Abstract
Opioid drugs like morphine and fentanyl are the gold standard for treating moderate to severe acute and chronic pain. However, opioid drug use can be limited by serious side effects, including constipation, tolerance, respiratory suppression, and addiction. For more than 100 years, we have tried to develop opioids that decrease or eliminate these liabilities, with little success. Recent advances in understanding opioid receptor signal transduction have suggested new possibilities to activate the opioid receptors to cause analgesia, while reducing or eliminating unwanted side effects. These new approaches include designing functionally selective ligands, which activate desired signaling cascades while avoiding signaling cascades that are thought to provoke side effects. It may also be possible to directly modulate downstream signaling through the use of selective activators and inhibitors. Separate from downstream signal transduction, it has also been found that when the opioid system is stimulated, various negative feedback systems are upregulated to compensate, which can drive side effects. This has led to the development of multi-functional molecules that simultaneously activate the opioid receptor while blocking various negative feedback receptor systems including cholecystokinin and neurokinin-1. Other novel approaches include targeting heterodimers of the opioid and other receptor systems which may drive side effects, and making endogenous opioid peptides druggable, which may also reduce opioid mediated side effects. Taken together, these advances in our molecular understanding provide a path forward to break the barrier in producing an opioid with reduced or eliminated side effects, especially addiction, which may provide relief for millions of patients.
Keywords: Opioid, Chronic Pain, Drug Discovery, Functional Selectivity, Novel Targets, Novel Strategies
Introduction
Chronic pain is a serious and growing health concern, with an estimated incidence of approximately one-third of the population of the United States, over 100 million people [1]. This imposes a serious economic cost in addition to the human cost, which has been estimated at US$ 600 billion in direct and economic costs every year [1]. Moderate to severe cancer and non-cancer chronic pain seriously degrades patient quality of life, and interferes with daily activities [2]. There are multiple drug classes used for the treatment of chronic pain, including non-steroidal anti-inflammatory drugs, tricyclic antidepressants (TCA), gabapentinoids, and finally opioid drugs (morphine, fentanyl, etc.), with progression between and within categories to stronger drugs dependent on patient response and the severity and type of the pain [3,4]. However, TCAs and gabapentinoids only work for 42 to 76 percent of the patient population, often leaving opioid drugs such as morphine and fentanyl the only efficacious treatment option for a large percentage of the patient population [4].
Despite their generally high efficacy, opioids can be ineffective in certain pain types and patient populations, including lowered effectiveness for treating neuropathic pain [5]. In addition, opioids induce a constellation of side effects, incuding dependence and addiction, tolerance, constipation, nausea, respiratory depression, somnolence, and mental clouding [3,6]. These side effects also diminish patient quality of life, decrease compliance, and are particularly problematic in long-term chronic users [7]. These limitations of chronic opioid therapy have spurred drug discovery efforts to develop new analgesic drugs with an improved therapeutic profile.
The first serious attempt to modify the chemical structure of morphine to reduce or eliminate addiction occurred in 1874 with the synthesis of diamorphine. Diamorphine was re-synthesized 23 years later and released by the Bayer company in 1898 under the trade name Heroin as a non-addictive morphine replacement and cough suppressant. As was later discovered, Heroin is more potent and more addictive than morphine, making these initial claims laughable in retrospect. However, this initial attempt with Heroin in many ways is emblematic of all future approaches to create non-addictive opioids, even as our methods greatly improved in sophistication. Today, the vast majority of opioid drugs are derivatives of the morphinan scaffold or re-formulations of previous drugs, while preserving the side-effect liabilities that limit the use of older opioids like morphine [8]. Some drugs have been developed which do not have these liabilities, such as ω-conotoxin, which are effective in treating intractable chronic pain. ω-Conotoxin acts by blocking calcium channels, thus blocking pain neuron conduction. However ω-conotoxin and similar drugs must be administered by the intrathecal route, and have a narrow therapeutic index, which is the difference in doses between that which produces the desired effect (analgesia) and that which produces undesired effects. This limits the use of these drugs to specific inpatient care scenarios [9]. There have been a few recent additions to the market which may buck this trend, as the multi-functional drugs tapentadol and tramadol both have moderate efficacy against pain with reduced addiction and abuse rates; however, these are some of the very few opioid drugs that show such promise [10]. Both drugs are mixed opioid receptor agonists and norepinephrine and/or serotonin reuptake inhibitors.
Clearly, new approaches are needed to overcome this fundamental limitation in the opioid drug discovery field. One such promising approach has taken shape over the last 10 to 15 years with a number of discoveries as to the molecular signaling mechanisms of the mu opioid receptor (MOR), the main target of opioid drugs. It has been shown that different signaling cascades are responsible for different aspects of the overall response, which has allowed us to differentiate analgesic signaling (e.g. GαI/O)from side-effect signaling (e.g. βarrestin2). Combined with other molecular discoveries, including negative feedback systems upregulated during opioid treatment, receptor heterodimerization, and methods to improve the drug-like properties of endogenous opioid peptides, these findings suggest new drug discovery approaches that are fundamentally different from previous target-based strategies. These novel approaches hold the potential to develop the opioid without side effects that we have been pursuing for more than a century.
Topics: Functional Selectivity
The idea of selectively targeting signaling cascades for the treatment of pain began in 1999 with the discovery from the Lefkowitz and Caron labs that βarrestin2 knockout (KO) mice had an enhanced anti-nociceptive response with morphine treatment [11]. This finding was provocative, but of course, patients cannot be treated by genetic KO, and βarrestin2 is a ubiquitous signaling regulator that regulates many G protein-coupled receptors (GPCRs). How could one selectively target βarrestin2 at the MOR?
The answer came from a separate discovery in molecular pharmacology at around the same time called functional selectivity (also “biased agonism” or “ligand-directed signaling”) [12]. Functional selectivity is the ability of certain ligands to activate the same receptor but to evoke different signaling cascades, likely through the induction of different receptor conformations [13]. This meant that in principle, a ligand could be designed or discovered, which could activate the MOR, but not recruit βarrestin2, recapitulating the benefits of the KO with a ligand that could be used in patients. Developing βarrestin2 biased ligands has generated intense interest and development in the intervening years, and has been expanded to targets beyond the MOR and chronic pain (see below). In addition, this molecular approach is amenable to targets beyond βarrestin2, and more targets for functionally selective drug discovery likely remain to be found, which is an area of great interest in our lab.
βarrestin2
As mentioned above, the initial finding in βarrestin2 KO mice was enhanced analgesia in response to morphine [11]. The first author of that paper, Laura Bohn, extended these initial findings in further studies demonstrating that βarrestin2 KO mice had reduced respiratory depression, constipation, dependence, and tolerance [6,14]. This represents an increase in the therapeutic index. One caveat from these studies is that the change in therapeutic index was not observed with high doses of morphine, or with any dose of the full MOR agonists fentanyl, methadone, or oxycodone, suggesting that strong activation of the system could override the effects of the loss of βarrestin2 on opioid side effects. Nonetheless, this improvement in the therapeutic index supported the development of functionally selective MOR agonists with low or no βarrestin2 recruitment. A strong effort from multiple labs led to the identification of multiple MOR biased ligands, including herkinorin [15,16] and TRV130 [17]. Other major development programs are also in progress and will be published soon (L. Bohn, conference presentation), and a large collaborative effort recently resulted in the publication of the MOR biased ligand PZM21 [18]. TRV130 and PZM21 have no appreciable differences in βarrestin2 recruitment or G protein coupling in vitro; however, PZM21 induces no tail-flick anti-nociception in mice, while TRV130 does so in rats (both produce anti-nociception in the hot plate assay). This unusual difference suggests that signaling effects beyond βarrestin2 may be involved in the comparative performance of these compounds.
All of these discovered ligands display strong bias against βarrestin2 recruitment (usually no efficacy at all) using widely accepted in vitro assays of βarrestin recruitment (DiscoveRx, TANGO, BRET). One of the limitations of this field is that there is no quantitative assay for βarrestin recruitment in vivo, making it difficult to evaluate these compounds outside of an artificial in vitro system. Indeed, depending on assay conditions, morphine can also display low to no βarrestin2 recruitment, demonstrating the importance of assay context in evaluating ligand bias [15,19]. Nonetheless, these biased compounds have replicated some of the expected findings from the βarrestin2 KO studies. PZM21 was found not to induce conditioned place preference and motor activation in mice, along with reduced (but not eliminated) constipation and no respiratory suppression [18]. TRV130, developed by the pharmaceutical company Trevena, also displayed reduced constipation and respiratory depression in mice [17,20]. TRV130 has advanced to clinical trials, the only βarrestin2 biased agonist to do so, where in small group Phase I trials it showed the potential for enhanced analgesia and reduced nausea [21,22]. In a larger Phase II trial, TRV130 did show potent and efficacious analgesia versus a sub-maximal dose of morphine. However, analysis of the adverse events experienced by the patients, including constipation, showed no apparent differences between TRV130 and morphine [23]. Of note, a fixed dosing regimen for TRV130 was used for these studies; dosing according to patient demand could potentially reduce side effects in comparison with morphine beyond what was seen in this trial. TRV130 has since advanced to Phase III trials, which will hopefully confirm whether or not a βarrestin2 biased ligand can treat pain in patients with reduced side effects. Also of note is that none of the biased ligands tested to date have been able to induce enhanced analgesia in preclinical or clinical testing, as was found in the original βarrestin2 KO paper [11]. Overall, there are some caveats and limitations to βarrestin2 as a drug discovery target, including the lack of a structure-activity relationship (SAR) for arrestin bias [18]. However, these are early days, and there is a great deal of excitement for the potential of this type of drug discovery.
Beyond the MOR, other receptors have also been studied for βarrestin2 biased signaling and drug discovery. Work by Chavkin and colleagues suggested that βarrestin2 mediates dysphoria and aversion seen with kappa opioid receptor (KOR) activation, and that a ligand biased against βarrestin2 could cause analgesia without dysphoria and aversion, and without typical MOR side effects [24,25]. A number of ligands biased against βarrestin2 have been found for the KOR, including 6’-guanidinonaltrindole, RB-64, and others [26-32]. RB-64 was particularly interesting, as it was shown to induce anti-nociception without the KOR side effects of anhedonia and motor dysfunction, while still causing conditioned place aversion [29]. These initial results are promising, but do suggest that targeting the KOR with a biased ligand may still cause aversion – more research is needed with different biased ligands to determine the future of the KOR as a target for functionally selective drug discovery. Other receptors are also being investigated for functionally selective drug discovery for conditions other than pain, including the dopamine receptors [33-36], ghrelin receptor [37], and others. Interestingly, for some of these systems, arrestin signaling is beneficial rather than detrimental, leading to an effort to find ligands biased for βarrestin2 [33].
Other Signaling Targets for Functionally Selective Drug Discovery
βarrestin2 has generated an intense level of interest, but there is every reason to believe that the same approach for functionally selective drug discovery can be applied to other signaling targets, and perhaps to even greater effect. While not many such targets have been identified, efforts by our lab and others to identify new signaling regulators of the MOR may provide more such targets in the future. We discuss a few known potential targets below.
It has been shown that chronic opioid administration results in a cellular response defined by increased adenylyl cyclase (AC) activity, termed AC superactivation. AC superactivation has been proposed to be a contributing factor to opioid-induced tolerance and dependence, suggesting that this mechanism should be targeted by functionally selective drug discovery to potentially reduce these side effects [38,39]. The protein kinase Raf-1, a partner in the Raf/MEK/ERK cascade, has been implicated in adenylyl cyclase superactivation at both the MOR and delta (DOR) opioid receptors in vitro [40,41]. It was later shown that knockdown of Raf-1 via siRNA attenuated both morphine-induced sensitization and tolerance in vivo, as well as thermal hyperalgesia – in which previously non-painful stimuli become painful [42,43]. Although MOR regulation involves a complex network of proteins (reviewed in [44]) and there is currently no literature on biased ligands with respect to Raf-1, identifying ligands that do or do not induce adenylyl cyclase activation via Raf-1 will aid in novel biased ligand development. An important caveat is that Raf-1 was shown to be AC subtype-dependent, superactivating AC V/VI; these subtypes may not be the most relevant AC subtypes to dependence and withdrawal in vivo.
The termination of MOR signaling is regulated in part by the slow GTPase activity of the Gα subunit, where GTP hydrolysis deactivates the G protein and allows for subsequent reactivation. Additionally, Regulators of G Protein Signaling (RGS) proteins act as GTPase accelerating proteins (GAP), enhancing the rate of GTP hydrolysis up to 100-fold [45]. These proteins thus act as negative regulators of GPCR signaling, and have been suggested to modulate the receptor response to opioids and other drugs of abuse [46].
There are more than 20 RGS proteins, and at least 11 have been implicated in MOR signaling in vitro and in vivo [45], although they appear to modulate MOR signaling in a variety of ways. For example, one study observed a decrease in morphine anti-nociception after knockdown of RGS2 and RGS3, but an increase after knockdown of RGS4, RGS7, RGS9, RGS12, RGS14, and RGS16, most significantly with RGS9 [47]. Interestingly, these RGS proteins appear to have selectivity in the GPCRs they modulate, as it was shown in SH-SY5Y cells that RGS19 selectively modulates MOR signaling but not DOR or the Nociceptin Receptor (NOP), and that RGS4 selectively modulates DOR signaling [48,49]. However, this appears to be system-dependent, because in other heterologous systems, RGS19 has differing effects on MOR, DOR, and NOP signaling. Lastly, some ligand-specific regulation by RGS proteins has been noted [50]. In this case, expression of an RGS-insensitive Gαo protein induced an increase in potency for the full efficacy agonist DAMGO, but increased both the potency and efficacy for the partial agonist morphine [50]. The diverse modulation RGS proteins exhibit, along with their suggested selectivity for receptors and ligands, make them ideal targets for biased compounds.
Direct Modulation of Downstream Signal Transduction
Designing functionally selective ligands is advantageous for several reasons, including that they impart the ability to modulate ubiquitously expressed signaling regulators only at a receptor target of interest. This can potentially reduce off-target effects as compared to targeting the signaling regulator itself. However, it is not always possible or desirable to pursue this approach. For instance, a signaling regulator could modulate signal transduction of a target cascade without being directly in the cascade in a way amenable to functionally selective drug discovery. Or, a target could be in the direct cascade, but for technical or other reasons, a functionally selective ligand cannot be found. In these cases, we may still be able to take advantage of the known signal transduction cascades in chronic pain by directly targeting signaling regulators with activators or inhibitors.
One such potential target is Heat shock protein 90 (Hsp90). Hsp90 is a chaperone protein, which is required for activation and stabilization of a variety of proteins [51]. It also has the ablity to modulate intracellular signal transduction molecules, including numerous kinases [51,52]. Hsp90 has been used as a target for anti-cancer drug discovery for over two decades. Compounds targeting Hsp90 can disrupt the activity of oncogenesis-related receptors, kinases and transcription factors [53]. A few previous studies reported that Hsp90 promotes diabetes-induced hypoalgesia and inflammation-induced allodynia, suggesting that Hsp90 is involved in regulating pain [54-56]. However, there have been only two studies conducted to investigate the role of Hsp90 specifically in MOR signal transduction and opioid response. Koshimizu et al. demonstrated that Hsp90 inhibition reduces adenylyl cyclase superactivation, a cellular marker of dependence [57]. A paper by the Devi group found that Hsp90 is upregulated by chronic morphine treatment in the pre-synaptic terminal, and that administration of the Hsp90 inhibitor geldanamycin in the periphery immediately prior to naloxone-precipitated withdrawal reduces the somatic signs of dependence and withdrawal [58]. This limited evidence from the literature does suggest that Hsp90 has a role in regulating pain, MOR signal transduction, and opioid behavioral responses. In our own work, we demonstrate that chronic Hsp90 inhibition worsens the opioid therapeutic index by decreasing tail flick and paw withdrawal anti-nociception, and increasing morphine-induced dependence and withdrawal (unpublished data, not shown). Taken together, this data suggets that Hsp90 modulators should be investigated for opioid co-therapy to improve the opioid therapeutic index.
In signaling kinases, previous studies have demonstrated that the activation of ERK1/2 and JNK MAPKs is associated with opioid-induced tolerance [59], dependence [60], hyperalgesia and allodynia [61,62], and constipation [63]. Similar studies also suggest that p38 MAPK is a key player in KOR-induced side effects, such as the aversive properties of stress and dysphoric behaviors [64-66]. Co-treatment with MOR and KOR agonists with inhibitors to ERK, JNK, or p38 may be a potential strategy to maximize the activity of MOR and KOR agonists without inducing opioid side effects. One caveat, however, is that these signaling kinases are broadly expressed and involved in many biological processes, which increases the risk of non-opioid side effects from targeting these molecules. Strategies such as protein-protein interaction disruptors or targeted brain delivery of inhibitors could impart more selectivity to targeting these molecules and reduce potential side effects.
Several reports have also suggested that opioids can bind to other receptors and activate the intracellular signaling pathways associated with these receptors. Watkins and colleagues have demonstrated that morphine and its metabolite can activate the toll-like receptor 4 (TLR4), which may be involved in the development of hyperalgesia, dependence, and allodynia [67-69]. Activating TLR4 signaling also induces the production of pro-inflammatory cytokines and prostaglandin E2, which can reduce the analgesic activity of opioids [70,71]. Selfridge et al. demonstrated that a naltrexone-based compound inhibited TLR4 activation, and increased and prolonged morphine analgesia [72]. These findings indicate that TLR4 and its downstream signaling mediators could also be targeted for direct inhibition to improve the opioid therapeutic index. The possibilities suggested here are not exhaustive, and many potential signaling regulators could be targeted by this approach as our knowledge of opioid signal transduction grows.
Multi-Functional Compounds
Over the past few decades, much basic science knowledge has been generated demonstrating how opioid-induced side effects are expressed. One major theme is that as the MOR system is chronically stimulated, other negative feedback systems are also upregulated to compensate. Several of these feedback systems have been identified, and shown to help drive opioid side effects. This has led to the idea of multi-functional compounds that target the MOR and a negative feedback system, to both activate the MOR to produce analgesia and antagonize the negative feedback system to reduce side effects. This is an example of basic science knowledge driving rational drug design from the beginning, rather than a blind target-based approach. Tapentadol and tramadol are examples of multi-functional drugs that have reached the market with evidence for reduced addiction liability, while a new generation of multi-functional drugs is in pre-clinical and early clinical development [10]. Both tapentadol and tramadol are mixed MOR agonists and norepineprhine and/or serotonin reuptake inhibitors.
Cholecystokinin
Porreca and colleagues found in several seminal studies that cholecystokinin(CCK)-containing neurons are a MOR negative feedback loop upregulated in the rostroventral medulla [5,13,73-79]. They found that CCK may drive the seemingly paradoxical phenomenon of opioid-induced hyperalgesia (OIH), promote neuropathic pain, drive opioid-induced reward, reduce opioid analgesic potency, and similar effects. Notably, treatment with a CCK antagonist could reverse some of these effects, suggesting that a multi-functional MOR agonist/CCK antagonist could be a beneficial therapeutic tool (i.e. [77]). This led to a program of drug development to make peptide and small molecule MOR agonist/CCK antagonist compounds, which have shown potent anti-nociception in chronic and acute pain models in mice and rats, along with some reduced side effects, notably reward [80-87] (F. Porreca, unpublished data).
Delta Opioid Receptor
The DOR has also been found to drive opioid-mediated side effects. Treatment with the DOR selective antagonist naltrindole, DOR antisense DNA, or DOR KO all reduced opioid-mediated tolerance, as well as other side effects, including dependence (depending on individual study) [88-90]. As above, this suggested the idea of a multi-functional MOR agonist/DOR antagonist to recapitulate these benefits. This has led to a peptide, peptidomimetic, and small molecule discovery and development effort by several labs, resulting in the production of molecules with the desired multi-functional properties [91-96]. These molecules generally did not produce tolerance, dependence, or conditioned place preference (i.e. reward) in rodents, while still producing potent and efficacious anti-nociception, validating the approach. These molecules will continue preclinical development, and will hopefully reach clinical testing soon.
One paradoxical finding in this field is that both MOR agonist/DOR antagonists as well as multi-functional MOR and DOR agonists are found to be beneficial. The MOR/DOR agonist MMP-2200 has been found to produce anti-nociception with reduced tolerance, dependence, and self-administration, suggesting lower abuse liability [97-99]. At this point, the mechanisms behind this seeming paradox are completely unknown, leaving us with the unusual situation that both multi-functional MOR agonist/DOR antagonist and MOR/DOR agonist drugs are valid targets for drug discovery to reduce opioid side effects.
Neurokinin Receptors
Pioneering work by Todd Vanderah and colleagues has strongly suggested that the neurokinin 1 (NK1) and, possibly, NK3 receptors drive opioid-mediated reward in the ventral tegmental area, as well as OIH and tolerance in other circuits (T. Vanderah, unpublished data) [100,101]. As above, this has suggested multi-functional MOR agonist/NK1 antagonists. A series of peptide ligands with this profile has been created, which shows anti-nociception with reduced tolerance and reward [102-107]. The further challenge for this target will be to make the initial peptide ligands druggable, by creating peptidomimetics or small molecules, or perhaps through novel peptide modifications such as glycosylation (see below). This will permit further development and clinical testing to truly validate the promise of this approach.
Beyond these initial targets, there are many potential targets for a multi-functional drug discovery approach, some of which remain to be discovered. Others which have been suggested include MOR agonist/KOR antagonist (YS. Lee, unpublished data), and MOR agonist/Cannabinoid1 or 2 agonist (T. Vanderah, unpublished data). Any system that is found to synergize with the opioid system or drive its side effects is amenable to this type of approach.
Opioid Receptor Heterodimers
The past decade has seen significant in vitro evidence indicating that GPCRs form functionally distinct heterodimer structures, with distinct biochemical and pharmacological properties compared to their homodimer or monomer counterparts. Heterodimer targets for pain most frequently consist of one opioid protomer (MOR or DOR), and a second GPCR protomer with a role in pain signal transduction, such as DOR, KOR [108], or CCR [109], among others. Conceptually, GPCR heterodimer ligands can be classified in two ways: 1) A single molecule consisting of two pharmacophores, separated by a linker of appropriate length, promoting simultaneous binding to both active sites, or 2) a single molecule that prefers one (or both) protomer(s) when in the heterodimer conformation. These distinctions are not necessarily mutually exclusive, but provide a useful tool for considering heterodimer studies involving pain and potential future drug discovery.
MOR-DOR Heterodimers
BRET studies found that the MOR forms heterodimers with DOR (MDOR) along with heterodimers between DOR and KOR (DKOR) in vitro [108]. Co-expression of MOR and DOR generates a novel pharmacological target with distinct receptor binding properties, indicating a unique receptor conformation [110] and unique signaling scaffolds relative to MOR or DOR homomers [111]. In vivo studies using mice co-expressing mCherry-MOR and GFP-DOR found each receptor throughout important regulatory pain regions such as the medulla, periaqueductal grey, and pons in brain [112], indicating that MDOR could be important in these regions. Their model was validated using pull-downs in the hippocampus to confirm that co-expression can lead to heterodimer complexes [112].
MDOR is potentially extensively expressed in the brain, with approximately 40 percent of neurons that express GFP-DOR co-expressing mCherry-MOR [112]. The functional consequences of MDOR remain difficult to dissect, although initial studies indicate that targeting MDOR may lead to improved analgesics with reduced side effects such as tolerance. A MDOR-specific antibody was developed to label and quantify MDOR in brain tissue [113], uncovering MDOR up-regulation after chronic morphine treatment in the medulla and other pain regulatory brain regions, and suggesting a potential role in tolerance requiring further investigation. Another group used the first transmembrane region of MOR (MORTM1) [114] to disrupt the MDOR heterodimer, which enhanced morphine analgesia and reduced anti-nociceptive tolerance. Together, these studies suggest that MDOR disruption may enhance analgesia and reduce tolerance.
Agonists with modest MDOR selectivity suggest that activating MDOR may be an important therapeutic target. Screening of a small molecule library revealed a modestly selective agonist, CYM51010, with 1:4:6 MDOR:MOR:DOR selectivity and efficacy in cells co-expressing MDOR versus MOR or DOR in a βarrestin2 recruitment assay [115]. The Portoghese group developed bivalent ligands containing a MOR agonist, oxymorphone, linked to the DOR antagonist naltrindole, with enhanced analgesia and reduced tolerance relative to morphine. Potency increased and tolerance decreased in a length-dependent manner [116], showing that linker lengths of 21 (MDAN-21) atoms were optimal. Follow-up studies revealed that MDAN-21 reduced MDOR internalization relative to mixed or single treatment with the pharmacophores, suggesting that reduced MDOR internalization may be the mechanism for reduced in vivo tolerance [117]. While internalization for CYM51010 has not been reported, the contrast of agonist activity at MOR and DOR compared to agonist at MOR and antagonist at DOR – not to mention the disruption studies – make the mechanism of MDOR-mediated effects unclear, even if these ligands offer promising results for analgesic development.
KOR-DOR Heterodimers
Another opioid heterodimer target for drug discovery is KOR and DOR (KDOR) [118]. A screening method using a chimeric Gα and intracellular Ca2+ in cells expressing DOR, KOR, or KDOR identified 6’GNTI as a five-fold selective ligand in cells co-expressing KOR and DOR, relative to KOR. Selective KDOR activation by 6’GNTI was further suggested when it was found that 6’GNTI only induces anti-nociception when injected into the spinal cord, not brain, while non-selective KOR agonists like U50,488 induce anti-nociception in both locations [119]. Paw withdrawal latency of 6’GNTI was reversed by either a peripherally injected DOR (naltrindole) antagonist or a KOR antagonist (nor-BNI) in models of inflammatory peripheral pain [120]. However, the in vitro system to characterize these compounds is fairly artificial, as it requires co-expression of a mutant Gq chimera protein, whereas opioids classically signal through Gαi/o. The spinal-mediated analgesia of 6’-GNTI is also dependent on bias against βarrestin signaling – that is, activation of G protein, but not βarrestin, at KOR was important for analgesia [111,119]. Synthetic analogues of 6’GNTI for KDOR selective agonists identified N-2′-Indolylnaltrexamine (INTA) as a candidate [121]. Interestingly, HEK293 cells co-expressing KOR and DOR did not result in significant βarrestin2 recruitment upon INTA treatment, suggesting that functional selectivity and heterodimer-mediated activity are intertwined. However, the low-level βarrestin2 recruitment by any ligand in their system suggests that these results could arise from experimental sensitivity. Regardless of the mechanism, INTA did not show conditioned place preference, suggesting a lack of reward after chronic treatment, as would be predicted for KOR biased agonists.
MOR-CCR5 Heterodimers
In CHO cells co-expressing MOR and CCR5 receptors, heterodimerization was found as assessed by co-immunoprecipitation studies. One component of morphine tolerance in inflammatory pain states is from chemokine release and hyperalgesia. Interestingly, the response to DAMGO (MOR agonist) or RANTES (CCR5 agonist) exhibited cross-desensitization, suggesting that these receptor systems interact [109]. DAMGO pretreatment reduced RANTES (CCL5)-promoted [35S]GTPγS coupling, while RANTES pre-incubation significantly reduced DAMGO-induced GTPγS coupling in co-expressing cells, suggesting a heterodimer. In this light, a bivalent ligand consisting of a MOR agonist and CCR5 antagonist was designed to study the effects of MOR-CCR5 heterodimers [122] on analgesia in inflammatory pain models. MC22 – a compound with a 22-atom spacer – improved anti-nociceptive potency 2000-fold over morphine in mice treated with lipopolysaccharide – a common model for inflammatory pain. While these papers did not include molecular pharmacology characterization of the compounds, the 22-atom linker length was significantly more potent in vivo than other linker lengths. Furthermore, relative to morphine and other linker lengths, MC22 showed a significant reduction in anti-nociceptive tolerance in chronic pain models. Due to the absence of molecular characterization, it is unclear how this molecule affects MOR-CCR5 signaling, but this remains a promising target for drug discovery.
Taken together, bivalent and heterodimer selective compounds at various opioid targets provide intriguing alternatives to minimize tolerance profiles and treat specific types of pain, such as neuropathic pain (MOR-CCR5). However, major limitations of the heteromer-mediated mechanism leading to reduced tolerance include: 1) The in vitro selectivity of these compounds is typically under 100-fold when molecular characterization is performed, and 2) many ligands – such as with MOR-CCR5 or MOR-DOR – connect a MOR agonist to an antagonist, and it remains unclear how the simultaneous occupation of an agonist and antagonist affects heterodimer signaling. Thus, further studies utilizing pharmacological tools that act as heterodimer antagonists or heterodimer agonists are required. Nonetheless, the therapeutic potential of MOR-DOR and MOR-CCR5 to develop molecules with reduced tolerance and side effect profiles is promising.
Making Endogenous Opioid Peptides Druggable
Endogenous opioid peptides such as endorphins, enkephalins, dynorphins, and endomorphins have been in focus as potential analgesic drug candidates for many years [123,124]. Although their in vivo efficacies are comparable to those of the alkaloid opiate derivatives in animal models of pain, and their side effect profiles are generally superior to small molecule drugs, the direct use of endogenous opioid peptides as analgesic drugs is still limited by their general lack of receptor selectivity (not true for all – especially endomorphins or targeting the KOR with dynorphins), short in vivo half-life, poor blood-brain barrier penetration, and poor bioavailability. To improve these pharmacological properties, several structural modification-based strategies have been developed and introduced in recent years [123,125]. We will briefly discuss these approaches below, placing emphasis on the advanced methodologies that may possibly result in bioavailable, potent, opioid peptide-based drug candidates in the near future.
Glycosylation
To increase bioavailability and blood-brain barrier permeability of endogenous opioid peptides, glycosylation of the peptide backbone by incorporation of glycosylated amino acids or sugar-amino acid moieties has been pursued. This is an attractive strategy, since glycosylation is one of the least toxic and most common post-translational modifications in vivo. This chemical intervention, using various types of carbohydrates, not only facilitates blood-brain barrier permeability of the target peptide in vivo, but occasionally also confers higher potency and proteolytic stability to the peptides [126,127]. This strategy has been successfully used for the generation of bioavailable β-endorphin, enkephalin, and endomorphin derivatives that are systemically stable, cross the blood-brain barrier, and have potent anti-nociception with often reduced side effects [127-129].
Cyclization
Cyclization of the peptide backbone of opioid peptides, a typical conformational constraint, is a widely used approach to enhance receptor selectivity and proteolytic stability, reduce off-target toxicity, and modulate binding activity. Since peptide analogs with appropriate conformational constraints tend to adopt specific conformations required for interaction at one receptor type but not another, conformational constraint has proved to be an extremely useful tool to dissect peptide binding characteristics. These backbone modifications include amide bond formation, disulfide-, hydrazine-, carbonyl- and amine bridges, or incorporation of cyclic amino acids or side chain conformational constraints. This can lead to very selective and potent agonist or antagonist ligands for all three types of opioid receptors [130-134]. Notably, a recent series of cyclized endomorphin derivatives from the Zadina group has shown great promise, with in vivo bioavailability, anti-nociception, and greatly reduced reward, abuse liability, and several other typical opioid induced side effects [135].
Other Modifications
Another structural modification is the N- or C-terminal conjugation of opioid peptides with lipids or incorporation of lipo-amino acids in the peptide sequence. This confers an amphipathic character to the opioid peptides which promotes interaction between the ligand and the lipid bilayer of the cell membrane, and possibly increases proteolytic stability and blood-brain barrier permeability [129,136,137].
Alternatively, opioid peptides can be packed into self-assembling monolayer vesicles or peptide-glutathione PEGylated-liposome nanobodies. These are attractive drug formulation approaches that enhance peripheral peptide transport and targeted delivery of opioid cargo into the central nervous system. Some advantages of this strategy are that the release of the encapsulated peptide can be strictly regulated, and the peptide is quasi-protected from proteolytic enzymes while it resides within the vesicle or nanobody. This approach has been widely used to target a variety of central nervous disorders with drugs that cannot normally go through the tightly closed blood-brain barrier [138-140].
Conclusions and Outlook
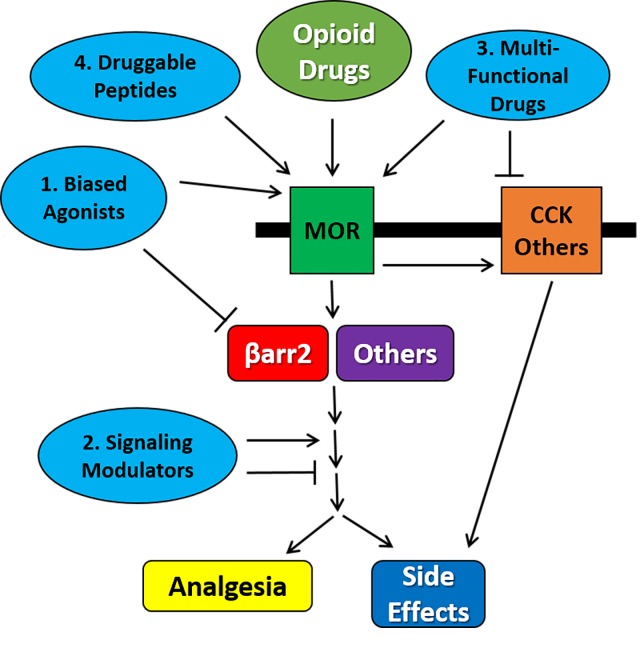
Historically, opioids are among the few efficacious options for the treatment of moderate to severe chronic pain, but are limited by significant side effects. More than a century of drug discovery and development has failed to generate a broadly applicable, high-efficacy opioid or other analgesic drug without these basic side effects. However, new findings in the molecular signaling of the MOR have suggested new approaches. As we determine which signaling cascades are responsible for which part of the response, those cascades can be targeted using functionally selective drug discovery, as for βarrestin2, or the molecules can be directly targeted using activators or inhibitors. Separately, multi-functional ligands can also be designed that will activate the MOR to promote analgesia, but antagonize other receptor systems that are upregulated during chronic opioid treatment and drive many side effects, including tolerance, dependence, and addiction. Opioid receptor heterodimers are also relatively new and attractive targets, but are very limited at this point by a lack of specific pharmacological tools. Lastly, endogenous peptide ligands, which are potent with few side effects but are poor drug candidates, may be chemically modified to make them stable and available to the brain for pain treatment. These approaches are summarized in Figure 1.
Figure 1.
Summary of Molecular Strategies for Improved Opioid Drug Discovery. Opioid drugs such as morphine and fentanyl activate the MOR, which in turns activates signal transduction cascades. These cascades include factors such as βarrestin2, and drive both analgesia and side effects. At the same time, MOR activation leads to activation of negative feedback receptor systems including CCK, which in turn drive the induction of opioid side effects. This process can be targeted by 1) biased ligands which activate the MOR while blocking or not activating negative signal transduction factors downstream such as βarrestin2; 2) signaling modulators which directly target intracellular signaling molecules to activate or inhibit and improve the opioid response; 3) multi-functional drugs which simultaneously activate the MOR to drive analgesia while blocking negative feedback receptor systems to reduce side effects; and 4) druggable endogenous opioid peptides which have a better activation profile than small molecule drugs like morphine.
All of these novel approaches are extremely promising, and may provide a way forward to break the barriers limiting pain drug discovery. However, by far the greatest limitation is that most of these approaches have generated molecules that have only been tested preclinically, in animal models. The biased ligand TRV130 and the multi-functional opioids tapentadol and tramadol are the only real compounds from these approaches that are in clinical testing or approved for human use. The coming years will provide a wealth of information as to the feasibility and translatability of these new approaches to human patients, as these drugs enter clinical trial testing.
Another potential limitation is that the targets for some of these approaches, particularly functional selectivity, are limited. Extensive additional basic science work is needed to flesh out the signal transduction mechanisms underlying the MOR, and the neural networks and interactions between the MOR and other receptor systems. This basic science exploration will provide the foundation for future development of these approaches to drug discovery and guarantee their future growth, maximizing our chances of successful translation.
Overall, however, the impact of these new approaches is difficult to understate. The need for new analgesic drugs without side effects, particularly addiction, is very pressing, both medically and socially. Drug overdose deaths with opioids have been steadily rising, and the United States now consumes 80 percent of the world’s opioid supply. The needs of pain patients are starting to be pitted against the need to control an emerging addiction and overdose epidemic. The invention of an analgesic without side effects, using these new approaches or any other, is crucially needed to avoid this clash where the patients are likely to be the victims.
Glossary
- MOR, KOR, DOR
Mu, Kappa, Delta Opioid Receptor
- NOP
Nociceptin Receptor
- TCA
tricyclic antidepressants
- GPCR
G Protein Coupled Receptor
- SAR
Structure-Activity Relationship
- AC
adenylyl cyclase
- RGS
Regulators of G Protein Signaling
- GAP
GTPase Activating Protein
- Hsp90
Heat shock protein 90
- TLR4
Toll-like Receptor 4
- CCK
cholecystokinin
- NK
neurokinin
Author's note
JS has performed contract research work for Depomed Inc. and Stealth Peptides, which are pharmaceutical companies developing pain drugs. However, none of the molecules or strategies utilized by either company are included in this review.
References
- Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Breivik H, Cherny N, Collett B. et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20(8):1420–1433. doi: 10.1093/annonc/mdp001. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence: Guidance. Cardiff (UK): National Collaborating Centre for Cancer (UK); 2012. Opioids in Palliative Care: Safe and Effective Prescribing of Strong Opioids for Pain in Palliative Care of Adults. [PubMed] [Google Scholar]
- Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206–219. doi: 10.1016/j.genhosppsych.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Malan TP Jr.. et al. Spinal and supraspinal mechanisms of neuropathic pain. Ann N Y Acad Sci. 2000;909:12–24. doi: 10.1111/j.1749-6632.2000.tb06673.x. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314(3):1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- Annemans L. Pharmacoeconomic impact of adverse events of long-term opioid treatment for the management of persistent pain. Clin Drug Investig. 2011;31(2):73–86. doi: 10.1007/BF03256935. [DOI] [PubMed] [Google Scholar]
- Woodcock J, Witter J, Dionne RA. Stimulating the development of mechanism-based, individualized pain therapies. Nat Rev Drug Discov. 2007;6(9):703–710. doi: 10.1038/nrd2335. [DOI] [PubMed] [Google Scholar]
- Duggan PJ, Tuck KL. Bioactive Mimetics of Conotoxins and other Venom Peptides. Toxins (Basel) 2015;7(10):4175–4198. doi: 10.3390/toxins7104175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart RC, Cicero TJ, Surratt HL. et al. Assessment of the abuse of tapentadol immediate release: the first 24 months. J Opioid Manag. 2012;8(6):395–402. doi: 10.5055/jom.2012.0139. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR. et al. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286(5449):2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M. et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320(1):1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Hruby VJ, Porreca F, Yamamura HI. et al. New paradigms and tools in drug design for pain and addiction. AAPS J. 2006;8(3):E450–E460. doi: 10.1208/aapsj080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60(1):58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA. et al. An opioid agonist that does not induce micro-opioid receptor--arrestin interactions or receptor internalization. Mol Pharmacol. 2007;71(2):549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidgewell K, Groer CE, Harding WW. et al. Herkinorin analogues with differential beta-arrestin-2 interactions. J Med Chem. 2008;51(8):2421–2431. doi: 10.1021/jm701162g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewire SM, Yamashita DS, Rominger DH. et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared to morphine. J Pharmacol Exp Ther. 2013 doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK. et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016:1–6. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, McDonald PH. Seeking Ligand Bias: Assessing GPCR Coupling to Beta-Arrestins for Drug Discovery. Drug Discov Today Technol. 2010;7(1):e37–e42. doi: 10.1016/j.ddtec.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XT, Pitis P, Liu G. et al. Structure-activity relationships and discovery of a G protein biased mu opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan- 9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem. 2013;56(20):8019–8031. doi: 10.1021/jm4010829. [DOI] [PubMed] [Google Scholar]
- Soergel DG, Subach RA, Burnham N. et al. Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain. 2014;155(9):1829–1835. doi: 10.1016/j.pain.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Soergel DG, Subach RA, Sadler B. et al. First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 2014;54(3):351–357. doi: 10.1002/jcph.207. [DOI] [PubMed] [Google Scholar]
- Viscusi ER, Webster L, Kuss M. et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157(1):264–272. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 2010;210(2):137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD. et al. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281(26):18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell KM, Frankowski KJ, Stahl EL. et al. Structure-Activity Relationship Studies of Functionally Selective Kappa Opioid Receptor Agonists that Modulate ERK 1/2 Phosphorylation While Preserving G Protein Over betaArrestin2 Signaling Bias. ACS Chem Neurosci. 2015 doi: 10.1021/acschemneuro.5b00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rives ML, Rossillo M, Liu-Chen LY. et al. 6'GNTI is a G protein-biased kappa opioid receptor agonist that inhibits arrestin recruitment. J Biol Chem. 2012 doi: 10.1074/jbc.C112.387332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Streicher JM. Functional Selectivity of 6'-guanidinonaltrindole (6'-GNTI) at Kappa Opioid Receptors in Striatal Neurons. J Biol Chem. 2013 doi: 10.1074/jbc.M113.476234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KL, Robinson JE, Zhu H. et al. The G protein-biased kappa-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J Pharmacol Exp Ther. 2015;352(1):89–109. doi: 10.1124/jpet.114.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMattio KM, Ehlert FJ, Liu-Chen LY. Intrinsic relative activities of kappa opioid agonists in activating Galpha proteins and internalizing receptor: Differences between human and mouse receptors. Eur J Pharmacol. 2015;761:235–244. doi: 10.1016/j.ejphar.2015.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet EL, Milon N, Heghinian MD. et al. Noribogaine is a G-protein biased kappa-opioid receptor agonist. Neuropharmacology. 2015;99:675–688. doi: 10.1016/j.neuropharm.2015.08.032. [DOI] [PubMed] [Google Scholar]
- White KL, Scopton AP, Rives ML. et al. Identification of novel functionally selective kappa-opioid receptor scaffolds. Mol Pharmacol. 2014;85(1):83–90. doi: 10.1124/mol.113.089649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Yost JM, Setola V. et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A. 2011;108(45):18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy JL, Free RB, Sibley DR. Identification of G protein-biased agonists that fail to recruit beta-arrestin or promote internalization of the D1 dopamine receptor. ACS Chem Neurosci. 2015;6(4):681–692. doi: 10.1021/acschemneuro.5b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller D, Kling RC, Skultety M. et al. Functionally selective dopamine D(2), D(3) receptor partial agonists. J Med Chem. 2014;57(11):4861–4875. doi: 10.1021/jm5004039. [DOI] [PubMed] [Google Scholar]
- Peterson SM, Pack TF, Caron MG. Receptor, Ligand and Transducer Contributions to Dopamine D2 Receptor Functional Selectivity. PLoS One. 2015;10(10):e0141637. doi: 10.1371/journal.pone.0141637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evron T, Peterson SM, Urs NM. et al. G Protein and beta-arrestin signaling bias at the ghrelin receptor. J Biol Chem. 2014;289(48):33442–33455. doi: 10.1074/jbc.M114.581397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Yue X, Varga EV, Stropova D. et al. Chronic morphine-mediated adenylyl cyclase superactivation is attenuated by the Raf-1 inhibitor, GW5074. Eur J Pharmacol. 2006;540(1-3):57–59. doi: 10.1016/j.ejphar.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Varga EV, Rubenzik M, Grife V. et al. Involvement of Raf-1 in chronic delta-opioid receptor agonist-mediated adenylyl cyclase superactivation. Eur J Pharmacol. 2002;451(1):101–102. doi: 10.1016/s0014-2999(02)02220-3. [DOI] [PubMed] [Google Scholar]
- Tumati S, Milnes TL, Yamamura HI. et al. Intrathecal Raf-1-selective siRNA attenuates sustained morphine-mediated thermal hyperalgesia. Eur J Pharmacol. 2008;601(1-3):207–208. doi: 10.1016/j.ejphar.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumati S, Roeske WR, Largent-Milnes T. et al. Sustained morphine-mediated pain sensitization and antinociceptive tolerance are blocked by intrathecal treatment with Raf-1-selective siRNA. Br J Pharmacol. 2010;161(1):51–64. doi: 10.1111/j.1476-5381.2010.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G. et al. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65(1):223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor J. mu-Opioid receptors and regulators of G protein signaling (RGS) proteins: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121(3):173–180. doi: 10.1016/j.drugalcdep.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor J. Regulator of G protein-signaling proteins and addictive drugs. Ann N Y Acad Sci. 2010;1187:341–352. doi: 10.1111/j.1749-6632.2009.05150.x. [DOI] [PubMed] [Google Scholar]
- Garzon J, Rodriguez-Diaz M, Lopez-Fando A. et al. RGS9 proteins facilitate acute tolerance to mu-opioid effects. Eur J Neurosci. 2001;13(4):801–811. doi: 10.1046/j.0953-816x.2000.01444.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu-Chen LY, Traynor JR. Differential modulation of mu- and delta-opioid receptor agonists by endogenous RGS4 protein in SH-SY5Y cells. J Biol Chem. 2009;284(27):18357–18367. doi: 10.1074/jbc.M109.015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Traynor JR. Modulation of mu-opioid receptor signaling by RGS19 in SH-SY5Y cells. Mol Pharmacol. 2013;83(2):512–520. doi: 10.1124/mol.112.081992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MJ, Harrison C, Zhong H. et al. Endogenous RGS protein action modulates mu-opioid signaling through Galphao. Effects on adenylyl cyclase, extracellular signal-regulated kinases, and intracellular calcium pathways. J Biol Chem. 2003;278(11):9418–9425. doi: 10.1074/jbc.M208885200. [DOI] [PubMed] [Google Scholar]
- Li J, Buchner J. Structure, function and regulation of the hsp90 machinery. Biomed J. 2013;36(3):106–117. doi: 10.4103/2319-4170.113230. [DOI] [PubMed] [Google Scholar]
- Ota A, Zhang J, Ping P. et al. Specific regulation of noncanonical p38alpha activation by Hsp90-Cdc37 chaperone complex in cardiomyocyte. Circ Res. 2010;106(8):1404–1412. doi: 10.1161/CIRCRESAHA.109.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Ramos KM, Loram LC. et al. Evidence for a role of heat shock protein-90 in toll like receptor 4 mediated pain enhancement in rats. Neuroscience. 2009;164(4):1821–1832. doi: 10.1016/j.neuroscience.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MJ, Li C, Yu C. et al. Inhibiting heat-shock protein 90 reverses sensory hypoalgesia in diabetic mice. ASN Neuro. 2010;2(4):e00040. doi: 10.1042/AN20100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Leung E, Gruner S. et al. Tamoxifen enhances the Hsp90 molecular chaperone ATPase activity. . PLoS One. 2010;5(4):e9934. doi: 10.1371/journal.pone.0009934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu TA, Tsuchiya H, Tsuda H. et al. Inhibition of heat shock protein 90 attenuates adenylate cyclase sensitization after chronic morphine treatment. Biochem Biophys Res Commun. 2010;392(4):603–607. doi: 10.1016/j.bbrc.2010.01.089. [DOI] [PubMed] [Google Scholar]
- Abul-Husn NS, Annangudi SP, Ma'ayan A. et al. Chronic morphine alters the presynaptic protein profile: identification of novel molecular targets using proteomics and network analysis. PLoS One. 2011;6(10):25535. doi: 10.1371/journal.pone.0025535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck EN, Ingram SL, Hermes SM. et al. Ligand-biased activation of extracellular signal-regulated kinase 1/2 leads to differences in opioid induced antinociception and tolerance. Behav Brain Res. 2016;298(Pt B):17–24. doi: 10.1016/j.bbr.2015.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi S, Gessi S, Varani K. et al. Morphine mediates a proinflammatory phenotype via mu-opioid receptor-PKCvarepsilon-Akt-ERK1/2 signaling pathway in activated microglial cells. Biochem Pharmacol. 2013;86(4):487–496. doi: 10.1016/j.bcp.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Popiolek-Barczyk K, Makuch W, Rojewska E. et al. Inhibition of intracellular signaling pathways NF-kappaB and MEK1/2 attenuates neuropathic pain development and enhances morphine analgesia. Pharmacol Rep. 2014;66(5):845–851. doi: 10.1016/j.pharep.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Sanna MD, Mello T, Ghelardini C. et al. Inhibition of spinal ERK1/2-c-JUN signaling pathway counteracts the development of low doses morphine-induced hyperalgesia. Eur J Pharmacol. 2015;764:271–277. doi: 10.1016/j.ejphar.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Duraffourd C, Kumala E, Anselmi L. et al. Opioid-induced mitogen-activated protein kinase signaling in rat enteric neurons following chronic morphine treatment. PLoS One. 2014;9(10):e110230. doi: 10.1371/journal.pone.0110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M. et al. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27(43):11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich JM, Messinger DI, Knakal CR. et al. Kappa Opioid Receptor-Induced Aversion Requires p38 MAPK Activation in VTA Dopamine Neurons. J Neurosci. 2015;35(37):12917–12931. doi: 10.1523/JNEUROSCI.2444-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton CC, Xu M, Chavkin C. Tyrosine phosphorylation of Kir3 following kappa-opioid receptor activation of p38 MAPK causes heterologous desensitization. J Biol Chem. 2009;284(46):31872–31881. doi: 10.1074/jbc.M109.053793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Loram LC, Zhang Y. et al. Evidence that tricyclic small molecules may possess toll-like receptor and myeloid differentiation protein 2 activity. Neuroscience. 2010;168(2):551–563. doi: 10.1016/j.neuroscience.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SS, Hutchinson MR, Rezvani N. et al. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165(2):569–583. doi: 10.1016/j.neuroscience.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis A, Grace PM, Wieseler J. et al. Morphine amplifies mechanical allodynia via TLR4 in a rat model of spinal cord injury. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Coats BD, Lewis SS. et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22(8):1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Ramos KM, Rodgers KM. et al. Activation of adult rat CNS endothelial cells by opioid-induced toll-like receptor 4 (TLR4) signaling induces proinflammatory, biochemical, morphological, and behavioral sequelae. Neuroscience. 2014;280:299–317. doi: 10.1016/j.neuroscience.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selfridge BR, Wang X, Zhang Y. et al. Structure-Activity Relationships of (+)-Naltrexone-Inspired Toll-like Receptor 4 (TLR4) Antagonists. J Med Chem. 2015;58(12):5038–5052. doi: 10.1021/acs.jmedchem.5b00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelowski CJ, Ossipov MH, Sun H. et al. Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain. 2000;87(3):265–273. doi: 10.1016/S0304-3959(00)00290-6. [DOI] [PubMed] [Google Scholar]
- Marshall TM, Herman DS, Largent-Milnes TM. et al. Activation of descending pain-facilitatory pathways from the rostral ventromedial medulla by cholecystokinin elicits release of prostaglandin-E(2) in the spinal cord. Pain. 2012;153(1):86–94. doi: 10.1016/j.pain.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols ML, Bian D, Ossipov MH. et al. Antiallodynic effects of a CCKB antagonist in rats with nerve ligation injury: role of endogenous enkephalins. Neurosci Lett. 1996;215(3):161–164. doi: 10.1016/0304-3940(96)12964-5. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Vanderah TW. et al. Induction of pain facilitation by sustained opioid exposure: relationship to opioid antinociceptive tolerance. Life Sci. 2003;73(6):783–800. doi: 10.1016/s0024-3205(03)00410-7. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Bernstein RN, Yamamura HI. et al. Enhancement of morphine antinociception by a CCKB antagonist in mice is mediated via opioid delta receptors. J Pharmacol Exp Ther. 1996;278(1):212–219. [PubMed] [Google Scholar]
- Xie JY, Herman DS, Stiller CO. et al. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25(2):409–416. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gardell S, Zhang D. et al. Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors. Brain. 2009;132(Pt 3):778–787. doi: 10.1093/brain/awn330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnes RS, Lee YS, Davis P. et al. Structure-activity relationships of bifunctional peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors. J Med Chem. 2006;49(10):2868–2875. doi: 10.1021/jm050921q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnes RS, Ying J, Kover KE. et al. Structure-activity relationships of bifunctional cyclic disulfide peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors. Peptides. 2008;29(8):1413–1423. doi: 10.1016/j.peptides.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon KE, Herman DS, Agnes RS. et al. Novel peptide ligands with dual acting pharmacophores designed for the pathophysiology of neuropathic pain. Brain Res. 2011;1395:1–11. doi: 10.1016/j.brainres.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby VJ, Agnes RS, Davis P. et al. Design of novel peptide ligands which have opioid agonist activity and CCK antagonist activity for the treatment of pain. Life Sci. 2003;73(6):699–704. doi: 10.1016/s0024-3205(03)00390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Agnes RS, Badghisi H. et al. Design and synthesis of novel hydrazide-linked bifunctional peptides as delta/mu opioid receptor agonists and CCK-1/CCK-2 receptor antagonists. J Med Chem. 2006;49(5):1773–1780. doi: 10.1021/jm05085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Agnes RS, Davis P. et al. Partial retro-inverso, retro, and inverso modifications of hydrazide linked bifunctional peptides for opioid and cholecystokinin (CCK) receptors. J Med Chem. 2007;50(1):165–168. doi: 10.1021/jm061268p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Fernandes S, Kulkarani V. et al. Design and synthesis of trivalent ligands targeting opioid, cholecystokinin, and melanocortin receptors for the treatment of pain. Bioorg Med Chem Lett. 2010;20(14):4080–4084. doi: 10.1016/j.bmcl.2010.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndungu JM, Cain JP, Davis JP. et al. Synthesis of constrained analogues of cholecystokinin/opioid chimeric peptides. Tetrahedron Lett. 2006;47(13):2233–2236. doi: 10.1016/j.tetlet.2006.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhamid EE, Sultana M, Portoghese PS. et al. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258(1):299–303. [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG. et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24(1):243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ikeda H, Tsuji M. et al. Antisense oligodeoxynucleotide to delta opioid receptors attenuates morphine dependence in mice. Life Sci. 1997;61(11):165–170. doi: 10.1016/s0024-3205(97)00620-6. [DOI] [PubMed] [Google Scholar]
- Ananthan S, Kezar HS 3rd, Carter RL. et al. Synthesis, opioid receptor binding, and biological activities of naltrexone-derived pyrido- and pyrimidomorphinans. J Med Chem. 1999;42(18):3527–3538. doi: 10.1021/jm990039i. [DOI] [PubMed] [Google Scholar]
- Ananthan S, Saini SK, Dersch CM. et al. 14-Alkoxy- and 14-Acyloxypyridomorphinans: Mixed Mu Agonist/Delta Antagonist Opioid Analgesics with Diminished Tolerance and Dependence Side Effects. J Med Chem. 2012;55(19):8350–8363. doi: 10.1021/jm300686p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand JP, Boyer BT, Mosberg HI. et al. The behavioral effects of a mixed efficacy antinociceptive peptide, VRP26, following chronic administration in mice. Psychopharmacology (Berl) 2016;233(13):2479–2487. doi: 10.1007/s00213-016-4296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AM, Griggs NW, Gao C. et al. Rapid Synthesis of Boc-2',6'-dimethyl-l-tyrosine and Derivatives and Incorporation into Opioid Peptidomimetics. ACS Med Chem Lett. 2015;6(12):1199–1203. doi: 10.1021/acsmedchemlett.5b00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland AA, Bender AM, Griggs NW. et al. Effects of N-Substitutions on the Tetrahydroquinoline (THQ) Core of Mixed-Efficacy mu-Opioid Receptor (MOR)/delta-Opioid Receptor (DOR) Ligands. J Med Chem. 2016;59(10):4985–4998. doi: 10.1021/acs.jmedchem.6b00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland AA, Yeomans L, Griggs NW. et al. Further Optimization and Evaluation of Bioavailable, Mixed-Efficacy mu-Opioid Receptor (MOR) Agonists/delta-Opioid Receptor (DOR) Antagonists: Balancing MOR and DOR Affinities. J Med Chem. 2015;58(22):8952–8969. doi: 10.1021/acs.jmedchem.5b01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery JJ, Raymond TJ, Giuvelis D. et al. In vivo characterization of MMP-2200, a mixed delta/mu opioid agonist, in mice. J Pharmacol Exp Ther. 2011;336(3):767–778. doi: 10.1124/jpet.110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson GW, Luginbuhl A, Dunbar C. et al. The mixed-action delta/mu opioid agonist MMP-2200 does not produce conditioned place preference but does maintain drug self-administration in rats, and induces in vitro markers of tolerance and dependence. Pharmacol Biochem Behav. 2015;132:49–55. doi: 10.1016/j.pbb.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Carmo GP, Polt R, Bilsky EJ. et al. Behavioral pharmacology of the mu/delta opioid glycopeptide MMP2200 in rhesus monkeys. J Pharmacol Exp Ther. 2008;326(3):939–948. doi: 10.1124/jpet.108.138180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Gardell LR, Wang R. et al. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005;116(3):276–288. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Zhang ET, King T. et al. Spinal NK-1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways. Pain. 2007;129(1-2):35–45. doi: 10.1016/j.pain.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri AK, Apostol CR, Wang Y. et al. Discovery of Novel Multifunctional Ligands with mu/delta Opioid Agonist/Neurokinin-1 (NK1) Antagonist Activities for the Treatment of Pain. J Med Chem. 2015;58(21):8573–8583. doi: 10.1021/acs.jmedchem.5b01170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largent-Milnes TM, Yamamoto T, Nair P. et al. Spinal or systemic TY005, a peptidic opioid agonist/neurokinin 1 antagonist, attenuates pain with reduced tolerance. Br J Pharmacol. 2010;161(5):986–1001. doi: 10.1111/j.1476-5381.2010.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair P, Yamamoto T, Cowell S. et al. Discovery of tripeptide-derived multifunctional ligands possessing delta/mu opioid receptor agonist and neurokinin 1 receptor antagonist activities. Bioorg Med Chem Lett. 2015;25(17):3716–3720. doi: 10.1016/j.bmcl.2015.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair P, Yamamoto T, Largent-Milnes TM. et al. Truncation of the peptide sequence in bifunctional ligands with mu and delta opioid receptor agonist and neurokinin 1 receptor antagonist activities. Bioorg Med Chem Lett. 2013;23(17):4975–4978. doi: 10.1016/j.bmcl.2013.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardanyan R, Kumirov VK, Nichol GS. et al. Synthesis and biological evaluation of new opioid agonist and neurokinin-1 antagonist bivalent ligands. Bioorg Med Chem. 2011;19(20):6135–6142. doi: 10.1016/j.bmc.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Nair P, Largent-Milnes TM. et al. Discovery of a potent and efficacious peptide derivative for delta/mu opioid agonist/neurokinin 1 antagonist activity with a 2',6'-dimethyl-L-tyrosine: in vitro, in vivo, and NMR-based structural studies. J Med Chem. 2011;54(7):2029–2038. doi: 10.1021/jm101023r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sun X, Bohn LM. et al. Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol Pharmacol. 2005;67(6):2173–2184. doi: 10.1124/mol.104.010272. [DOI] [PubMed] [Google Scholar]
- Chen C, Li J, Bot G. et al. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483(2-3):175–186. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Kabli N, Martin N, Fan T. et al. Agonists at the delta-opioid receptor modify the binding of micro-receptor agonists to the micro-delta receptor hetero-oligomer. Br J Pharmacol. 2010;161(5):1122–1136. doi: 10.1111/j.1476-5381.2010.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21(10):2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G. et al. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Func. 2015;220(2):677–702. doi: 10.1007/s00429-014-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I. et al. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3(131):ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Zhang ZN, Guan JS. et al. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron. 2011;69(1):120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Gomes I, Fujita W, Gupta A. et al. Identification of a mu-delta opioid receptor heteromer-biased agonist with antinociceptive activity. Proc Natl Acad Sci U S A. 2013;110(29):12072–12077. doi: 10.1073/pnas.1222044110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL. et al. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005;102(52):19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekkirala AS, Kalyuzhny AE, Portoghese PS. An immunocytochemical-derived correlate for evaluating the bridging of heteromeric mu-delta opioid protomers by bivalent ligands. ACS Chem Biol. 2013;8(7):1412–1416. doi: 10.1021/cb400113d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M, Fong J, Jones RM. et al. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci U S A. 2005;102(102):9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Streicher JM, Groer CE. et al. Functional selectivity of 6'-guanidinonaltrindole (6'-GNTI) at kappa-opioid receptors in striatal neurons. J Biol Chem. 2013;288(31):22387–22398. doi: 10.1074/jbc.M113.476234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Sanchez TA. et al. Regulation of kappa-opioid receptor signaling in peripheral sensory neurons in vitro and in vivo. J Pharmacol Exp Ther. 2011;338(1):92–99. doi: 10.1124/jpet.110.177493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Naour M, Lunzer MM, Powers MD. et al. Putative kappa opioid heteromers as targets for developing analgesics free of adverse effects. J Med Chem. 2014;57(15):6383–6392. doi: 10.1021/jm500159d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgun E, Javed MI, Lunzer MM. et al. Inhibition of Inflammatory and Neuropathic Pain by Targeting a Mu Opioid Receptor/Chemokine Receptor5 Heteromer (MOR-CCR5). J Med Chem. 2015;58(21):8647–8657. doi: 10.1021/acs.jmedchem.5b01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich JV, McLaughlin JP. Opioid Peptides: Potential for Drug Development. Drug Discov Today Technol. 2012;9(1):e23–e31. doi: 10.1016/j.ddtec.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior: 2013. Peptides. 2014;62:67–136. doi: 10.1016/j.peptides.2014.09.013. [DOI] [PubMed] [Google Scholar]
- De Marco R, Janecka A. Strategies to Improve Bioavailability and In Vivo Efficacy of the Endogenous Opioid Peptides Endomorphin-1 and Endomorphin-2. Curr Top Med Chem. 2015;16(2):141–155. doi: 10.2174/1568026615666150817103635. [DOI] [PubMed] [Google Scholar]
- Jones EM, Polt R. CNS active O-linked glycopeptides. Front Chem. 2015;3:30. doi: 10.3389/fchem.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, St Louis L, Knapp BI. et al. Can amphipathic helices influence the CNS antinociceptive activity of glycopeptides related to beta-endorphin? J Med Chem. 2014;57(6):2237–2246. doi: 10.1021/jm400879w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsky EJ, Egleton RD, Mitchell SA. et al. Enkephalin glycopeptide analogues produce analgesia with reduced dependence liability. J Med Chem. 2000;43(13):2586–2590. doi: 10.1021/jm000077y. [DOI] [PubMed] [Google Scholar]
- Varamini P, Toth I. Lipid- and sugar-modified endomorphins: novel targets for the treatment of neuropathic pain. Front Pharmacol. 2013;4:155. doi: 10.3389/fphar.2013.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecka A, Kruszynski R. Conformationally restricted peptides as tools in opioid receptor studies. Curr Med Chem. 2005;12(4):471–481. doi: 10.2174/0929867053362983. [DOI] [PubMed] [Google Scholar]
- Perlikowska R, Piekielna J, Fichna J. et al. Pharmacological properties of novel cyclic pentapeptides with micro-opioid receptor agonist activity. Med Chem. 2014;10(2):154–161. doi: 10.2174/157340641002140131161135. [DOI] [PubMed] [Google Scholar]
- Mollica A, Davis P, Ma SW. et al. Synthesis and biological activity of the first cyclic biphalin analogues. Bioorg Med Chem Lett. 2006;16(2):367–372. doi: 10.1016/j.bmcl.2005.09.080. [DOI] [PubMed] [Google Scholar]
- Bartosz-Bechowski H, Davis P, Zalewska T. et al. Cyclic enkephalin analogs with exceptional potency at peripheral delta opioid receptors. J Med Chem. 1994;37(1):146–150. doi: 10.1021/jm00027a018. [DOI] [PubMed] [Google Scholar]
- Remesic M, Lee YS, Hruby VJ. Cyclic Opioid Peptides. Curr Med Chem. 2016;23(13):1288–1303. doi: 10.2174/0929867323666160427123005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadina JE, Nilges MR, Morgenweck J. et al. Endomorphin analog analgesics with reduced abuse liability, respiratory depression, motor impairment, tolerance, and glial activation relative to morphine. Neuropharmacology. 2016;105:215–227. doi: 10.1016/j.neuropharm.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Cros CD, Toth I, Blanchfield JT. Lipophilic derivatives of leu-enkephalinamide: in vitro permeability, stability and in vivo nasal delivery. Bioorg Med Chem. 2011;19(4):1528–1534. doi: 10.1016/j.bmc.2010.12.042. [DOI] [PubMed] [Google Scholar]
- Pasha S, Gupta K. Various drug delivery approaches to the central nervous system. Expert Opin Drug Deliv. 2010;7(1):113–135. doi: 10.1517/17425240903405581. [DOI] [PubMed] [Google Scholar]
- Popov M, Abu Hammad I, Bachar T. et al. Delivery of analgesic peptides to the brain by nano-sized bolaamphiphilic vesicles made of monolayer membranes. Eur J Pharm Biopharm. 2013;85(3 Pt A):381–389. doi: 10.1016/j.ejpb.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Rip J, Gaillard PJ. et al. Enhanced brain delivery of the opioid peptide DAMGO in glutathione pegylated liposomes: a microdialysis study. Mol Pharm. 2013;10(5):1533–1541. doi: 10.1021/mp300272a. [DOI] [PubMed] [Google Scholar]
- Rip J, Chen L, Hartman R. et al. Glutathione PEGylated liposomes: pharmacokinetics and delivery of cargo across the blood-brain barrier in rats. J Drug Target. 2014;22(5):460–467. doi: 10.3109/1061186X.2014.888070. [DOI] [PMC free article] [PubMed] [Google Scholar]