Abstract
Uric acid, generated from the metabolism of purines, has both proven and emerging roles in human disease. Serum uric acid in humans is determined by production and by the net balance of reabsorption and secretion in kidney and intestine. In the human kidney, epithelial reabsorption dominates over secretion, such that in normal subjects there is at least 90% net reabsorption of filtered urate resulting in a fractional excretion of <10%. Tranilast, an anti‐inflammatory drug with pleiotropic effects, has a marked hypouricemic, uricosuric effect in humans. We report here that tranilast is a potent inhibitor of [14C]‐urate transport mediated by the major reabsorptive urate transporters (URAT1, GLUT9, OAT4, and OAT10) in Xenopus oocytes; this provides an unequivocal molecular mechanism for the drug's uricosuric effect. Tranilast was found to inhibit urate transport mediated by URAT1 and GLUT9 in a fully reversible and noncompetitive (mixed) manner. In addition, tranilast inhibits the secretory urate transporters NPT1, OAT1, and OAT3 without affecting the secretory efflux pump ABCG2. Notably, while benzbromarone and probenecid inhibited urate as well as nicotinate transport, tranilast inhibited the urate transport function of URAT1, GLUT9, OAT4, OAT10, and NPT1, without significantly affecting nicotinate transport mediated by SMCT1 (IC 50 ~1.1 mmol/L), SMCT2 (IC 50 ~1.0 mmol/L), and URAT1 (IC 50 ~178 μmol/L). In summary, tranilast causes uricosuria by inhibiting all the major reabsorptive urate transporters, selectively affecting urate over nicotinate transport. These data have implications for the treatment of hyperuricemia and gout, the pharmacology of tranilast, and the structure‐function analysis of urate transport.
Keywords: Benzbromarone, GLUT9, gout, hyperuricemia, tranilast, URAT1, uric acid
Abbreviations
- ABCG2
ATP‐binding cassette subfamily G, member 2
- AD
Alzheimer's disease
- BHB
β‐hydroxy butyrate
- Gluc
Gluconate
- GLUT
Glucose transporter
- Lac
Lactate
- MRP4
multidrug resistance protein 4
- MS
Multiple sclerosis
- MSU
Monosodium urate
- Nico
Nicotinate
- NPT
Sodium phosphate transporter
- OAT
Organic anion transporter
- PAH
para‐aminohippurate
- PD
Parkinson's Disease
- PZA
Pyrazinoic acid
- Sal
Salicylate
- SLC
solute carrier gene
- SMCT
Na+‐dependent monocarboxylate transporters
- SUA
Serum uric acid
- URAT1
Urate transporter‐1
- α‐KG
α‐ketoglutarate
Introduction
The causative link between hyperuricemia and disease is well established for gout, a common and excruciatingly painful inflammatory arthritis (Choi et al. 2005). Hyperuricemia has also been linked to the pathogenesis of gout‐associated comorbidities, including metabolic syndrome, hypertension (Perlstein et al. 2006; Feig et al. 2008; Forman et al. 2009), and diabetic nephropathy (Doria and Krolewski 2011). Hyperuricemia has an opposite, protective effect in neurodegenerative disease, including Parkinson's Disease (PD), multiple sclerosis (MS) (Hooper et al. 1998), and Alzheimer's disease/dementia (Spitsin and Koprowski 2010). Higher circulating uric acid levels thus reduce the risk of PD (Weisskopf et al. 2007) and the rate of disease progression (Ascherio et al. 2009).
In humans, 70–80% of serum urate is excreted in urine and the remaining 20–30% is secreted by the intestine. In the kidney, reabsorptive and secretory transporters maintain the balance between proximal tubular reabsorption and secretion (Mandal and Mount 2015). Approximately 90% of the urate filtered by renal glomeruli is reabsorbed into the blood by transepithelial transport in the renal proximal tubule, with ~10% fractional excretion; reabsorption thus dominates over secretion in the human kidney. Urate reabsorption in the proximal tubule involves the coordinated activity of several transporters. Sodium‐dependent reabsorption of organic monocarboxylates by apical Na+‐dependent monocarboxylate transporters SMCT1 and SMCT2, encoded by the SLC5A8 and SLC5A12 genes, respectively (Coady et al. 2004; Srinivas et al. 2005), increases the intracellular concentration of monocarboxylate anions that can then exchange with luminal urate via urate‐anion exchangers. Increases in the circulating concentrations of the SMCT substrates nicotinate, pyrazinoate, lactate, and ketones result in hyperuricemia (Gibson and Doisy 1923; Shapiro and Hyde 1957; Goldfinger et al. 1965; Gershon and Fox 1974), due to increased apical uptake of these filtered anions, increased intracellular concentrations in proximal tubular cells, and augmented urate‐anion exchange (Guggino and Aronson 1985). URAT1, encoded by SLC22A12 gene, is the dominant apical urate/anion exchanger in humans; loss‐of‐function mutations in SLC22A12 are associated with hypouricemia and hyperuricosuria. The “orphan” organic anion transporter (OAT) “ORCTL3″ (OAT10) has also been shown to mediate urate‐nicotinate exchange (Bahn et al. 2008). In addition, providing further heterogeneity, human OAT4 reportedly functions as an apical urate‐anion exchanger (Hagos et al. 2007), exchanging urate with divalent organic anions.
Multiple genome‐wide association studies (GWAS) have implicated variability in the SLC2A9 (solute carrier gene family‐2, member 9) gene that encodes GLUT9 (glucose transporter 9) in determining serum urate concentration (SUA) (Doring et al. 2008; Vitart et al. 2008; Wallace et al. 2008). GLUT9 mediates urate exit at the basolateral membrane during reabsorption of urate in the proximal tubule. Separate basolateral urate transporters, OAT1 (organic anion transporter 1) and OAT3, encoded by SLC22A6 and SLC22A8, respectively, function in urate secretion (Eraly et al. 2008), transporting urate from blood into proximal tubular cells for secretion at the apical membrane. Urate secretion at the apical membrane is mediated by ATP‐driven efflux pumps MRP4 (multi‐drug resistance protein 4) (Van Aubel et al. 2004) or ABCG2 (Matsuo et al. 2009; Woodward et al. 2009) and/or electrogenic apical urate transporters NPT1/Oatv1 (encoded by the SLC17A1 gene) (Jutabha et al. 2003; Iharada et al. 2010) and NPT4 (encoded by the SLC17A3 gene) (Jutabha et al. 2010).
Uricosuric drugs such as benzbromarone (Enomoto et al. 2002), probenecid (Enomoto et al. 2002), fenofibrate (Uetake et al. 2010), lesinurad (Fleischmann et al. 2014), and losartan (Iwanaga et al. 2007) have been shown to inhibit URAT1, without assessing the effects on the entire panel of reabsorptive urate transporters. Antiuricosuric agents (e.g., nicotinate, pyrazinoate) can serve as the exchanging anion from inside tubule cells, thereby enhancing urate transport by URAT1 and OAT10 through trans‐stimulation (Guggino and Aronson 1985; Mandal and Mount 2015); at higher concentrations, these anions can also be uricosuric, due to extracellular cis‐inhibition at the apical membrane. Notably, although literature comparisons can certainly be made, there has been no comprehensive study of the interactions between specific uricosurics and anti‐uricosurics with all the various reabsorptive and secretory urate transporters.
Urate‐lowering therapy is a mainstay in the management of gout. Currently available urate‐lowering drugs in the U.S. include allopurinol, a purine analog that inhibits the enzyme xanthine oxidase; probenecid, a urate transport inhibitor; and febuxostat, a nonpurine inhibitor of xanthine oxidase. A substantial fraction of patients with gout fail to achieve adequate urate lowering with the current available drugs, indicating a need for alternative medications. Tranilast [N‐(3,4‐dimethoxycinnamoyl) anthranilic acid], an effective anti‐allergic drug developed in Japan, has been widely used for more than 40 years in Asia for the clinical treatment of bronchial asthma, atopic rhinitis, atopic dermatitis, and keloids. Tranilast causes potent reduction in SUA in healthy human subjects, at least partially due to uricosuric effects (Sundy and Kitt 2010). It also reportedly suppresses inflammation induced by monosodium urate (MSU) crystals in vivo (Serafini and Emerling 2010), with potential dual utility for “flare prophylaxis” (Borstad et al. 2004) during urate reduction.
This study was initiated to clarify the molecular mechanisms of the uricosuric effect of tranilast. A perceived limitation of other reports regarding uricosuric agents was the exclusive focus on URAT1, hence we studied the interaction of tranilast with all the reabsorptive urate transporters, in addition to a representative subset of secretory transporters. A secondary goal afforded by this approach was thus an across‐the‐board comparison of transport characteristics for multiple urate transporters, studied contemporaneously in the same expression system.
Materials and Methods
Complementary RNA (cRNA) expression in Xenopus oocytes
Studies in animals have been carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health, and were approved by the Institution's Animal Care and use Committee or local equivalent. Mature female Xenopus laevis frogs (NASCO, Fort Atkinson, MI) were subjected to partial ovariectomy under tricane (Ethyl 3‐aminobenzoatemethanesulfonate, SIGMA St Louis, MO) anesthesia (0.17% for 15–20 min) as described previously (Mount et al. 1999). In brief, a small incision was made in the abdomen and a lobe of ovary was removed. Subsequently, the oocytes were prewashed for 5 min in Ca2+‐free ND96 medium (96 mmol/L NaCl, 2 mmol/L KCl, 1 mmol/L MgCl2, and 5 mmol/L HEPES, pH 7.4) to remove blood and damaged tissue. Oocytes were then defolliculated by treatment with 3.5 mg/mL collagenase A (Roche, Indianapolis, IN) in Ca2+‐free ND96 medium for 60–70 min with gentle agitation at room temperature (25°C). After this treatment, oocytes were washed four times in Ca2+‐free ND96 medium, and then incubated in isotonic Ca2+‐containing ND96 medium (96 mmol/L NaCl, 2.0 mmol/L KCl, 1.8 mmol/L CaCl2, 1.0 mmol/L MgCl2 and 5 mmol/L Hepes, pH 7.4) containing 2.5 mmol/L pyruvate, and gentamycin (10 μg/mL).
The indicated full‐length cDNAs were subcloned into the pGEMHE oocyte expression plasmid, 3′ of a T7 promoter and flanked by Xenopus laevis‐specific 5′‐ and 3′‐UTRs. Plasmid DNA was linearized at the 3′‐end of cDNAs by Not1, Nhe1, or EcoR1 digestion, and cRNA was in vitro transcribed by using T7 RNA polymerase (mMESSAGE mMACHINE; Ambion, Austin, TX) following the suppliers protocol. Isopropanol‐precipitated, in vitro transcribed capped cRNA was washed with 70% ethanol, dried and then dissolved in sterile nuclease‐free water. The yield and RNA integrity of the capped cRNA samples were assessed by spectroscopy (at 260 nm) and 1% agarose‐formaldehyde gel electrophoresis, respectively. All cRNA samples were stored frozen in aliquots at −80°C until used. About 18 h after isolation, oocytes were injected with 50 nL of sterile water, 50 mmol/L tris pH 7.4, or 50 nL of a cRNA solution in 50 mmol/L tris buffer (pH 7.4) containing 25 ng of the indicated cRNA using fine‐tipped micropipettes by a microinjector (World Precision Instrument Inc. Sarasota, FL). The injected oocytes were incubated in isotonic ND96 medium (pH 7.4) containing 1.8 mmol/L CaCl2, 2.5 mmol/L pyruvate, and gentamycin (10 μg/mL) at 16–18°C for approximately 48 h to allow expression of protein from injected cRNA.
Western blotting
Total cellular protein for western blot analysis was prepared from groups of ~100 X. laevis oocytes injected with relevant cRNAs that were transcribed in vitro from related constructs. After 48 h of expression of protein from the injected cRNA, oocytes were transferred to 1.7 mL polypropylene microfuge tubes on ice and were lysed using a Teflon homogenizer in lysis buffer (50 mmol/L Tris‐HCl, pH 7.5, 50 mmol/L NaCl, 1 mmol/L EDTA, pH 8, 1% Triton X‐100) supplemented with protease inhibitors cocktail (Roche, Indianapolis, IN). After clearing the lysate off yolk and cellular debris by centrifugation at 2665 g for 10 min, the supernatant was stored at −80°C. Western blotting was performed using affinity‐purified rabbit polyclonal anti‐SLC2A9/GLUT9, anti‐URAT1, or anti‐OAT10 antibodies (MBL; Medical & Biological Laboratories Co. Ltd.) at a titer of 1:1000. Total lysates of proteins were fractionated using 7.5% SDS/PAGE gel electrophoresis (BIO‐RAD, Hercules, CA). Proteins were transferred to polyvinylidene difluoride (PVDF) membrane (BIO‐RAD) at 100 V for 3 h. The membrane was blocked in 5% nonfat dried milk in TBST. Primary antibodies were diluted in 5% milk in TBST and incubated with the membrane at room temperature for 2 h with continuous gentle shaking. Blots were washed in TBST and probed with an HRP‐conjugated secondary antibody (BIO‐RAD) in TBST containing 5% fat‐free milk for 1 h at RT. The membrane was then washed four times with TBST and chemiluminescence performed using ECL (PIERCE; Rockford, IL), following standard protocols.
Transport assays
For uptake experiments, oocytes were washed four times with ND96 medium (pH 7.4) without pyruvate and gentamycin. After approximately 60 min of starvation, oocytes were preincubated in the indicated isotonic uptake medium [(96 mmol/L NaCl, 2.0 mmol/L KCl, 1.8 mmol/L CaCl2, 1.0 mmol/L MgCl2, and 5 mmol/L Hepes, pH 7.4) or (98 mmol/L KCl, 1.8 mmol/L CaCl2, 1.0 mmol/L MgCl2, and 5 mmol/L Hepes, pH 7.4) or Cl‐ free, uptake medium (100 mmol/L Na‐gluconate, k‐gluconate or, NMDG‐gluconate, 2 mmol/L k‐gluconate, 1 mmol/L Mg‐gluconate, 1 mmol/L Ca‐gluconate, 10 mmol/L HEPES, pH 7.4,) for about 30 min in the absence or presence of indicated concentration of drugs [tranilast (Nuon Therapeutics Inc. San Francisco, CA), benzbromarone (SIGMA), or probenecid (SIGMA)] and then incubated in the same medium containing [14C]urate/[14C]nicotinate (40 μmol/L) in the absence or presence of indicated concentration of drugs or organic anions for 1 h. In other sets of experiment, control or indicated cRNA‐injected protein‐expressing oocytes were preinjected with 50 nL of water or unlabeled test organic anions [100 mmol/L pyrazine carboxylate (PZA), pH 7.4] using fine‐tipped micropipettes and then transferred to the isotonic medium (86 mmol/L NaCl, 10 mmol/L PZA, 2.0 mmol/L KCl, 1.8 mmol/L CaCl2, 1.0 mmol/L MgCl2 and 5 mmol/L Hepes, pH 7.4) containing 10 mmol/L PZA or indicated other organic anions and incubated for 2 h for recovery before the uptake assay. After 60 min of incubation in the indicated uptake medium containing [14C]urate/[14C]nicotinate (40 μmol/L) at room temperature [25°C] in a horizontal shaker‐incubator, oocytes (15–20 in each group) were washed three times with the ice‐cold uptake medium to remove external radioisotope.
For [14C]‐urate efflux studies, oocytes expressing ABCG2 were preinjected with 50 nL of 1500 μmol/L [14C]‐urate dissolved in efflux medium (ND96, pH 7.4). Preinjected oocytes were then incubated in ND96 medium for 1 h at 16°C for recovery. After incubation, the oocytes were washed in cold ND96 medium four times to remove any external adhering [14C]‐urate from the oocytes and then subjected to efflux for 1 h at room temperature (~25°C) in ND96 medium (pH 7.4) in the absence or presence of drug.
Radioisotope content of each individual oocyte was measured by scintillation counter after solubilization in 0.3 mL of 10% (v/v) SDS and addition of 2.5 mL of scintillation fluid. All uptake experiments included at least 15 oocytes in each experimental group, with multiple controls as appropriate; statistical significance for individual experiments was defined as two‐tailed P < 0.05, and results were reported as means ± S.E. All the uptake experiments shown were performed at least three times for confirmation; data for each figure are from a single representative experiment. Statistical analyses including linear regressions were done using SigmaPlot software.
Results
Tranilast inhibits urate transport mediated by URAT1
Multiple uricosuric drugs have been shown to inhibit human URAT1, one of three urate/anion exchangers at the apical membrane of human proximal tubule cells. URAT1 is thought to be the dominant urate‐anion exchanger in proximal tubular urate reabsorption, given that recessive loss‐of‐function mutations in the SLC22A12 gene encoding URAT1 are associated with hypouricemia (Enomoto et al. 2002). To test the transport function of URAT1 in vitro, we preinjected URAT1 cRNA into Xenopous oocytes, examined URAT1 protein expression by western blotting, and then measured the [14C]‐urate uptake activity of the URAT1 in various isotonic uptake media at an extracellular [14C]‐urate concentration of 40 μmol/L. We found that in isotonic ND96 medium (pH 7.4), the URAT1‐expressing oocytes showed about 5–6 fold higher [14C]‐urate uptake activity over the water‐injected control oocytes (Fig. 1A and B). When the Na+ in ND96 medium was substituted by K+ or Li+, the [14C]‐urate uptake activity of URAT1 was only slightly (P < 0.01) affected (Fig. 1B) which was in agreement with the previous observation (Enomoto et al. 2002) that urate transport via URAT1 is sodium‐independent. In chloride‐free medium (pH 7.4), URAT1 showed 17–21 fold higher [14C]‐urate uptake activity over the water‐injected control oocytes (Fig. 1B) suggesting either a capacity for urate/Cl− exchange mechanism in URAT1 or unrelated cis‐inhibitory effects of extracellular Cl−. Antiuricosuric anions are thought to increase urate reabsorption by URAT1 through their trans‐stimulatory effects; therefore, we evaluated the trans‐stimulatory effect of preloaded intracellular pyrazinoate (PZA), nicotinate, lactate, p‐aminohippurate, β‐hydroxy butyrate, and salicylate on urate uptake via URAT1. When URAT1‐expressing oocytes were preloaded with nonlabeled PZA or nicotinate using a fine micropipette or by preincubating isotonic medium for 3–4 h, urate uptake activity was stimulated to 70–75 fold higher than PZA‐ or nicotinate‐preloaded control oocytes (Fig. 1C), which was consistent with previous observations (Enomoto et al. 2002). We did not however detect any significant trans‐stimulatory effect of preloaded intracellular lactate, para‐aminohippurate (PAH), and β‐hydroxybutyrate (BHB) on URAT1‐mediated urate uptake (Fig. 1C), perhaps due to metabolism of microinjected lactate and other anions. We also found that the URAT1‐mediated urate uptake was not cis‐inhibited by 10 mM extracellular organic anions such as lactate (Fig. 1D), pyruvate, BHB, PAH, α‐ketoglutarate (α‐KG), formate, oxalate, citrate, succinate, and maleate (data not shown), despite cis‐inhibition by 10 mmol/L extracellular nicotinate, PZA, and salicylate (Fig. 1D).
Figure 1.

Tranilast inhibits urate transport mediated by the human urate transporter URAT1 expressed in Xenopus laevis oocytes. (A) Time course of [14C]‐urate uptake by control and URAT1‐expressing oocytes. [14C]‐urate uptake by URAT1‐expressing oocytes was trans‐stimulated by preinjecting with 50 nL of 100 mmol/L PZA or nicotinate (Nico) 2 h before [14C]‐urate uptake experiment. Urate uptakes in the control oocytes were identical, resulting in superimposed data. Inset, western blot analysis of URAT1 protein expressed in oocytes. (B) [14C]‐urate transport properties of URAT1: voltage sensitivity and Na+‐dependence was examined by replacing extracellular NaCl by KCl or LiCl. Chloride effects were also examined; for the 0 Cl− bath, NaCl was replaced by Na‐gluconate (Na‐Gluc), KCl by K‐gluconate (K‐Gluc), MgCl2 by Mg‐gluconate, and CaCl2 by Ca‐gluconate. *P < 0.001 compared with NaCl (ND96). (C) Trans‐stimulatory effects of preloaded organic anions on [14C]‐urate uptake by URAT1‐expressing oocytes: [14C]‐urate uptake rate via URAT1 was measured in URAT1‐expressing oocytes preloaded with 50 nL of 100 mmol/L Lac, Nico, PZA, PAH, BHB or salicylate (Sal). *P < 0.001 compared with water‐injected control; NS, not significant. (D). Inhibition of [14C]urate by URAT1 in the presence of extracellular organic anions or uricosuric drugs, including tranilast: the uptake of [14C]‐urate (40 μmol/L) by URAT1‐expressing oocytes in exchange of preloaded intracellular nicotinate was determined after 1 h in the absence or presence of inhibitors (uricosuric drugs or organic anions) that were added to the extracellular medium (pH 7.4) at the indicated concentrations. *P < 0.001 compared with DMSO. Tran, Benz, Prob, DMSO. Data are mean ± S.E. with n = 12–15. (E) The 50% inhibitory concentration (IC 50) curve of tranilast for [14C]‐urate uptake‐mediated by URAT1 in oocytes preloaded with nicotinate. PZA, pyrazine carboxylate; PAH, para‐aminohippurate; Tran, tranilast; Benz, benzbromarone; Prob, probenecid; DMSO, dimethylsulfoxide.
We then proceeded to examine the effect of varying concentrations of tranilast on URAT1‐mediated [14C]‐urate uptake. We found that tranilast inhibited basal URAT1‐mediated [14C]‐urate uptake, that is, uptake in the absence of PZA or nicotinate preloading, in a dose‐dependent manner with a 50% inhibitory concentration (IC50) of ~20 μmol/L (data not shown). We also found that tranilast very efficiently inhibited URAT1‐mediated [14C]‐urate/PZA or [14C]‐urate/nicotinate exchange (Fig. 1D) in a dose‐dependent manner with an IC50 of ˜21 μmol/L (Fig. 1E). In parallel, we found that both benzbromarone (100 μmol/L) and probenecid (1 mmol/L) also efficiently inhibited URAT1‐mediated [14C]‐urate/PZA or [14C]‐urate/nicotinate exchange (Fig. 1D) with an IC50 of benzbromarone ~0.45 μmol/L for URAT1‐mediated [14C]‐urate uptake or [14C]‐urate/PZA exchange (data not shown).
Tranilast inhibits urate transport mediated by GLUT9a
Human GLUT9‐mediated urate transport has been shown to be electrogenic and affected by changes in membrane potential (Anzai et al. 2008; Bibert et al. 2009; Witkowska et al. 2012). GLUT9 has two isoforms with divergent N‐terminal cytoplasmic domains, generated by transcriptional initiation at alternative promoters; these isoforms have equivalent urate transport activity when expressed in Xenopus laevis oocytes (Anzai et al. 2008). We sought to investigate the effect of tranilast on GLUT9a‐mediated [14C]‐urate uptake in Xenopus laevis oocytes. We found that the [14C]‐urate uptake activity of GLUT9a‐expressing oocytes was 37–39 fold higher than that of water‐injected control oocytes (Fig. 2A and B) in isotonic ND96 uptake medium at an extracellular [14C]‐urate concentration of 40 μmol/l [14C]‐urate. The urate uptake activity of GLUT9a in depolarized oocytes (Fig. 2B), induced by the replacement of extracellular Na+ by K+, was found to be 140–150 fold higher than control oocytes. The complete removal of Cl− ion from the extracellular medium also increased the urate uptake activity of GLUT9a, to130–140 fold over control (Fig. 2B). This urate uptake was efficiently inhibited by both tranilast (100 μmol/L) and benzbromarone (100 μmol/L) but only about 60% inhibited by probenecid (1.0 mmol/L) (Fig. 2 C and D); PZA, nicotinate, and salicylate had no effect on GLUT9a activity (Fig. 2C). Tranilast inhibited GLUT9a‐mediated [14C]‐urate uptake in a dose‐dependent manner with an IC50 of ~15.6 μmol/L (Fig. 2E); for benzbromarone, the IC50 was ~14.2 μmol/L (data not shown). The IC50 of tranilast for GLUT9a remained unchanged by membrane depolarization, that is, the drug was equally effective under both membrane‐polarized and depolarized conditions (data not shown).
Figure 2.

Tranilast inhibits urate transport mediated by the human urate transporter GLUT9a. (A) Time course of [14C]‐urate uptake by control and GLUT9a‐expressing oocytes. Inset, western blot analysis of GLUT9a protein expressed in oocytes. (B) [14C]urate transport properties of GLUT9a: voltage sensitivity and Na+‐dependence was examined by replacing extracellular NaCl by KCl or LiCl. We also assessed [14C]‐urate uptake by GLUT9a in the absence of extracellular Cl− ion (0 Cl−); in 0 Cl− bath, NaCl was replaced by Na‐gluconate (Na‐Gluc), KCl by k‐gluconate (K‐Gluc), MgCl2 by Mg‐gluconate, and CaCl2 by Ca‐gluconate. Asterisk, P < 0.001 compared with NaCl (ND96); NS, not significant. (C) Inhibition of [14C]‐urate uptake by GLUT9a in the presence of antiuricosuric or uricosuric drugs: The uptake of [14C]‐urate (40 μmol/L) by GLUT9a‐expressing oocytes was determined in complete absence of Na+ (NaCl of ND96 medium was replaced by KCl) after 1 h in the absence or presence of inhibitors (uricosuric drugs) which were added to the extracellular medium (pH 7.4) at the indicated concentrations. *P < 0.001 compared with DMSO. (D) Inhibition of [14C]‐urate uptake by GLUT9a in depolarized cells. The uptake of [14C]‐urate (40 μmol/L) by GLUT9a‐expressing oocytes was determined in the complete absence of Na+ (NaCl of ND96 medium was replaced by KCl) after 1 h in the absence or presence of inhibitors (uricosuric drugs) at the indicated concentrations. *P < 0.001 compared with DMSO. Tran, Benz Prob, Sal, DMSO. Data are mean ± S.E. with n = 12–15. Data are mean ± S.E. with n = 12–15. (D) The 50% inhibitory concentration (IC 50) curve of tranilast for [14C]‐urate uptake via GLUT9a. Tran, tranilast; Benz, benzbromarone; Prob, probenecid; Sal, salicylate; DMSO, dimethylsulfoxide.
Tranilast inhibits urate transport mediated by OAT4
Human OAT4 reportedly functions as an apical urate/dicarboxylate exchanger at the apical membrane of renal proximal tubule cells (Hagos et al. 2007). We sought to investigate whether tranilast inhibits [14C]‐urate uptake activity of OAT4 in Xenopus laevis oocytes that express OAT4 protein. We found that the OAT4‐mediated [14C]‐urate uptake was approximately twofold higher than that of water‐injected control oocytes (Fig. 3A) in ND96 medium. Preloading with intracellular maleate had a modest trans‐stimulatory effect on OAT4‐mediated urate uptake (Fig. 3A) compared to other preloaded organic anions. We also found that the OAT4‐mediated trans‐stimulation of urate uptake by maleate was efficiently cis‐inhibited by 10 mM extracellular maleate with much less cis‐inhibition by 10 mmol/L succinate or α‐KG (Fig. 3B). OAT4‐mediated [14C]‐urate uptake was very efficiently inhibited by tranilast (100 μmol/L), benzbromarone (100 μmol/L), but only 80% inhibited by probenecid (1 mmol/L) (Fig. 3B). The IC50 of tranilast for [14C]‐urate uptake by OAT4 was found to be ~22 μmol/L (data not shown).
Figure 3.

Tranilast inhibits urate transport mediated by the human urate/dicarboxylate exchanger OAT4. (A) The uptake of [14C]‐urate by OAT4‐expressing oocytes was performed in ND96 medium (pH 7.4) at ~25°C for 1 h. Na+‐dependence was examined by replacing extracellular NaCl by KCl. The [14C]‐urate uptake by OAT4‐expressing oocytes was found to be significantly trans‐stimulated when OAT4‐expressing oocytes were preinjected with 50 nL of 100 mmol/L maleate (Mal); preinjection with 50 nL of 100 mmol/L succinate (Succi), α‐ketogluterate (α‐KG), nicotinate (Nico), or PZA had no significant effects. Asterisk, P < 0.001 compared with water‐injected control. (B) The inhibition of [14C]‐urate uptake by OAT4 was examined in the presence of extracellular organic anions (for cis‐inhibition) or uricosuric drugs at the indicated concentrations. Asterisk, P < 0.001 compared with DMSO/NaCl(ND96). Tran, Benz, Prob, DMSO. Data are mean ± S.E. with n = 12–15. PZA, pyrazine carboxylate; Tran, Tranilast; Benz, Benzbromarone; Prob, probenecid; DMSO, dimethylsulfoxide; Lac, lactate; Nico, nicotinate.
Tranilast inhibits urate transport mediated by OAT10
The hORCTL3/OAT10 (human organic cation transporter like 3/organic anion transporter‐10, encoded by SLC22A13) has been characterized as a urate transporter and high‐affinity nicotinate exchanger (Bahn et al. 2008); it functions in apical urate reabsorption in the proximal tubule. We sought to investigate whether tranilast inhibits [14C]‐urate uptake activity of OAT10 in Xenopus laevis oocytes that express OAT10 protein. We found that the OAT10‐mediated [14C]‐urate uptake was ~4–5 fold higher than that of water‐injected control oocytes (Fig. 4A–C) in ND96 medium. Preloading with the intracellular organic anions, PZA, nicotinate or BHB, had a modest trans‐stimulatory effect on OAT10‐mediated urate uptake (Fig. 4B). In chloride‐free medium (pH 7.4), OAT10 showed negligible [14C]‐urate uptake activity (Fig. 4C) indicating OAT10‐mediated urate transport is dependent on extracellular Cl−, which is in marked contrast with the behavior of URAT1. OAT10‐mediated [14C]‐urate uptake was very efficiently inhibited by both tranilast (100 μmol/L) and benzbromarone (100 μmol/L), however, probenecid (1.0 mmol/L) inhibited about 65% of the urate transport activity of OAT10 (Fig. 4D). The IC50 of tranilast for [14C]‐urate uptake by OAT10 was found to be ~31 μmol/L, and for benzbromarone, it was ~20 μmol/L (data not shown).
Figure 4.

Tranilast inhibits urate transport mediated by the human urate transporter OAT10. (A) Time course of [14C]‐urate uptake by control and URAT1‐expressing oocytes. [14C]‐urate uptake by OAT10‐expressing oocytes was trans‐stimulated by preinjecting with 50 nL of 100 mmol/L PZA 2 h. Inset, western blot analysis of OAT10 protein expressed in oocytes. (B) Trans‐stimulatory effects of preloaded organic anions on [14C]‐urate uptake by OAT10‐expressing oocytes: [14C]‐urate uptake rate mediated by OAT10 was measured in OAT10‐expressing oocytes preloaded with 50 nL of 100 mmol/L Lac, Nico, PZA, PAH, BHB, α‐ketoglutarate (α‐KG) or succinate (Succi). *P < 0.001 compared with water‐injected control. (C) [14C]‐urate transport properties of OAT10: voltage sensitivity and Na+‐dependence was examined by replacing extracellular NaCl by KCl or LiCl. Existence of urate/Cl− exchange mechanism or Cl− dependence or cis‐inhibitory effects of extracellular Cl− on was verified by [14C]‐urate uptake via OAT10 in the absence of extracellular Cl− ion (0 Cl−). In 0 Cl− bath, NaCl was replaced by Na‐gluconate (Na‐Gluc), KCl by k‐gluconate (K‐Gluc), MgCl2 by Mg‐gluconate, and CaCl2 by Ca‐gluconate. Asterisk, P < 0.001 compared with NaCl (ND96); NS, not significant. (D) Inhibition of [14C]‐urate by OAT10 in the presence of extracellular organic anions or uricosuric drugs: The uptake of [14C]‐urate (40 μmol/L) by OAT10‐expressing oocytes in exchange of preloaded intracellular PZA was determined after 1 h in the absence or presence of inhibitors (uricosuric drugs) that were added to the extracellular medium at the indicated concentrations. *P < 0.001 compared with NaCl (ND96). Tran, Benz, Prob, DMSO. Data are mean ± S.E. with n = 12–15. (E) The 50% inhibitory concentration (IC 50) curve of tranilast for [14C]‐urate uptake via OAT10 in oocytes preloaded with PZA. PZA, pyrazine carboxylate; PAH, para‐aminohippurate; Lac, lactate; Nico, nicotinate; PZA, pyrazine carboxylate; Tran, tranilast; Benz, benzbromarone; Prob, probenecid; DMSO, dimethylsulfoxide.
Tranilast inhibits urate transport via URAT1 and GLUT9a in a reversible, noncompetitive (mixed) manner
To determine the reversibility of urate transport inhibition, oocytes expressing URAT1 or GLUT9a were preincubated with 100 μmol/L tranilast and then subjected to [14C]‐urate uptake for 1 h after washing and removal of the inhibitor. We found almost no inhibition of urate transport mediated by URAT1 (Fig. S1) or GLUT9a (Fig. S2) after removal of tranilast from the uptake medium. When similar experiments were performed in parallel for benzbromarone, the urate uptake was substantially inhibited even in the absence of additional benzbromarone (Figs. 1, 2). These results indicate that tranilast reversibly inhibited URAT1‐ or GLUT9a‐mediated urate transport, whereas inhibition of URAT1‐ and GLUT9a‐mediated urate transport by benzbromarone was not immediately reversible.
To further characterize the mechanism of inhibition of urate transport caused by tranilast, we examined the kinetics of both URAT1 and GLUT9a‐mediated [14C]‐urate uptake for 1 h at room temperature [25°C], using increasing concentrations of [14C]‐urate in ND96 medium (pH 7.4) containing no inhibitor, 50 μmol/L cold urate, or 15–40 μmol/L tranilast (Fig. 5A and B) and then analyzing the mode of inhibition after Eadie–Hofstee linearizations (Fig. 5C and D). The results shown in Figure 5C demonstrate that the inhibition of URAT1‐mediated urate transport by tranilast closely resembles a noncompetitive inhibition (mixed) model since the V max was decreased from 233.4 to 120.1 pmoles/oocyte/h and the apparent K m (K m app) was decreased from 548 μmol/L to 522 μmol/L by 20 μmol/L of tranilast. In the presence of 40 μmol/L of tranilast, the V max was decreased from 233.4 to 63.5 pmoles/oocyte/h and the apparent K m (K m app) was decreased from 548 μmol/L to 460 μmol/L. The inhibition constant (K i) of tranilast for URAT1‐mediated urate uptake was determined to be 21.33 ± 2.2 μmol/L. As expected for a noncompetitive mechanism (Cheng and Prusoff 1973), the inhibition constant is equivalent to the measured IC50 for URAT1.
Figure 5.
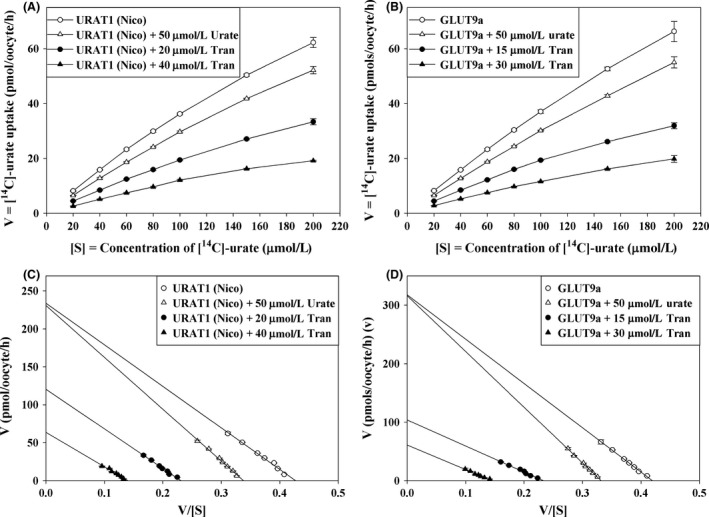
Tranilast inhibits urate transport activity of URAT1 and GLUT9a in a noncompetitive (mixed) manner. (A) The kinetic curve of URAT1‐mediated [14C]‐urate uptake in the absence or presence of inhibitor (cold urate or tranilast). (B) The kinetic curve of GLUT9a‐mediated [14C]‐urate uptake in the absence or presence of inhibitor (cold urate or tranilast). (C) Eadie–Hofstee plot of URAT1‐mediated [14C]‐urate uptake in the absence or presence of inhibitor. (D) Eadie–Hofstee plot of GLUT9a‐mediated [14C]‐urate uptake in the absence or presence of inhibitor. V, the [14C]‐urate uptake rate in pmol/oocyte/h; V/S, [14C]‐urate uptake rate per concentration (μmol/L) of [14C]‐urate; open circle, in the absence of inhibitor; open circle, in the absence of inhibitor; open triangle, in the presence 50 μmol/L cold urate; closed circle 15 or 20 μmol/L tranilast; closed triangle 30 or 40 μmol/L tranilast. Tran, Benz, Prob, SalDMSO, W, oocytes were washed out of drugs with uptake medium. Data are mean ± S.E. with n = 12–15. Tran, Tranilast; Benz, Benzbromarone; Prob, probenecid; Sal, salicylate; DMSO, dimethylsulfoxide; W, oocytes were washed out of drugs with uptake medium.
For GLUT9a, (Fig. 5D) the V max was decreased from 317.7 to 108.4 pmoles/oocyte/h and the apparent K m (K m app) was decreased from 757.5 μmol/L to 452.6 μmol/L by 15 μmol/L of tranilast. In the presence of 30 μmol/L of tranilast, the Vmax was decreased from 317.7 to 61 pmoles/oocyte/h and the apparent K m (K m app) was decreased from 757.51 μmol/L to 420 μmol/L. Therefore, the results shown in Figure 5D demonstrate that the inhibition of GLUT9a‐mediated urate transport by tranilast also closely resembles a noncompetitive (mixed) inhibition model. The inhibition constant (K i) of tranilast for GLUT9a‐mediated urate uptake was determined to be 17.13 ± 1.3 μmol/L. As expected for a noncompetitive mechanism (Cheng and Prusoff 1973), the inhibition constant is equivalent to the measured IC50 for GLUT9a.
URAT1‐mediated nicotinate transport is resistant to tranilast
Since organic anions such as nicotinate and pyrazine carboxylate (PZA) were found to trans‐stimulate as well as cis‐inhibit URAT1‐mediated urate uptake (Fig. 1 A, C and D), we sought to determine whether extracellular [14C]‐nicotinate is transported by URAT1 and whether this activity is sensitive to tranilast. We found that the URAT1‐expressing oocytes showed ~2 fold higher [14C]‐nicotinate uptake activity over water‐injected control oocytes in ND96 medium at an extracellular [14C]‐nicotinate concentration of 40 μmol/L; this activity was time‐dependent (Fig. S3), Na+‐independent, and inhibited by extracellular Cl− (Fig.S4). Preloading with PZA had a significant trans‐stimulatory effect (about 19–20 fold higher than the water‐injected control oocytes) on URAT1‐mediated [14C]‐nicotinate uptake (Fig. 6A) compared to other preloaded organic anions. We then examined the effect of varying concentrations of tranilast on URAT1‐mediated [14C]‐nicotinate/PZA exchange. Surprisingly, we found that URAT1‐mediated [14C]‐nicotinate uptake was significantly resistant to tranilast (Fig. 6B) with an IC50 of ~190 μmol/L (Fig. 6C), whereas [14C]‐nicotinate uptake remained very sensitive to benzbromarone (IC50 ~0.42 micromol/L, i.e. using the Greek for micro as in all the other IC50s) and probenecid (data not shown). The URAT1‐mediated [14C]‐nicotinate uptake was also cis‐inhibited by the presence of extracellular urate, PZA, or salicylate (data not shown).
Figure 6.

Functional characterization of human URAT1 as a nicotinate transporter; effects of uricosuric drugs. (A) Trans‐stimulatory effects of preloaded organic anions on [14C]‐nicotinate uptake by URAT1‐expressing oocytes: [14C]‐nicotinate transport rate via URAT1 was measured in URAT1‐expressing oocytes preloaded with 50 nL of 100 mmol/L Lac, PZA, PAH, BHB, or urate. *P < 0.001 compared with water‐injected control; NS, not significant. (B) Inhibition of [14C]‐nicotinate by URAT1 in the presence of extracellular urate or uricosuric drugs: The uptake of [14C]‐nicotinate (40 μmol/L) by URAT1‐expressing oocytes in exchange of preloaded intracellular PZA was determined after 1 h in the absence or presence of inhibitors (uricosuric drugs) that were added to the extracellular medium (pH 7.4) at the indicated concentrations. *P < 0.001 compared with DMSO/NaCl(ND96). Tran, Benz, Prob, DMSO. (C) The 50% inhibitory concentration (IC 50) curve of tranilast for [14C]‐nicotinate uptake by URAT1‐expressing oocytes preinjected with 50 nL of 100 mmol/L PZA 2 h before [14C]‐nicotinate uptake experiment. Data are mean ± S.E. with n = 12–15. BHB, β‐hydroxy butyrate; PAH, para‐aminohippurate; Lac, lactate, PZA, pyrazine carboxylate; PAHe , para‐aminohippurate; Tran, Tranilast; Benz, Benzbromarone.
SMCT1‐ and SMCT2‐mediated nicotinate transport is resistant to tranilast
SMCT1 (encoded by SLC5A8) and SMCT2 (encoded by SLC5A12) are sodium‐coupled renal monocarboxylate transporters (SMCTs), the former high‐affinity and the latter low‐affinity (Coady et al. 2004; Srinivas et al. 2005). SMCT1 and SMCT2 transport a variety of monocarboxylates, including nicotinate, lactate, and pyruvate, in a Na+‐dependent manner (Coady et al. 2004; Srinivas et al. 2005). The transport of urate via URAT1 is known to be driven by the intracellular nicotinate or PZA concentration, such that by loading cells with these substrates, SMCT1 and SMCT2 collaborate in the proximal tubule with URAT1 and OAT10 in apical urate reabsorption (Mandal and Mount 2015). We sought to investigate the effect of tranilast on SMCT1‐ and SMCT2‐mediated [14C]‐nicotinate uptake in oocytes. We first examined the [14C]‐nicotinate uptake activity of SMCT1 and SMCT2 expressed in Xenopous oocytes 48 h after injection of the respective cRNA. We found that SMCT1‐mediated [14C]‐nicotinate uptake activity was Na+‐, Ca2+‐, and Cl− ion‐dependent and 150–160 fold higher than the water‐injected control oocytes in ND96 medium (Fig. 7A). The SMCT2‐mediated [14C]‐nicotinate uptake activity was primarily Na+ ion‐dependent and 17–18 fold higher than the water‐injected control oocytes in ND96 medium (Fig. 7B). Both SMCT1 and SMCT2‐mediated [14C]‐nicotinate uptake were found to be very resistant to tranilast (IC50~1.1 mmol/L for SMCT1 and IC50~1.0 mmol/L for SMCT2) (data not shown) but sensitive to benzbromarone (IC50~49.6 μmol/L for SMTC1 and ~61.3 μmol/L for SMCT2) (data not shown). SMCT1 was significantly inhibited by 1.0 mmol/L probenecid, whereas SMCT2 remained almost unaffected (Fig. 7C).
Figure 7.

Functional and pharmacological characterization of human nicotinate transporters, hSMCT1, SMCT2, and OAT10. The uptake of [14C]‐nicotinate (40 μmol/L) by SMCT1‐, SMCT2‐ or OAT10‐expressing oocytes was performed in ND96 medium (pH 7.4) at ~25°C. [14C]‐nicotinate transport properties of nicotinate transporters: voltage sensitivity and Na+‐dependence was examined by replacing extracellular NaCl by KCl or LiCl. Chloride ion‐dependence was also assessed; in 0 Cl− bath, NaCl was replaced by Na‐gluconate (Na‐Gluc), KCl by K‐gluconate (K‐Gluc), MgCl2 by Mg‐gluconate, and CaCl2 by Ca‐gluconate. (A) [14C]‐nicotinate transport properties of human SMCT1 (B) [14C]nicotinate transport properties of human SMCT2. *P < 0.001 compared with NaCl (ND96). (C) Inhibition of [14C]‐nicotinate uptake via SMCT1, and SMCT2 in the presence of uricosuric drugs added into the extracellular medium (pH 7.4) at the indicated concentrations. P < 0.01 compared with NaCl (ND96); NS, not significant. (D) [14C]‐nicotinate transport properties of human OAT10 in oocytes preloaded with PZA. *P < 0.001 compared with uptake in the absence of inhibitor; NS, not significant. (E) Inhibition of [14C]‐nicotinate uptake via OAT10 (preloaded with PZA) in the presence of uricosuric drugs added into the extracellular medium (pH 7.4) at the indicated concentrations. P < 0.01 compared with NaCl (ND96); NS, not significant. Tran, Benz, Prob, DMSO. Data are mean ± S.E. with n = 12–15. PZA, pyrazine carboxylate; Tran, Tranilast; Benz, Benzbromarone; Prob, probenecid; DMSO, dimethylsulfoxide.
OAT10‐mediated nicotinate transport is sensitive to tranilast
Since OAT10 is a high‐affinity nicotinate exchanger (Bahn et al. 2008), we sought to investigate the effect of tranilast on OAT10‐mediated [14C]‐nicotinate uptake through its expression in Xenopus laevis oocytes. We first examined the [14C]‐nicotinate uptake activity of OAT10 expressed in Xenopous oocytes 48 h after preinjection of the respective cRNA. We found that the human OAT10‐mediated [14C]‐nicotinate uptake was 17–18 fold higher than the water‐injected control oocytes (Fig. 7D) in ND96 medium. In chloride‐free medium (pH 7.4), OAT10 showed very little [14C]‐nicotinate uptake activity over the water‐injected control oocytes (Fig. 7D) indicating that, in comparison to URAT1, OAT10‐mediated [14C]‐nicotinate uptake activity is Cl− ion‐dependent.
The [14C]‐nicotinate uptake activity of OAT10 was found to be inhibited by both tranilast (100 μmol/L) and benzbromarone (100 μmol/L)) (Fig. 7E). Tranilast was found to inhibit OAT10‐mediated [14C]‐nicotinate uptake with an IC50 of ~42 μmol/L (data not shown). When OAT10‐expressing oocytes were preloaded with nonlabeled PZA, the [14C]‐nicotinate uptake activity increased to 118–120 fold higher than PZA‐preloaded control oocytes (Fig. 7E and F) in ND96 medium. Tranilast also inhibited OAT10‐mediated enhanced [14C]‐nicotinate/PZA exchange with the IC50 of ˜43.1 μmol/L (Fig. S5). In parallel, benzbromarone was found to inhibit OAT10‐mediated [14C]‐nicotinate/PZA exchange with the IC50 of ~22.8 μmol/L (data not shown). Therefore, the OAT10‐mediated nicotinate transport is more sensitive to tranilast which is in marked contrast with that of SMCT1, SMCT2, and URAT1 which are resistant to tranilast (IC50 > 185 μmol/L).
Tranilast inhibits urate transport mediated by NPT1
The results of our previous experiments have clearly established that tranilast inhibits almost all the known urate transporters in the urate reabsorptive pathway. Human NPT1 (Na+‐phosphate transporter‐1, encoded by SLC17A1) has been reported to transport organic anions such as urate, PAH, aspirin, and salicylate in a voltage‐driven and Cl−‐dependent manner (Iharada et al. 2010). NPT1 is expressed in the apical membrane of renal tubular cells and mediates urate secretion. We investigated whether tranilast inhibits [14C]‐urate uptake activity of NPT1 in Xenopus laevis oocytes. NPT1‐mediated [14C]‐urate uptake was found to be effectively inhibited by tranilast (100 μmol/L) and benzbromarone (100 μmol/L) but only partially inhibited by probenecid (1.0 mmol/L) (Fig. 8). The IC50 of tranilast for [14C]‐urate uptake by NPT1 was ~18.9 μmol/L and for benzbromarone it was ~17.1 μmol/L (data not shown).
Figure 8.

Tranilast inhibits urate transport mediated by the human urate transporter NPT1. The uptake of [14C]‐urate by NPT1‐expressing oocytes was performed in ND96 medium (pH 7.4) at ~25°C for 1 h. The [14C]‐urate uptake by NPT1‐expressing oocytes was found little trans‐stimulated when NPT1‐expressing oocytes were preinjected with 50 nL of 100 mmol/L nicotinate (Nico) 2 h before [14C]‐urate uptake experiment. The [14C]‐urate uptake by NPT1 was found substantially inhibited in the presence of extracellular organic anions or uricosuric drugs. Organic anions or uricosuric drugs were added to the extracellular medium (pH 7.4) at the indicated concentrations. *P < 0.001 compared with uptake in the presence of DMSO or in the absence of inhibitor. Tran, Benz, Prob, DMSO, PZA, Sal. Data are mean ± S.E. with n = 12–15. PZA, pyrazine carboxylate; Tran, Tranilast; Benz, Benzbromarone; Prob, probenecid; DMSO, dimethylsulfoxide.
ABCG2‐mediated urate efflux is not affected by tranilast
The ATP‐binding cassette, subfamily G, member 2 (ABCG2) protein has been recently characterized as a high‐capacity urate secretion transporter (Woodward et al. 2009; Matsuo et al. 2012) expressed on the apical membrane of human renal proximal tubular cells (Huls et al. 2008), in addition to enterocytes and hepatocytes. We investigated the effect of tranilast (added in the efflux assay medium) on the [14C]‐urate efflux activity of ABCG2 expressed in Xenopus laevis oocytes. For [14C]‐urate efflux studies, control and ABCG2‐expressing oocytes were preinjected with 50 nL of 500 μmol/L [14C]‐urate and then incubated in the in ND96 medium (pH 7.4) for 1 h for recovery before efflux experiment. Preinjected oocytes were washed to remove any residual extracellular [14C]‐urate and then subjected to [14C]‐urate efflux for 1 h at room temperature (~25°C) in the absence or presence of tranilast. The results of this urate efflux experiment show that human ABCG2‐mediated urate efflux remained unaffected by tranilast at even 100 μmol/L concentration (Fig. 9), whereas benzbromarone (100 μmol/L) inhibited ~48% and probenecid (1.0 mmol/L) inhibited ~20% of urate transport activity of ABCG2. The mutation (Q141K) in ABCG2 causing hyperuricemia and gout (Woodward et al. 2009) showed ~50% of the urate transport activity of wild‐type ABCG2 (Fig. 9).
Figure 9.

Urate efflux mediated by the human urate transporter ABCG2 is unaffected by tranilast. Control or ABCG2‐expressing oocytes were preinjected with 50 nL of 500 μmol/L 14C]‐urate [dissolved in efflux medium (ND96, pH 7.4)], incubated in ND96 medium for 1 h at 16°C for recovery. Oocytes were washed in cold efflux medium four times and then subjected to efflux for 1 h at room temperature (~25°C) in ND96 medium (pH 7.4) in the absence or presence of drug. The amount of [14C]‐urate retained in each oocyte after 60 min of efflux is shown here. *P < 0.001 compared with urate efflux in the absence of any inhibitor. Tran, Benz, Prob, DMSO. All data are mean ± S.E.M. with n = 12–15. PZA, pyrazine carboxylate; Tran, Tranilast; Benz, Benzbromarone; Prob, probenecid; DMSO, dimethylsulfoxide. Prob, probenecid; DMSO, dimethylsulfoxide.
Tranilast inhibits urate transport mediated by OAT3 and OAT1
Basolateral entry of urate into proximal tubule cells via OAT1 and OAT3 mediates the first step of urate secretion by this nephron segment (Eraly et al. 2008). We investigated the effect of tranilast on [14C]‐urate uptake activity of OAT3 in Xenopous laevis oocytes. We found that the OAT3‐mediated [14C]‐urate uptake was ~5 fold higher than the water‐injected control oocytes (Fig. 10A) in ND96 medium. Preloaded intracellular organic anion such as PZA and nicotinate did not have any effect on its [14C]‐urate uptake (data not shown). The OAT3‐mediated urate uptake was however almost completely cis‐inhibited by extracellular 10 mmol/L PZA, nicotinate or salicylate (Fig. 10A). OAT3‐mediated urate uptake was also effectively inhibited by 50 μmol/L tranilast, with an IC50 of ~15.0 μmol/L (data not shown), 100 μmol/L benzbromarone, and 1.0 mmol/L probenecid (Fig. 10A). We also found that OAT1‐mediated urate uptake was ~5 fold higher than the water‐injected control oocytes (Fig. 10B) in ND96 medium and it was effectively inhibited by tranilast (100 μmol/L), benzbromarone (100 μmol/L) and probenecid (1.0 mmol/L) (Fig. 10B).
Figure 10.

Tranilast inhibits urate transport mediated by urate transporter OAT3 and OAT1. The uptake of [14C]‐urate (40 μmol/L) was performed in ND96 medium (pH 7.4) at ~25°C for 1 h. (A) The [14C]‐urate uptake by mouse OAT3 in oocytes remained unaffected if oocytes were preinjected with 50 nL of 100 mmol/L PZA but cis‐inhibited by 10 mmol/L PZA, nicotinate (Nico), or salicylate (Sal). The [14C]‐urate uptake by OAT3‐expressing oocytes was effectively inhibited by 50 μmol/L tranilast (Tran), 100 μmol/L benzbromarone (Benz) or 1.0 mmol/L probenecid (Prob). (B) The [14C]‐urate uptake by human OAT1 in oocytes was effectively inhibited by 100 μmol/L tranilast (Tran), 100 μmol/L benzbromarone (Benz) or 1.0 mmol/L probenecid (Prob). Data are mean ± S.E. with n = 12–15. *P < 0.001 compared with uptake in the presence of DMSO or in the absence of inhibitor. PZA, pyrazine carboxylate; Tran, Tranilast; Benz, Benzbromarone; Prob, probenecid; DMSO, dimethylsulfoxide.
Discussion
In humans, the reabsorption of filtered urate by the renal proximal tubule predominates over proximal tubular urate secretion, such that even modest inhibition of reabsorptive urate transport can cause uricosuria (Roch‐Ramel and Guisan 1999). Tranilast, an anti‐inflammatory drug, causes a potent reduction in SUA in human subjects, at least partially due to uricosuria (Sundy and Kitt 2010), hence we have investigated the effect of this drug on reabsorptive urate transporters. Here, we report that tranilast inhibits urate transport mediated by all the major reabsorptive urate transporters, as expressed in Xenopus oocytes. Tranilast inhibited urate transport by URAT1 and GLUT9 in a reversible and noncompetitive (mixed) manner. Tranilast failed to significantly inhibit nicotinate transport by URAT1 and the Na+‐dependent nicotinate transporters SMCT1 and SMCT2. Finally, tranilast also inhibited several key secretory urate transporters, with the exception of ABCG2.
Prior to studying the effect of tranilast on transporters, we first verified, optimized, and compared the urate transport characteristics of multiple urate and nicotinate transporters, given the complex interactions between these transporters with both uricosurics and antiuricosurics. Overall, we found substantial condition‐dependent differences in the activity of specific transporters, with considerable opportunity for optimization of function in oocytes. There are also substantial differences in the relative activity of the various reabsorptive and secretory transporters in Xenopus oocytes. We found that URAT1 can operate as a urate/nicotinate or urate/PZA exchanger, using the intracellular pool of nicotinate or PZA, and that this urate transport activity was independent of Na+ and putative changes in membrane potential. PZA is a metabolite of pyrazinamide, a drug used for treatment of tuberculosis and a key pharmacological probe for renal urate transport (Mandal and Mount 2015). The potent antiuricosuric action of pyrazinamide can thus be explained by trans‐activation mechanism of urate/PZA exchange by intracellular PZA (Guggino and Aronson 1985), transported into the cell by Na+‐dependent transporters (Manganel et al. 1985) (SMCT1 and SMCT2) where it “trans‐activates” apical exchange with luminal urate (Mandal and Mount 2015). In the absence of extracellular Cl− urate, the uptake activity of URAT1 was stimulated, potentially due to the lack of cis‐inhibition by Cl−. In contrast to prior reports (Enomoto et al. 2002), we did not find trans‐stimulatory effects of preloaded intracellular lactate, nor did we detect cis‐inhibitory effects of 10 mM extracellular lactate on urate uptake by URAT1. We did however find that nicotinate is a substrate for URAT1, via direct measurement of [14C]‐nicotinate uptake, an activity of URAT1 that had not previously been established.
As observed for URAT1, the trans‐stimulatory effect of intracellular niocotinate or PZA on urate uptake by OAT10 was also significant. Notably, the absence of extracellular Cl− was found to abrogate urate and nicotinate uptake activity of OAT10, which is in direct contrast to URAT1. In this respect, OAT10 is more similar to OAT1, which is also stimulated by the presence of extracellular Cl− (Rizwan et al. 2007). Unlike URAT1 and OAT10, OAT4 appears to function by exchanging urate with divalent anions; in particular, the trans‐stimulatory effect of intracellular maleate on urate uptake by OAT4 was significant. With respect to basolateral urate transporters, urate transport mediated by GLUT9a was markedly stimulated by cell membrane depolarization, as reported previously (Anzai et al. 2008; Bibert et al. 2009; Witkowska et al. 2012). Urate uptake mediated by the basolateral transporter OAT3, thought to play a role in urate secretion (Eraly et al. 2008), was not trans‐stimulated by intracellular niocotinate, PZA, or α‐KG. The urate uptake activity of OAT3 was however cis‐inhibited by the presence of extracellular monovalent anions (10 mM), including nicotinate, PZA, and salicylate.
The monocarboxylate transporters SMCT1 and SMCT2 play important indirect roles in urate reabsorption (Mandal and Mount 2015). Although the SMCT1‐ and SMCT2‐mediated [14C]‐nicotinate uptake activity was primarily Na+‐dependent, we found significant reduction in nicotinate uptake activity of SMCT1 and SMCT2 in the absence of extracellular Cl−, suggesting SMCT1‐ and SMCT2‐mediated nicotinate transport is dependent on extracellular Cl−. This extends and confirms the prior electrophysiological data for SMCT1, showing Cl−‐dependence without evidence of direct Cl− transport (Coady et al. 2004). Others have reported an absence of chloride dependence in SMCT1 (Miyauchi et al. 2004), which is refuted by our data and that of Coady et al. (2004).
Our studies on the effect of tranilast on multiple urate transporters revealed several novel actions of the drug. The most important observations are: (1) Tranilast effectively inhibited urate transport activities of the major reabsorptive urate transporters URAT1, GLUT9, OAT4, and OAT10; this provides an unequivocal molecular explanation for the drug's uricosuric effect; (2) Tranilast did not inhibit the nicotinate transport mediated by URAT1, SMCT1, and SMCT2, even at higher concentrations (>180 μmol/L). However, OAT10‐mediated nicotinate transport was comparatively sensitive (IC50 of ~43.1 μmol/L) to tranilast; (3) Inhibition of URAT1 and GLUT9a by benzbromarone and probenecid was not reversible in this system, whereas tranilast interacts with these transporters in a reversible, noncompetitive manner. (4) Tranilast also inhibited the secretory transporters NPT1, OAT1, and OAT3, with no effect on ABCG2‐mediated urate efflux.
Since 90% of uric acid from glomerular filtrate is reabsorbed in the renal proximal tubule, drugs that inhibit reabsorptive urate transporters in vivo are potent uricosurics. Inhibition of secretory urate transporters by uricosurics has less effect on urate excretion, given the dominance of urate reabsorption in the kidney and the very low fractional excretion of urate in humans (Roch‐Ramel and Guisan 1999). For example, although probenecid inhibits apical OAT10/URAT1/OAT4, it also inhibits the apical secretory transporters NPT1 (Jutabha et al. 2003), NPT4 (Jutabha et al. 2010) and the basolateral secretory transporters OAT1 and OAT3; probenecid is however a widely utilized uricosuric agent (Roch‐Ramel and Guisan 1999). Notably, however, via the inhibition of secretory transporters tranilast has the capacity to affect in vivo homeostasis of urate, including compartmentalization kinetics in the central nervous system and distribution in intracellular versus extracellular compartments. Mechanistically, the inhibition of urate transport mediated by multiple urate transporters from different gene families suggests the existence of analogous interaction sites for urate and tranilast. Urate transport mediated by URAT1 was specifically inhibited by <50 μmol/L tranilast, without affecting its nicotinate transport activity. This novel finding suggests distinct but likely overlapping binding sites in URAT1, one for urate and the other for nicotinate, which are sufficiently dissimilar to differentially interact with tranilast. Further studies are required to clarify interaction sites for tranilast, nicotinate, and urate in URAT1; it also remains to be determined whether other potent uricosurics differentially affect urate and nicotinate transport by URAT1.
The inhibitory action of tranilast on urate transport by URAT1 and GLUT9 was abrogated by washing oocytes, suggesting that the interaction is reversible; this differs from the behavior of benzbromarone. The results of Eadie–Hofstee linearizations of urate uptake kinetics revealed that the inhibition of URAT1 and GLUT9a‐mediated urate uptake by tranilast closely resembles a mixed noncompetitive inhibition model. Notably, the GLUT9 and URAT1 proteins exhibit minimal homology with one another. The noncompetitive inhibition (mixed) model for both urate transporters suggests that tranilast binds to a site other than the urate translocation sites, with indirect allosteric effects on urate transport.
The urate‐lowering and uricosuric effect of tranilast (Sundy and Kitt 2010) is evidently explained by the potent inhibition of multiple reabsorptive urate transporters (URAT1, OAT10, OAT4, and GLUT9). Although inhibition of any of these transporters would cause uricosuria, we postulate that GLUT9 inhibition is particularly influential in the effect of tranilast, given the more severe uricosuric phenotype for patients with GLUT9‐deficient, versus URAT1‐deficient, renal hypouricemia (Dinour et al. 2010). GLUT9 is the exclusive exit pathway for urate during reabsorption, whereas there is considerable heterogeneity for the apical entry mechanism. The urate‐lowering effect of tranilast could additionally involve inhibition of xanthine oxidase, already linked to its effect on the generation of reactive oxygen species (Miyachi et al. 1987). Notably, the two classical inhibitors of xanthine oxidase, febuxostat, and oxypurinol (the active metabolite of allopurinol) also interact with URAT1 (Iwanaga et al. 2005; Jutabha and Anzai 2014), such that additional interactions of tranilast with xanthine oxidase would not be an unexpected property for a uricosuric drug.
The previously reported effects of tranilast include antiproliferative effects (Rogosnitzky et al. 2012), activation of the nuclear aryl hydrocarbon receptor (Hu et al. 2013), inhibition of TGF‐beta (Tao et al. 2011), inhibition of cation channels (Mihara et al. 2013), mast cell stabilization (Sastre et al. 2013), and modulation of tryptophan metabolism (Munn et al. 1998). It has therapeutic uses and therapeutic potential in a wide variety of disorders, including for example diabetic nephropathy (Mifsud et al. 2003). To the extent that some of these disorders, including diabetic nephropathy (Rosolowsky et al. 2008; Ficociello et al. 2010; Doria and Krolewski 2011), are linked to hyperuricemia, it is tempting to speculate that some of the systemic therapeutic benefits of tranilast are mediated by its urate‐lowering action. In this regard, it is equally interesting that hyperuricemia is in turn linked to changes in tryptophan metabolism (Liu et al. 2011), providing indirect links between these actions of tranilast.
Authorship Contributions
Participated in research design: Asim K. Mandal, David B. Mount. Conducted experiments: Asim K. Mandal, Andria Foster. Contributed new reagents or analytic tools: Adriana Mercado, Kambiz Zandi‐Nejad. Performed data analysis: Asim K. Mandal, David B. Mount. Wrote or contributed to the writing of the manuscript: Asim K. Mandal, David B. Mount.
Disclosure
None declared.
Supporting information
Figure S1. Tranilast inhibits urate transport activity of URAT1 in a reversible manner in Xenopus laevis oocytes.
Figure S2. Tranilast inhibits urate transport activity of GLUT9a in a reversible manner in Xenopus laevis oocytes.
Figure S3. Functional characterization of human URAT1 as a nicotinate transporter.
Figure S4. [14C]‐nicotinate transport properties of URAT1: voltage sensitivity and Na+‐dependence was examined by replacing extracellular NaCl by KCl or LiCl.
Figure S5. The 50% inhibitory concentration (IC50) curve of tranilast for [14C]‐nicotinate uptake via OAT10 in oocytes preloaded with PZA.
Acknowledgements
We thank Owen Woodward for the gift of ABCG2 cDNAs. Funded by the NIH (DK 57708, DK070756, and AR065968) and by a grant from Nuon Therapeutics.
Mandal A. K., Mercado A., Foster A., Zandi‐Nejad K., Mount D. B.. Uricosuric targets of tranilast, Pharma Res Per, 5(2), 2017, e00291, doi: 10.1002/prp2.291
References
- Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, Jin CJ, et al. (2008). Plasma urate level is directly regulated by a voltage‐driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem 283: 26834–26838. [DOI] [PubMed] [Google Scholar]
- Ascherio A, LeWitt PA, Xu K, Eberly S, Watts A, Matson WR, et al. (2009). Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol 66: 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn A, Hagos Y, Reuter S, Balen D, Brzica H, Krick W, et al. (2008). Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J Biol Chem 283: 16332–16341. [DOI] [PubMed] [Google Scholar]
- Bibert S, Hess SK, Firsov D, Thorens B, Geering K, Horisberger JD, et al. (2009). Mouse GLUT9: evidences for a urate uniporter. Am J Physiol Renal Physiol 297: F612–F619. [DOI] [PubMed] [Google Scholar]
- Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA (2004). Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol 31: 2429–2432. [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH (1973). Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22: 3099–3108. [DOI] [PubMed] [Google Scholar]
- Choi HK, Mount DB, Reginato AM (2005). Pathogenesis of gout. Ann Intern Med 143: 499–516. [DOI] [PubMed] [Google Scholar]
- Coady MJ, Chang MH, Charron FM, Plata C, Wallendorff B, Sah JF, et al. (2004). The human tumour suppressor gene SLC5A8 expresses a Na+‐monocarboxylate cotransporter. J Physiol 557(Pt 3): 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinour D, Gray NK, Campbell S, Shu X, Sawyer L, Richardson W, et al. (2010). Homozygous SLC2A9 mutations cause severe renal hypouricemia. J Am Soc Nephrol 21: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria A, Krolewski AS (2011). Diabetes: lowering serum uric acid levels to prevent kidney failure. Nat Rev Nephrol 7: 495–496. [DOI] [PubMed] [Google Scholar]
- Doring A, Gieger C, Mehta D, Gohlke H, Prokisch H, Coassin S, et al. (2008). SLC2A9 influences uric acid concentrations with pronounced sex‐specific effects. Nat Genet 40: 430–436. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, et al. (2002). Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417: 447–452. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Vallon V, Rieg T, Gangoiti JA, Wikoff WR, Siuzdak G, et al. (2008). Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genomics 33: 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig DI, Soletsky B, Johnson RJ (2008). Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 300: 924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficociello LH, Rosolowsky ET, Niewczas MA, Maselli NJ, Weinberg JM, Aschengrau A, et al. (2010). High‐normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6‐year follow‐up. Diabetes Care 33: 1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann R, Kerr B, Yeh LT, Suster M, Shen Z, Polvent E, et al. (2014) Pharmacodynamic, pharmacokinetic and tolerability evaluation of concomitant administration of lesinurad and febuxostat in gout patients with hyperuricaemia. Rheumatology (Oxford).53: 2167–2174. [DOI] [PubMed] [Google Scholar]
- Forman JP, Choi H, Curhan GC (2009). Uric acid and insulin sensitivity and risk of incident hypertension. Arch Intern Med 169: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon SL, Fox IH (1974). Pharmacologic effects of nicotinic acid on human purine metabolism. J Lab Clin Med 84: 179–186. [PubMed] [Google Scholar]
- Gibson HV, Doisy EA (1923). A note on the effect of some organic acids upon the uric acid excretion of man. J Biol Chem 55: 605–610. [Google Scholar]
- Goldfinger S, Klinenberg E Jr, Seegmiller JE (1965). Renal Retention of Uric Acid Induced by Infusion of Beta‐Hydroxybutyrate and Acetoacetate. N Engl J Med 272: 351–355. [DOI] [PubMed] [Google Scholar]
- Guggino SE, Aronson PS (1985). Paradoxical effects of pyrazinoate and nicotinate on urate transport in dog renal microvillus membranes. J Clin Invest 76: 543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A (2007). Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol 18: 430–439. [DOI] [PubMed] [Google Scholar]
- Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, et al. (1998). Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA 95: 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Zhao J, Pei G (2013). Activation of aryl hydrocarbon receptor (ahr) by tranilast, an anti‐allergy drug, promotes miR‐302 expression and cell reprogramming. J Biol Chem 288: 22972–22984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huls M, Brown CD, Windass AS, Sayer R, van den Heuvel JJ, Heemskerk S, et al. (2008). The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int 73: 220–225. [DOI] [PubMed] [Google Scholar]
- Iharada M, Miyaji T, Fujimoto T, Hiasa M, Anzai N, Omote H, et al. (2010). Type 1 sodium‐dependent phosphate transporter (SLC17A1 Protein) is a Cl(‐)‐dependent urate exporter. J Biol Chem 285: 26107–26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga T, Kobayashi D, Hirayama M, Maeda T, Tamai I (2005). Involvement of uric acid transporter in increased renal clearance of the xanthine oxidase inhibitor oxypurinol induced by a uricosuric agent, benzbromarone. Drug Metab Dispos 33: 1791–1795. [DOI] [PubMed] [Google Scholar]
- Iwanaga T, Sato M, Maeda T, Ogihara T, Tamai I (2007). Concentration‐dependent mode of interaction of angiotensin II receptor blockers with uric acid transporter. J Pharmacol Exp Ther 320: 211–217. [DOI] [PubMed] [Google Scholar]
- Jutabha P, Anzai N (2014). Inhibitory effect of febuxostat, a non‐purine xanthine oxidase inhibitor, on renal urate transporters. J Am Soc Nephrol 25: 652A. [Google Scholar]
- Jutabha P, Kanai Y, Hosoyamada M, Chairoungdua A, Kim do K, Iribe Y, et al. (2003). Identification of a novel voltage‐driven organic anion transporter present at apical membrane of renal proximal tubule. J Biol Chem 278: 27930–27938. [DOI] [PubMed] [Google Scholar]
- Jutabha P, Anzai N, Kitamura K, Taniguchi A, Kaneko S, Yan K, et al. (2010). Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate. J Biol Chem 285: 35123–35132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sun X, Di D, Quan J, Zhang J, Yang X (2011). A metabolic profiling analysis of symptomatic gout in human serum and urine using high performance liquid chromatography‐diode array detector technique. Clin Chim Acta 412(23–24): 2132–2140. [DOI] [PubMed] [Google Scholar]
- Mandal A, Mount DB (2015). The Molecular Physiology of Uric Acid Homeostasis. Annual Rev Physiol 77: 323–345. [DOI] [PubMed] [Google Scholar]
- Manganel M, Roch‐Ramel F, Murer H (1985). Sodium‐pyrazinoate cotransport in rabbit renal brush border membrane vesicles. Am J Physiol 249(3 Pt 2): F400–F408. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Ikebuchi Y, et al. (2009) Common defects of ABCG2, a high‐capacity urate exporter, cause gout: a function‐based genetic analysis in a Japanese population. Sci Transl Med 1: 5ra11. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Takada Y, et al. (2012). Identification of ABCG2 dysfunction as a major factor contributing to gout. Nucleosides, Nucleotides Nucleic Acids 30: 1098–1104. [DOI] [PubMed] [Google Scholar]
- Mifsud S, Kelly DJ, Qi W, Zhang Y, Pollock CA, Wilkinson‐Berka JL, et al. (2003). Intervention with tranilast attenuates renal pathology and albuminuria in advanced experimental diabetic nephropathy. Nephron Physiol 95: 83–91. [DOI] [PubMed] [Google Scholar]
- Mihara H, Suzuki N, Yamawaki H, Tominaga M, Sugiyama T (2013). TRPV2 ion channels expressed in inhibitory motor neurons of gastric myenteric plexus contribute to gastric adaptive relaxation and gastric emptying in mice. Am J Physiol Gastrointest Liver Physiol 304: G235–G240. [DOI] [PubMed] [Google Scholar]
- Miyachi Y, Imamura S, Niwa Y (1987). The effect of tranilast of the generation of reactive oxygen species. J Pharmacobiodyn 10: 255–259. [DOI] [PubMed] [Google Scholar]
- Miyauchi S, Gopal E, Fei YJ, Ganapathy V (2004). Functional identification of SLC5A8, a tumor suppressor down‐regulated in colon cancer, as a Na(+)‐coupled transporter for short‐chain fatty acids. J Biol Chem 279: 13293–13296. [DOI] [PubMed] [Google Scholar]
- Mount DB, Mercado A, Song L, Xu J, George AL Jr, Delpire E, et al. (1999). Cloning and characterization of KCC3 and KCC4, new members of the cation‐chloride cotransporter gene family. J Biol Chem 274: 16355–16362. [DOI] [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. (1998). Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281: 1191–1193. [DOI] [PubMed] [Google Scholar]
- Perlstein TS, Gumieniak O, Williams GH, Sparrow D, Vokonas PS, Gaziano M, et al. (2006). Uric acid and the development of hypertension: the normative aging study. Hypertension 48: 1031–1036. [DOI] [PubMed] [Google Scholar]
- Rizwan AN, Krick W, Burckhardt G (2007). The chloride dependence of the human organic anion transporter 1 (hOAT1) is blunted by mutation of a single amino acid. J Biol Chem 282: 13402–13409. [DOI] [PubMed] [Google Scholar]
- Roch‐Ramel F, Guisan B (1999). Renal Transport of Urate in Humans. News Physiol Sci 14: 80–84. [DOI] [PubMed] [Google Scholar]
- Rogosnitzky M, Danks R, Kardash E (2012). Therapeutic potential of tranilast, an anti‐allergy drug, in proliferative disorders. Anticancer Res 32: 2471–2478. [PubMed] [Google Scholar]
- Rosolowsky ET, Ficociello LH, Maselli NJ, Niewczas MA, Binns AL, Roshan B, et al. (2008). High‐normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol 3: 706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre E, Caracuel L, Xavier FE, Balfagon G, Blanco‐Rivero J (2013). Opposite effect of mast cell stabilizers ketotifen and tranilast on the vasoconstrictor response to electrical field stimulation in rat mesenteric artery. PLoS ONE 8: e73232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini TA, Emerling DE (2010) Tranilast suppresses inflammation induced by monosodium urate (MSU) crystals in vivo. EULAR: AB0054. [Google Scholar]
- Shapiro M, Hyde L (1957). Hyperuricemia due to pyrazinamide. Am J Med 23: 596–599. [DOI] [PubMed] [Google Scholar]
- Spitsin S, Koprowski H (2010). Role of uric acid in Alzheimer's disease. J Alzheimers Dis 19: 1337–1338. [DOI] [PubMed] [Google Scholar]
- Srinivas SR, Gopal E, Zhuang L, Itagaki S, Martin PM, Fei YJ, et al. (2005). Cloning and functional identification of slc5a12 as a sodium‐coupled low‐affinity transporter for monocarboxylates (SMCT2). Biochem J 392(Pt 3): 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundy JS, Kitt M (2010) Tranilast, a novel potential treatment for the chronic management of hyperuricemia in gout, reduces serum uric acid (SUA) in healthy subjects. EULAR: SAT0369. [Google Scholar]
- Tao Y, Hu L, Li S, Liu Q, Wu X, Li D, et al. (2011). Tranilast prevents the progression of chronic cyclosporine nephrotoxicity through regulation of transforming growth factor beta/Smad pathways. Transplant Proc 43: 1985–1988. [DOI] [PubMed] [Google Scholar]
- Uetake D, Ohno I, Ichida K, Yamaguchi Y, Saikawa H, Endou H, et al. (2010). Effect of fenofibrate on uric acid metabolism and urate transporter 1. Intern Med 49: 89–94. [DOI] [PubMed] [Google Scholar]
- Van Aubel RA, Smeets PH, Van Den Heuvel JJ, Russel FG (2004). Human organic anion transporter mrp4 (abcc4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal Physiol 288: F327–333. [DOI] [PubMed] [Google Scholar]
- Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, et al. (2008). SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 40: 437–442. [DOI] [PubMed] [Google Scholar]
- Wallace C, Newhouse SJ, Braund P, Zhang F, Tobin M, Falchi M, et al. (2008). Genome‐wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet 82: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, O'Reilly E, Chen H, Schwarzschild MA, Ascherio A (2007). Plasma urate and risk of Parkinson's disease. Am J Epidemiol 166: 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowska K, Smith KM, Yao SY, Ng AM, O'Neill D, Karpinski E, et al. (2012). Human SLC2A9a and SLC2A9b isoforms mediate electrogenic transport of urate with different characteristics in the presence of hexoses. Am J Physiol Renal Physiol 303: F527–F539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M (2009). Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci USA 106: 10338–10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Tranilast inhibits urate transport activity of URAT1 in a reversible manner in Xenopus laevis oocytes.
Figure S2. Tranilast inhibits urate transport activity of GLUT9a in a reversible manner in Xenopus laevis oocytes.
Figure S3. Functional characterization of human URAT1 as a nicotinate transporter.
Figure S4. [14C]‐nicotinate transport properties of URAT1: voltage sensitivity and Na+‐dependence was examined by replacing extracellular NaCl by KCl or LiCl.
Figure S5. The 50% inhibitory concentration (IC50) curve of tranilast for [14C]‐nicotinate uptake via OAT10 in oocytes preloaded with PZA.


