At low magnification the arterial lumen was compressed and dislocated by a thickening of the tunica media of the vessel wall, that is replaced by fibrotic tissue with conspicuous hemosiderin deposits and vessels proliferation (A). At high magnification a heavy chronic inflammatory infiltrate including numerous eosinophils was present (B) and small irregular vessels proliferation (C) with prominent endothelial cells were also evident (D).
Two months after excision of the tumor, follow-up was uneventful and no recurrence was detected.
Ethical approval
The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Informed consent
Informed consent has been obtained from all individuals included in this study.
3 Discussion
When EH occurred in the subcutis and deep soft tissue, the presence of a medium-sized vessel mainly an artery, in continuity with or close to the lesion, is reported in more than half of the cases described in the largest series [4, 13].
However, the development of EH inside a large muscular artery is rarely seen. About 22 cases have been reported, involving in order of decreasing frequency: temporal artery [14–20], radial artery [21–24], brachial artery [11, 12], ulnar artery [25, 26], axillary artery [27, 28], subclavian artery [29], facial artery [30], post-auricular artery [31], popliteal artery [32], common carotid artery [33] and occipital artery [12]. The lesion can grow entirely in the lumen of the blood vessel developing occlusive symptoms (such as weakening of the pulse in peripheral arteries) or it can originate from the vascular wall and developing outside with compression of adjacent structures. Radiologically, it can present as a solid nodule close to the vessel wall or it can more frequently cause aneurysmal changes.
We present the case of a 33-year-old man who developed a solid nodule in the left brachial artery, 5 months after a blood test. Although the benign nature of the lesion was confirmed by a biopsy, a complete excision was performed because of the rapid enlargement and the development of compressive symptoms.
Only other two cases of EH with brachial involvement have been previously reported in literature. Arnold et al reported the case of a 20-year-old female of Turkish descent presenting with multiple papules on left arm and hand initially diagnosed as pyogenic granuloma. The lesions recurred after few months from the excision with a similar skin distribution but with also a brachial artery involvement. Clinically this arterial involvement was accompanied by a decreased in the strength of radial pulse in the left arm and US and MRI demonstrated a hypodense structure 2.7×1.6×1.4 mm in size, seated around the artery without infiltration of the vessel. Histological features were coherent with EH [11].
Vandy et al described the case of a 26-year-old Caucasian woman presented with a painless mass in her left upper arm. There were no associated skin lesions as in our case and arterial duplex imaging and MRI demonstrated a 1.6 diameter by 2 cm long well defined mass surrounding the brachial artery [12].
All three cases presented as a solid mass surrounding the artery of 2–3.1 cm in greatest axis. The case described by Vandy et al. was totally asymptomatic and surgery was performed for diagnostic purpose, after inconclusive fine-needle aspiration cytology.
Clinical presentation of our case was initially very similar to that of Vandy’s case, but excision was necessary because the lesion enlarged and become symptomatic due to compression of artery and median nerve.
Arnold’s case showed a peculiar clinical presentation with recurrent and multiple skin lesions involving the left arm and hand and with a weakening of the radial homolateral pulse. Notably despite its benign etiology, EH still carries a 33% local recurrence rate [1]. Eosinophilia was absent in all cases. Our patient had had a blood test in the same site 5 months before whereas no history of trauma was present in the other two cases.
Circumstantial description of histological features of EH associated with brachial artery was not reported in cases described by Arnold and Vandy. In the present case the lesion appeared to be centered in the media of the vessel, with some muscular fibers conserved both in the adventitial and luminal front. Intimal thickening could be observed but the lumen was preserved. Intra-luminal growth and destruction of the arterial wall is mainly described in temporal arteries and dermal vessels [14]. We can speculate that the larger caliber and high blood flow that characterized brachial artery and similar muscular arteries could preserve these vessels from occlusion by EH.
Differential diagnoses of EH include both benign and malignant disease. Kimura disease and EH were previously considered to be part of a single disease spectrum, but now it is clear that they are two different entities. Kimura disease is usually located in the subcutaneous tissue of the head and neck, with systemic lymphadenopathy, marked eosinophilia, and elevated serum immunoglobulin E level. Histopathology shows eosinophil and lymphocyte infiltration with obvious lymphoid follicles and a lack of epithelioid endothelial cell proliferation [2].
Organized thrombosis implies a vessel obstruction by fibrin and connective tissue embedding numerous neovessels. The thrombus recanalization could show multiple, small, endothelium-lined papillary structures with hyaline stalks, which have been named intravascular papillary endothelial hyperplasia (Masson’s tumor). However, vessels with irregular lumen and plump epithelioid cells are typically absent. This is true also for reaction to insect bite and vaccines that may present inflammatory infiltrate similar to EH but not the prominent vascular proliferation [2].
Other important differential diagnoses for EH include epithelioid hemangioendothelioma and angiosarcoma. Angiosarcomas could be easily distinguished from EH for presenting nuclear atypia with hyperchromatism, mitotic activity and scarce eosinophils in the inflammatory infiltrate [2].
Epithelioid hemangioendothelioma represents a well-differentiated angiosarcoma, so notwithstanding cytological similarities with EH, vascular proliferation show nuclear atypia, infiltrative margins and lacks inflammatory component [2].
Treatment for EH comprises both medical and surgical options. Medical treatment includes mainly radiotherapy as low-dose irradiation, intralesional steroid injections, laser, chemotherapy and criotherapy [11]. This kind of therapies could have a role in dermal lesion and in selected settings but not in deep-sited or large vessel lesions. Although the disease is benign, surgical excision appears to be the treatment of choice because a histological analysis is necessary for diagnosis.
Fine needle aspiration for initial diagnosis is often inconclusive [12, 34]. Tru-cut could be diagnostic as in our case, and could allow a wait-and-see policy. Nevertheless we cannot exclude that the relative rapid enlargement observed after biopsy was actually due to intralesional bleeding consequent to surgical procedure.
4 Conclusion
Summarizing, for preoperative evaluation of EH involving large arteries, eco color doppler sonography gives reliable, cost-effective details and allows to establish the relation of the lesion with the wall vessel. Otherwise RMI, even if performed with contrast agent, could fail to demonstrate this relationship given however a major precision on it border referring to the around anatomical structure. In solid lesion tru-cut represents a useful tool to join a correct diagnosis avoiding surgery, but it could cause hemorrhagic and inflammatory changes with rapid enlargement of the lesion. In symptomatic cases radical surgery still represent the treatment of choice and it should be performed with the cooperation of a vascular surgeon.
Footnotes
Conflict of interest statement: Authors state no conflict of interest
References
- 1.Fletcher CDM World Health Organization., and International Agency for Research on Cancer. World Health Organization classification of tumours. 4th ed. Lyon: IARC Press; 2013. WHO classification of tumours of soft tissue and bone; p. 468. [Google Scholar]
- 2.Armed Forces Institute of Pathology (U.S.) and Universities Associated for Research and Education in Pathology. Atlas of tumor pathology. 1–2 Washington: Armed Forces Institute of Pathology; 1991. (Third series). [Google Scholar]
- 3.Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81(1):1–14. doi: 10.1111/j.1365-2133.1969.tb15914.x. [DOI] [PubMed] [Google Scholar]
- 4.Fetsch JF, Weiss SW. Observations concerning the pathogenesis of epithelioid hemangioma (angiolymphoid hyperplasia) Mod Pathol. 1991;4(4):449–55. [PubMed] [Google Scholar]
- 5.Rosai J. Angiolymphoid hyperplasia with eosinophilia of the skin. Its nosological position in the spectrum of histiocytoid hemangioma. Am J Dermatopathol. 1982;4(2):175–84. doi: 10.1097/00000372-198204000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Fetsch JF, et al. Epithelioid hemangioma of the penis: a clinicopathologic and immunohistochemical analysis of 19 cases, with special reference to exuberant examples often confused with epithelioid hemangioendothelioma and epithelioid angiosarcoma. Am J Surg Pathol. 2004;28(4):523–33. doi: 10.1097/00000478-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Park Y, Chung J, Cho CG. Angiolymphoid hyperplasia with eosinophilia of the tongue: report of a case and review of the literature. Oral Oncol. 2002;38(1):103–6. doi: 10.1016/s1368-8375(01)00020-3. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes BF, et al. Epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia) of the orbit: a case report. J Med Case Rep. 2007;1:30. doi: 10.1186/1752-1947-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang M, Lloyd WC, O’Hara M. Angiolymphoid hyperplasia with eosinophilia: an unusual presentation in a child. J AAPOS. 2008;12(3):302–4. doi: 10.1016/j.jaapos.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Evans HL, Raymond AK, Ayala AG. Vascular tumors of bone: A study of 17 cases other than ordinary hemangioma, with an evaluation of the relationship of hemangioendothelioma of bone to epithelioid hemangioma, epithelioid hemangioendothelioma, and high-grade angiosarcoma. Hum Pathol. 2003;34(7):680–9. doi: 10.1016/s0046-8177(03)00249-1. [DOI] [PubMed] [Google Scholar]
- 11.Arnold M, et al. Unilateral angiolymphoid hyperplasia with eosinophilia involving the left arm and hand. J Cutan Pathol. 1999;26(9):436–40. doi: 10.1111/j.1600-0560.1999.tb01871.x. [DOI] [PubMed] [Google Scholar]
- 12.Vandy F, et al. Angiolymphoid hyperplasia involving large arteries. J Vasc Surg. 2008;47(5):1086–9. doi: 10.1016/j.jvs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. A clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12(5 Pt 1):781–96. doi: 10.1016/s0190-9622(85)70098-9. [DOI] [PubMed] [Google Scholar]
- 14.Hsiao HT, Wu YH. Intra-arterial angiolymphoid hyperplasia with eosinophilia of the temporal artery: report of two cases and review of the literature. Dermatol Sinica. 2012;30:97–100. [Google Scholar]
- 15.Aurello P, et al. Angiolymphoid hyperplasia with eosinophilia: a rare artery lesion. Anticancer Res. 2003;23(3C):3069–72. [PubMed] [Google Scholar]
- 16.Chopra P, Handoo A, Parakh R. Epithelioid hemangioma of the temporal artery: a case report. Indian J Pathol Microbiol. 2007;50(3):595–8. [PubMed] [Google Scholar]
- 17.Grum F, et al. High-resolution color-coded sonography in angiolymphoid hyperplasia with eosinophilia presenting as temporal arteritis. Circulation. 2010;121(8):1045–6. doi: 10.1161/CIR.0b013e3181d38e01. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura H, et al. Epithelioid hemangioma of the temporal artery clinically mimicking temporal arteritis. Pathol Int. 1999;49(9):831–5. doi: 10.1046/j.1440-1827.1999.00949.x. [DOI] [PubMed] [Google Scholar]
- 19.Koubaa W, et al. Intra-arterial angiolymphoid hyperplasia with eosinophilia. J Cutan Pathol. 2008;35(5):495–8. doi: 10.1111/j.1600-0560.2007.00837.x. [DOI] [PubMed] [Google Scholar]
- 20.Poilpre M, et al. [A nodule of the left temporal artery]. Ann Pathol. 1999;19(1):53–4. [PubMed] [Google Scholar]
- 21.Khaira HS, Deshmukh NS, Vohra RK. Angiolymphoid hyperplasia presenting as a radial artery aneurysm. Eur J Vasc Endovasc Surg. 1999;17(2):178–9. doi: 10.1053/ejvs.1998.0645. [DOI] [PubMed] [Google Scholar]
- 22.Morton K, Robertson AJ, Hadden W. Angiolymphoid hyperplasia with eosinophilia: report of a case arising from the radial artery. Histopathology. 1987;11(9):963–9. doi: 10.1111/j.1365-2559.1987.tb01902.x. [DOI] [PubMed] [Google Scholar]
- 23.Sandbank J, et al. Angiolymphoid hyperplasia with eosinophilia (epithelioid hemangioma) J Cardiovasc Surg (Torino) 1991;32(3):370–2. [PubMed] [Google Scholar]
- 24.Reed RJ, Terazakis N. Subcutaneous angioblastic lymphoid hyperplasia with eosinophilia (Kimura’s disease) Cancer. 1972;29(2):489–97. doi: 10.1002/1097-0142(197202)29:2<489::aid-cncr2820290239>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 25.Krapohl BD, et al. A rare vasoproliferative lesion: angiolymphoid hyperplasia with eosinophilia of the hand. Br J Plast Surg. 2003;56(2):168–70. doi: 10.1016/s0007-1226(03)00084-5. [DOI] [PubMed] [Google Scholar]
- 26.Ozcanli H, et al. Angiolymphoid hyperplasia: a case of a rare arterial involvement and successful recurrence treatment with laser therapy. J Eur Acad Dermatol Venereol. 2007;21(8):1106–7. doi: 10.1111/j.1468-3083.2006.02094.x. [DOI] [PubMed] [Google Scholar]
- 27.Kukreja N, Koslowski M, Insall R. Angiolymphoid hyperplasia with eosinophilia presenting as an axillary artery aneurysm. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.02.2011.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhat SP, et al. Angiolymphoid hyperplasia with eosinophilia presenting as a giant axillary artery aneurysm. Vascular. 2010;18(1):49–52. doi: 10.2310/6670.2009.00041. [DOI] [PubMed] [Google Scholar]
- 29.Selvaraj AD, et al. An unusual etiology of a subclavian artery aneurysm. Vasc Med. 2009;14(4):377–9. doi: 10.1177/1358863X08101857. [DOI] [PubMed] [Google Scholar]
- 30.Kimura Y, et al. Angiolymphoid hyperplasia with eosinophilia arising from the facial artery. J Laryngol Otol. 2003;117(7):570–3. doi: 10.1258/002221503322113067. [DOI] [PubMed] [Google Scholar]
- 31.Cornelius RS, Biddinger PW, Gluckman JL. Angiolymphoid hyperplasia with eosinophilia of the head and neck. AJNR Am J Neuroradiol. 1995;16(4 Suppl):916–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Ghotbi R, Sotiriou A, Mlinaric M. [Epithelioid hemangioma of the popliteal artery]. Chirurg. 1999;70(12):1494–6. doi: 10.1007/pl00002584. [DOI] [PubMed] [Google Scholar]
- 33.Abrahim MJ, Gregory ND, Chennupati SK. Epithelioid hemangioma of the internal carotid artery: a case report supporting the reactive pathogenesis hypothesis of this vascular tumor. Int J Pediatr Otorhinolaryngol. 2014;78(7):1186–9. doi: 10.1016/j.ijporl.2014.04.050. [DOI] [PubMed] [Google Scholar]
- 34.Chow LT, Yuen RW, Tsui WM, Ma TK, Chow WH, Chan SK. Cytologic features of Kimura’s disease in fine-needle aspirates. A study of eight cases. Am J Clin Pathol. 1994;102:316–21. doi: 10.1093/ajcp/102.3.316. [DOI] [PubMed] [Google Scholar]