Abstract
The orange carotenoid protein (OCP) and fluorescence recovery protein (FRP) are present in many cyanobacteria, and regulate an essential photoprotection cycle in an antagonistic manner as a function of light intensity. We characterized the oligomerization states of OCP and FRP by using native mass spectrometry, a technique that has the capability of studying native proteins under a wide range of protein concentrations and molecular masses. We found that dimeric FRP (dFRP) is the predominant state at protein concentrations ranging from 3 μM to 180 μM, and that higher order oligomers gradually form at protein concentrations above this range. The OCP, however, demonstrates significantly different oligomerization behavior. Monomeric OCP (mOCP) dominates at low protein concentrations, with an observable population of dimeric OCP (dOCP). The ratio of dOCP to mOCP, however, increases proportionally with the protein concentration. Higher order OCP oligomers form at protein concentrations beyond 10 μM. Additionally, native mass spectrometry coupled with ion mobility allowed us to measure protein collisional cross sections (CCS) and interrogate the unfolding of different FRP and OCP oligomers. We found that monomeric FRP exhibits a one-stage unfolding process, which could be correlated with its C-terminal bent crystal structure. The structural domain compositions of FRP and OCP are compared and discussed.
TOC image
Introduction
Protein oligomerization pertains to 35% or more of the proteins in a cell.1 This behavior is advantageous for protein evolution because it opens opportunities for new function and control. The strength and duration of association depend on the nature of the protein and various experimental variables (e.g., T, pH, and concentration).2 Oligomerization also occurs for those photosynthetic proteins that are involved in solar energy capture and storage. An example is chlorophyll a-chlorophyll c2-peridinin-protein (apcPC), one of the major light harvesting complexes in the dinoflagellate Symbiodinium. Size exclusion chromatography (SEC), blue native gel electrophoresis (BN-PAGE), and native mass spectrometry (MS) all demonstrate that it exists as a trimer.3 Nondenaturing electrophoresis of light-harvesting complex 2 from a purple bacterium revealed the presence of dimers, trimers, and even supercomplexes.4 These examples provide valuable insights into the higher-order assemblies of and interactions among the different components of a photosynthetic apparatus. The oligomerization states of many photosynthetic proteins, whether they be monomers, oligomers, or co-exist as multi-oligomeric states, however, still remain unclear.
Photosynthesis starts with light-energy absorption by pigment-protein antenna complexes and continues with energy transfer to reaction centers for photochemistry. The regulation of energy transfer is crucial for sustainable photosynthesis because excess energy, if not properly dissipated, may damage the photosynthetic machinery and lead to photo inhibition and cell death.5
In many cyanobacteria, the excess excitation energy is absorbed by phycobilisome (PBSs) antenna complexes and dissipated through a nonradiative pathway. This pathway starts from the absorption of blue light by the orange carotenoid protein (OCP) to induce a color and conformational change of the OCP from an orange (OCPO) to a red form (OCPR).6 The OCPR is competent for PBS binding, forming a PBS-OCPR quenching complex. Quenching is terminated by the action of a fluorescence recovery protein (FRP), which facilitates the conversion of OCPR back to OCPO, thereby expelling OCP from the PBS. The OCP is the first photoactive protein identified to use a carotenoid as the photo-responsive chromophore.7 The crystal structure of OCP reveals an antiparallel homodimer.8,9 Each monomer is composed of two domains that encompass a keto-carotenoid: an α-helical N-terminal domain (NTD) and an α helix/β sheet C-terminal domain (CTD). Although dimerization of OCP may be an artifact of crystallization,9 native MS, which does not require protein crystallization, reveals the existence of both dimeric and monomeric OCP.10
The oligomerization of FRP, like that of OCP, is also not well understood. Most of the FRP sequences in cyanobacterial strains contain 106 to 111 amino acids. In Synechocystis sp. PCC 6803, the first Met (GTG) encoded by the slr1964 gene can be considered as the first Met of the FRP and the fourth Met coincides with the first Met of most of the homologs. Using a series of Synechocystis mutants, Gwizdala et al. compared “shorter” and “longer” versions of FRP.11 The results suggest that the longer version FRP (beginning at Met1) synthesized in Synechocystis is less active than the shorter version, and that the starting Met for the shorter version is Met26.11 Remarkably, FRP exists in two different oligomeric states in the same crystal unit: dimeric and tetrameric, and the dimer is in two different conformations. Based on co-immunoprecipitation and docking simulation results, one dimeric FRP (dFRP) appears to be the active form whereas the tetrameric form may be inactive.12
Both OCP and FRP are soluble proteins, and their oligomeric states remain an open question. Here we report the use of native MS to probe the oligomeric state of FRP and OCP in solution and to investigate further how concentration affects the oligomeric states of those proteins. Native MS is emerging for partial characterization of proteins, especially large protein assemblies that are recalcitrant to crystallization.13,14 Although native MS does not provide resolution at the atomic level, its broad mass range and ability to analyze multiple species simultaneously make it a powerful tool for interrogating protein and cofactor stoichiometries, protein topologies, and ligand-protein interactions.15
One classical example is the characterization of the 4-oxalocrotonate tautomerase protein, which was originally reported to be a pentamer.16 A hexamer, however, was later observed with X-ray crystallography,17 consistent with native MS18. Determination of protein oligomeric states by native MS, however, is challenging to interpret when oligomers dissociate during desolvation or form as non-specific adducts in the spray.19 The former possibility can be avoided by carefully optimizing sample cone and collisional voltages to maintain the complex intact. For the latter, protein concentration seems to be an important factor. For example, native MS identified urease as an (αβ)12 assembly that readily disassembles into (αβ)3 subunits, supporting an ((αβ)3)4 architecture, in accord with the crystal structure, which revealed an (αβ)12 assembly. At higher concentrations, urease forms 24-, 36-, and even 48-mers in the gas phase, probably as non-specific adducts without any biological relevance.20 Insulin, as another example, not only forms well-defined oligomers in its native state, but also aggregates and gives amyloid fibrils.21 Native MS shows that association of insulin is concentration-dependent, and the relative proportions of the species detected at the different protein concentrations enable the estimation of association constants, affording a value that is close to that reported by Porker and Biswas.22
Additionally, native MS can be coupled with ion mobility (IM) to separate ions based on their size and shape and to provide collisional cross section (CCS) that can be compared with those from the crystal structure and from theory.23,24 Increasing the collisional voltage causes protein ion unfolding as seen by an increase in the CCS; thus, native MS and IM can provide insights in conformational dynamics of protein higher order structures.25,26 Our goal in this work is to utilize native MS, IM, and collision-induced unfolding to probe the oligomerization states of native FRP and OCP.
Materials and Methods
Expression of FRP in E. coli
The full-length version of FRP (Slr1964) without its stop codon was amplified by using the primers (FRPndeIF and FRPEcoRIR, Table S1) from genomic DNA of Synechocystis sp. PCC 6803 and cloned into the pET21a vector. The insertion sites are NdeI and EcoRI as indicated by the primer names. The truncated (short) version of FRP starting from the second open reading frame (i.e., MLQTAEA) was generated by using primers (FRPSF and FRPSR, Table S1). Cell culture and protein induction were performed according to previously reported methods.12
FRP purification
E. coli cells were lysed by sonication in 20 mM Tris buffer (pH 7.5) buffer A supplemented with protease inhibitor cocktail and DNase (Sigma, St. Louis, MO) and 200 mM NaCl. The cell lysate was clarified by centrifugation at 25,000 × g, and the supernatant was loaded onto a HisTrap HP column (GE healthcare, Marlborough, MA). The His6-tagged FRP was eluted with buffer A containing 300 mM imidazole and further purified by gel filtration chromatography by using a HiPrep Sephacryl S-200 HR (GE healthcare, Marlborough, MA) column and an isocratic flow of buffer A. The purity of FRP was confirmed by SDS–PAGE by using a precast gradient gel (Any kD™ Mini-PROTEAN, Bio-Rad, CA). The concentration of FRP was determined by 280 nm absorption on a NanoDrop spectrophotometer (Thermo Scientific, MA, USA).
OCP purification
OCP was isolated from Synechocystis sp. PCC 6803 by using the procedure of Zhang, et al.10
Native MS and IM-MS Analysis of FRP
The purified FRP sample was washed with 400 mM ammonium acetate (pH 8.0) in a 10 kDa molecular weight cut off filter (Vivspin, Goettingen, Germany). The original buffer and salts were removed after 10 cycles of washing. The FRP sample was introduced into the ESI source of a Waters Synapt G2 mass ESI Q-TOF (Electrospray ionization-quadrupole time of flight, Waters Corporation, Milford, MA) mass spectrometer by using commercial borosilicate emitters with extra coating (ES387, Hudson, New Hampshire, Thermo Scientific). To investigate the effects of concentration on the oligomeric state of FRP, the concentrations of the introduced FRP were varied. The backing pressure was adjusted to 5 mBar for transferring large protein ions. The sample cone voltage was 20 V. The collisional energy for the trap region was manipulated to observe the dissociation of FRP. For IM experiments, the gas flow rate was 35 mL/min, the ion mobility separation (IMS) wave height was 20 V, and the IMS wave velocity was 500 m/s. The data were output from MassLynx (Waters Corporation, Milford, MA) and plotted by Origin (OriginLab Corporation, Northampton, MA). The IM experiment was calibrated with protein standards (ubiquitin and myoglobin) by using published protocols. The drift-time information from native MS ion-mobility experiments was converted into CCS by considering the molecular weight and charge state of the protein assemblies.27, 28
Native MS of OCP
OCP was analyzed on the same instrument with the same parameters as mentioned above. The samples were washed with 400 mM ammonium acetate solution (pH 8.0) and analyzed at a series of different concentrations. To be consistent with FRP analysis, the pH value of the buffer was adjusted to be 8.0 instead of 6.8 as our previous study.10 Because the dimer to monomer ratio can vary slightly depending on the instrument conditions, this concentration analysis was conducted in rapid succession.
Results and discussion
Oligomeric state of FRP
To probe the oligomeric state of FRP, we performed a native MS experiment by using one stock FRP sample diluted in series to concentrations of 3, 5, 10, 40, and 180 μM. (Figure 1). Considering the estimated pI (isoelectric point) of FRP is approximately 6.49 from amino acid component analysis 29, we adjusted the pH of NH4Ac buffer to 8.0 to avoid precipitation.
Fig. 1.
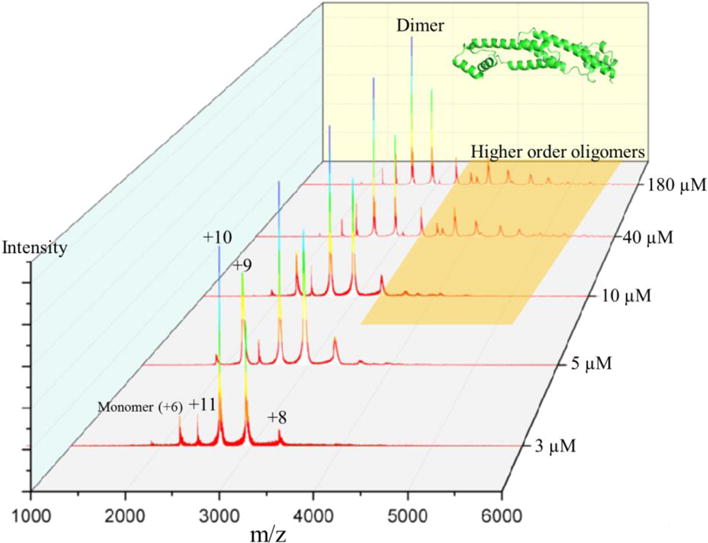
Native mass spectrum of FRP diluted in series to concentrations of 180, 40, 10, 5, and μM.
The underlying premise of native MS is that ionization of protein complexes from aqueous solution preserves the native structure, at least for the time scale of MS. The sample in the capillary is held at high electric potentials, and droplets containing high charge are drawn out to form the “Taylor cone”.30 The droplet size is reduced by a potential gradient, aided by collisions with a nebulizing gas, until the “Rayleigh limit” is reached.31 When the protein concentration is high, a significant number of droplets will contain more than one protein or protein complex.14 Adopting low concentrations of proteins for native MS analysis avoids the formation of non-specific adducts. A calculation, based on “18 nm” average droplet size30, shows that most droplets are empty and a few contain one protein when the protein concentration is 50 μM.14 In this study, the lowest concentration we used is 3 μM, as lower concentrations lead to a low signal-to-noise spectrum (data not shown) and a less confident interpretation. A distribution of dFRP carrying different charges was identified at all concentrations. (m/z = 2199.65 at +12, m/z = 2399.74 at +11, m/z =2639.57 at +10, m/z = 2932.73 at +9 and m/z = 3299.0881 at +8 (experimental MW = 26,385.23 Da). The peak representing the +12 charge state is slightly higher, however, than the +11 charge state of dimeric FRP at 3 μM, inconsistent with a bell-shaped distribution of charge states. This suggests the presence of small amounts of monomeric FRP (Figure 1) because the peaks representing dFRP carrying +12 charge and monomeric FRP (mFRP) carrying +6 charge overlap. This trace amount of monomer is likely generated during desolvation and transmission to the mass spectrometer, as we could observe no prominent peaks representing mFRP when we decreased the protein concentration. Furthermore, peaks representing mFRP increased when we increased the collisional voltage, confirming that the small amount of mFRP can be generated in the transmission process (Figure 2).
Fig. 2.
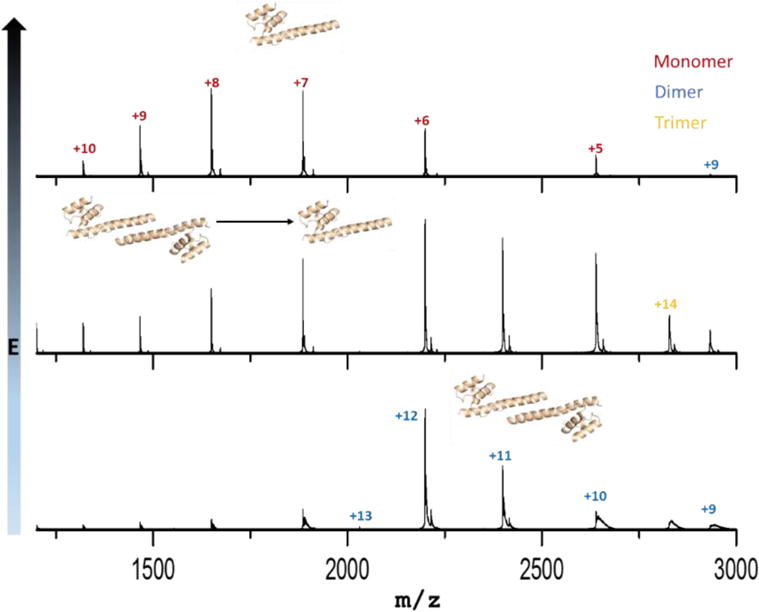
Collisional-induced dissociation of dimeric FRP. Trap collisional voltage were set at 18 V, 38 V and 58 V from bottom to top.
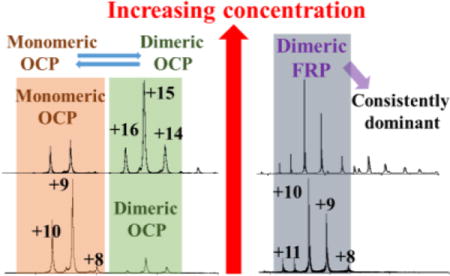
We found that dFRP is consistently the dominant state in native MS at all protein concentrations, and the charge states appear as a bell-shaped curve distribution. Trimeric and tetrameric FRP forms gradually appeared in the spectrum when the concentration increased. Furthermore, higher order oligomers—up to octamers—form at 180 μM (Figure 3). Aside from the peaks representing mFRP and dFRP, those for the higher order protein oligomers decease in relative abundance as the oligomer increases in size; tetrameric FRP is no exception. In other words, no special behavior was observed for tetrameric FRP. Although X-ray crystallography favors the tetramer12, that species is unlikely to be of any biological relevance. Instead, the tetramer is concentration-driven and forms more readily at high concentration.
Fig. 3.
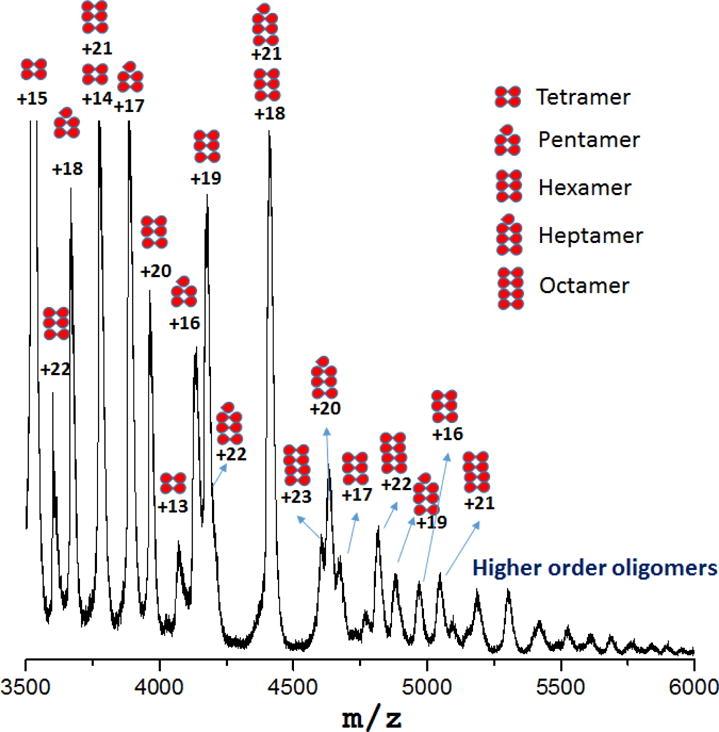
Tetrameric, pentameric, hexameric, heptameric and octameric FRP detected by native MS at a protein concentration of 180 μM.
The MW of the protein complex in the native state, from a calibration curve of SEC, shows FRP to exist as a trimer32; however, this was later revised to a dimer12. The discrepancies may be due to the proteins (calibrants or the sample) existing in various conformations, as protein shapes do not necessarily correlation linearly with MW.33 The FRP crystal structure showed that two conformations exist as dimer and one as a tetramer.12
Oligomeric states of OCP
Although a dimer is revealed by crystallography, OCP elutes as a monomer by SEC in both its active (red) and inactive (orange) states9,34, suggesting that the dimerization of OCP may not be biologically relevant. Thermodynamic calculations of binding energy suggest that it is not a stable assembly and only 3 of the 22 amino acids making up the dimerization interface are absolutely conserved.9 Because determining protein oligomeric states under native conditions can be challenging especially in vitro, and biases can be introduced by different techniques (e.g., band broadening during SEC), we used native MS to compare the oligomeric state of OCP at various protein concentrations to that of FRP, as a reference point and as a follow-up to our previous report10. We found bell-shaped distributions of mOCP (+9, +10 and +11, keto-cartenoid included) and dOCP (+14, +15 and +16, keto-cartenoid included), in agreement with our previous report.10 Moreover, the relative abundance of dOCP to mOCP increased proportionally to the concentration, as shown in Figure 4. This result suggests that the dimerization of OCP (also revealed by crystallography) could be the consequence of the high concentrations of protein sample required for both protein crystallography8,34 and for some native mass spectrometry experiments35.
Fig. 4.
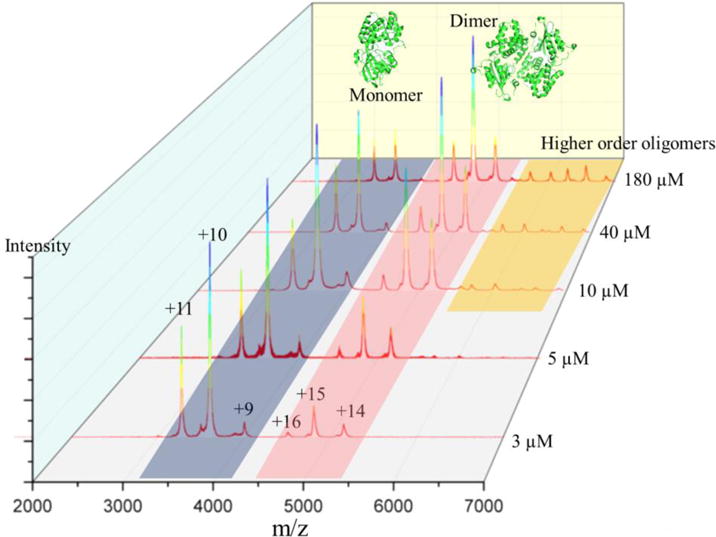
Native mass spectrum of OCP diluted to concentrations of 180, 40, 10, 5, and 3 μM. Structure of monomeric and dimeric OCP are shown as PDB 1M98.
Thus, OCP has a high tendency to dimerize, depending on the protein concentration in vitro, and perhaps in vivo. Of note is that OCP can form higher order oligomers just as FRP (Figure 5). In our previous report, we found the dimer-to-monomer ratio is different for the red and orange states of the protein.10 Whether the functional form of OCP is a monomer or a dimer still remains unclear. In our first report in which we used native MS, we could detect a monomer-to-dimer transition. How this phenomenon is related to physiological function, and how the dimer-monomer transition could benefit biosensor designs are questions for future studies.
Fig. 5.
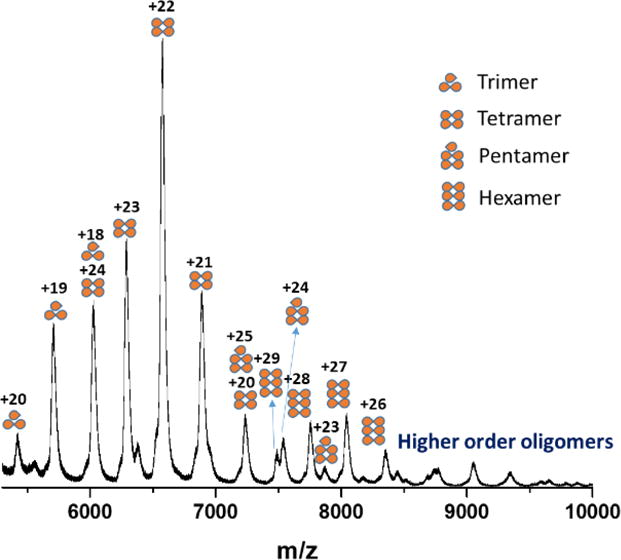
Trimeric, tetrameric, pentameric and hexameric OCP detected by native MS at 180 μM.
Ion mobility of FRP
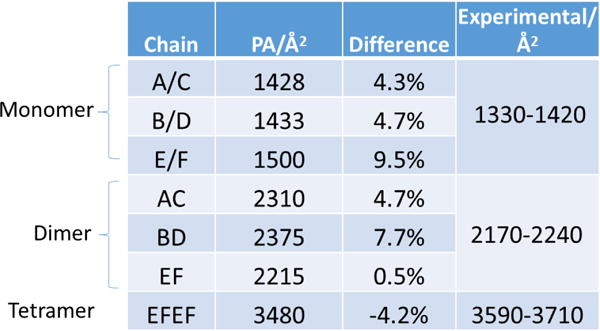
Unfolded or large protein complex ions undergo more collisions with the neutral gas in an IM tube and, therefore, exhibit a larger CCS than folded or small protein complex ions.36 To look for folded or unfolded conformers in the gas phase, we calibrated the ion mobility instrument with denatured protein standards according to previous reports (calibration curve is shown in supplementary Figure S1).27,28 We then calculated CCS values by a projection approximation (PA) method. This method calculates the averaged projected area, ignoring the scattering and long-range interactions between the neutral gas and the ions.37 Whereas the resulting CCS is usually underestimated by this method,38 an empirically scaled PA, proposed by Ruotolo and Robinson, can correct for this. Its accuracy in predicting protein CCS was previously verified.39, 40 The CCS values for FRP as determined by IM-MS in this study, and the scaled PA values of FRP (PDB 4JDX), along with the measured values, are listed in Table 1. The predicted CCS values agree generally with the measured values. It is worth noting that the calculated E/F chain (comprising the tetrameric FRP in PDB 4JDX) dimer is only 0.5% different from the measured one.
Table 1.
Theoretical (scaled PA) and experimental CCS values; the experimental CCS values are calculated from peak widths at 10% peak height.
|
Gas-phase unfolding of FRP under collisional activation
Although the measured CCS agrees well with the theoretical CCS, FRP clearly exhibits a positive linear relationship between charge state and CCS, especially for the monomer and dimer (see supplementary Fig. S2). When FRP carries an increasing number of charges, the protein partially unfolds, and this “enlarged CCS effect” is more obvious for mFRP and dFRP, as a relatively high percentage of domains are affected compared to higher order FRP oligomers. Multiple studies support a linear relationship between charge state and CCS41–43, which is in agreement with our results.
Robinson and co-workers using IM-MS44 reported experimental evidence of long-lived, unfolded non-covalent complexes. The intermediates in the dissociation pathway can be correlated with increases in CCS indicating that the protein complex undergoes both high-order structure refolding and monomer unfolding prior to dissociation. Thus, an unfolding heat map can provide information on the stability and flexibility of protein complex. Here we observed stable unfolding intermediates for all the oligomeric FRP samples in the gas phase of the mass spectrometer (Figure 6 and supplementary Fig. S2).
Fig. 6.
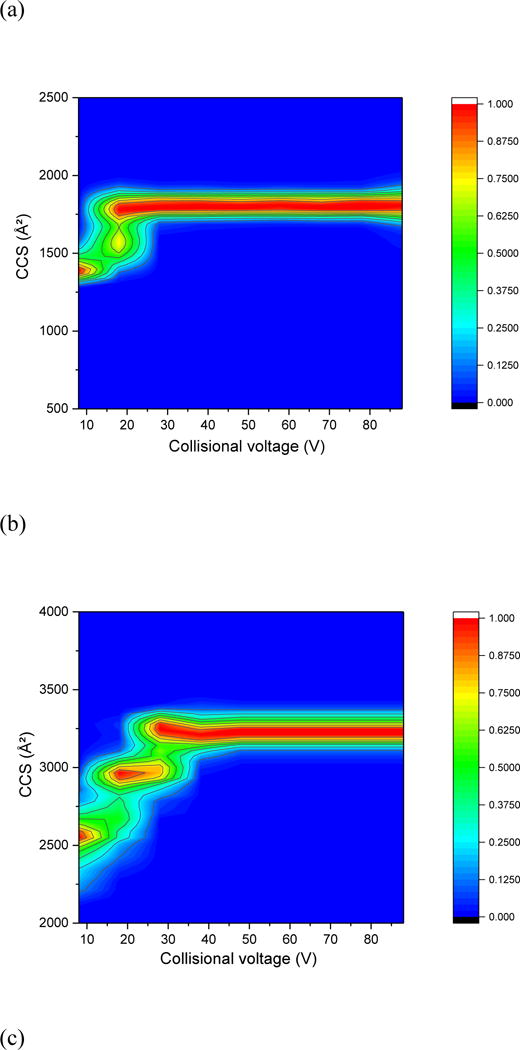

Collision-induced unfolding heat map of FRP (a) monomer at +4 charge state, (b) dimer at +11 charge state, (c) trimer at +13 charge state, (d) tetramer at +15 charge state.
Interestingly, monomeric FRP exhibits a roughly one-stage unfolding process, whereas the dimer undergoes a two-stage process, and the trimer and tetramer a three-stage unfolding process. Usually, the more complex the protein domains are, the more diversified the conformations it can assume before reaching a Coulombic repulsion limit. The one-step unfolding of mFRP, as shown in the unfolding heat map, might represent the movement of C-terminal region, which is “bent” toward an antiparallel alpha helix bundle. The two-step unfolding process of dFRP then reflects the unfolding of individual FRP C-terminal domains, while maintaining the interface between two subunits. The dramatic change of CCS for different charge states (See supplementary Fig. 2S) is as large as the intermediates observed during the increasing of collisional voltage (Figure 6) and suggests that dFRP retains highly flexibile and comprises multiple conformations during the unfolding process.
The unfolding heat maps of pentameric, hexameric, heptameric, and octameric FRP (Fig. S3) show that as the complexity of interacting domains is increased, fewer intermediate states exist. Specifically, heptameric and octameric FRP exhibit relatively rigid structures in the gas phase. Dimeric state FRP was proposed to be the functional state 12; thus, any form of higher order FRP in the native environment, if present in vivo, would require some structural flexibility to dissociate into the active dimer. Thus, these higher order FRP oligomers cannot back dissociate into dimeric FRP owing to the rigid structure. This reinforces our proposal that the higher order oligomers of FRP are artifacts formed at high protein concentrations.
Conclusions
Oligomerization, which is important in conformation, function, and stability of proteins, can be studied by native MS. Indeed, native MS illuminates the structure and oligomeric state of OCP and FRP, which are crucial participants in the cyanobacterial photoprotection cycle. Although FRP’s oligomeric state and active form remained largely enigmatic until a crystal structure was determined, up to three conformations exist in the crystal structure: two separate dimers and a tetramer. Likewise, the native state of OCP, whether monomeric or dimeric, has also been controversial.
Using native MS, we found FRP to be predominantly dimeric independent of the protein concentration, although a small fraction of higher order oligomers forms at higher protein concentration. OCP, in contrast, exists as both monomer and dimer, and the relative abundance of dOCP increases with increasing protein concentration. Moreover, an unfolding heat map determined by collisional activation and IM-MS shows a one-step unfolding process for mFRP, two-step for dFRP, and three-step for trimeric and tetrameric FRP. Higher order FRP oligomers have rather rigid structures, strongly suggesting they are of little relevance in vivo. Native MS offers new insights into the biological assemblies of FRP and OCP, especially for their oligomeric states, building a foundation for future structure-function analyses of this important photo protection mechanism.
Supplementary Material
Acknowledgments
Funding
This research was funded by the U.S. Department of Energy (DOE), office of Basic Energy Sciences, Photosynthetic Systems (PS) Program (Grant DE-FG02-07ER15902 to REB). Instrumentation was made available by the Photosynthetic Antenna Research Center (PARC), an Energy Frontier Research Center funded by the U.S. Department of Energy (DOE), office of Basic Energy Sciences (Grant DE-SC0001035). The research was also supported by the National Institute of General Medical Sciences (Grant 2P41GM103422 to MLG). YL was supported jointly by the PS and NIH grants, HL and JK were supported by the PS grant, and RS and HZ were supported by the PARC grant. All cell growth and sample preparation was supported by the PS grant.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Calibration curve CCS vs. New Td., CCS of FRP at different charge states, heat map of FRP pentamer, hexamer, heptamer and octamer; primers used in the construction of full length and truncated version of FRP in pET21a.
References
- 1.Goodsell DS, Olson AJ. Structural Symmetry and Protein Function. Annual Review of Biophysics and Biomolecular Structure. Annu Rev Biophys Biomol Struct. 2000;29(1):105–153. doi: 10.1146/annurev.biophys.29.1.105. [DOI] [PubMed] [Google Scholar]
- 2.Ali MH, Imperiali B. Protein oligomerization: How and why. Bioorg Med Chem. 2005;13(17):5013–5020. doi: 10.1016/j.bmc.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 3.Jiang J, Zhang H, Orf GS, Lu Yb, Xu W, Lucas B, Harrington LB, Liu H, Lo CS, Blankenship RE. Evidence of functional trimeric chlorophyll a/c2-peridinin proteins in the dinoflagellate Symbiodinium. BBA-Bioenergetics. 2014;1837:1904–1912. doi: 10.1016/j.bbabio.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 4.D’Amici GM, Rinalducci S, Murgiano L, Italiano F, Zolla L. Oligomeric Characterization of the Photosynthetic Apparatus of Rhodobacter sphaeroides R26.1 by Nondenaturing Electrophoresis Methods. J Proteome Res. 2010;9:192–203. doi: 10.1021/pr9005052. [DOI] [PubMed] [Google Scholar]
- 5.Nishiyama Y, Allakhverdiev SI, Murata N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. BBA-Bioenergetics. 2006;1757:742–749. doi: 10.1016/j.bbabio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Wilson A, Punginelli C, Gall A, Bonetti C, Alexandre M, Routaboul J, Kerfeld CA, Grondelle RV, Robert B, Kennis JMK, Kirilovsky D. A photoactive carotenoid protein acting as light intensity sensor. Proc Natl Acad Sci U S A. 2008;105:12075–12080. doi: 10.1073/pnas.0804636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerfeld CA, Kirilovsky D. Structural, mechanistic and genomic insights into OCP-mediated photoprotection. Adv Bot Res. 2013;65:1–26. [Google Scholar]
- 8.Kerfeld CA, Sawaya MR, Brahmandam V, Cascio D, Ho KK, Trevithick-Sutton CC, Krogmann DW, Yeates TO. The Crystal Structure of a Cyanobacterial Water-Soluble Carotenoid Binding Protein. Structure. 2003;11:55–65. doi: 10.1016/s0969-2126(02)00936-x. [DOI] [PubMed] [Google Scholar]
- 9.Wilson A, Kinney JN, Zwart PH, Punginelli C, D’Haene S, Perreau F, Klein MG, Kirilovsky D, Kerfeld CA. Structural Determinants Underlying Photoprotection in the Photoactive Orange Carotenoid Protein of Cyanobacteria. J Biol Chem. 2010;285:18364–18375. doi: 10.1074/jbc.M110.115709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Liu H, Niedzwiedzki DM, Prado M, Jiang J, Gross ML, Blankenship RE. Molecular Mechanism of Photoactivation and Structural Location of the Cyanobacterial Orange Carotenoid Protein. Biochemistry. 2014;53:13–19. doi: 10.1021/bi401539w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gwizdala M, Wilson A, Omairi-Nasser A, Kirilovsky D. Characterization of the Synechocystis PCC 6803 Fluorescence Recovery Protein involved in photoprotection. BBA – Bioenergetics. 2013;1827:348–354. doi: 10.1016/j.bbabio.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Sutter M, et al. Crystal structure of the FRP and identification of the active site for modulation of OCP-mediated photoprotection in cyanobacteria. Proc Natl Acad Sci U S A. 2013;110:10022–10027. doi: 10.1073/pnas.1303673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Cui W, Gross ML, Blankenship RB. Native mass spectrometry of photosynthetic pigment–protein complexes. FEBS Letters. 2013;587(8):1012–1020. doi: 10.1016/j.febslet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benesch JL, Ruotolo BT, Simmons DA, Robinson CV. Protein Complexes in the Gas Phase: Technology for Structural Genomics and Proteomics. Chem Rev. 2007;107:3544–3567. doi: 10.1021/cr068289b. [DOI] [PubMed] [Google Scholar]
- 15.Marcoux J, Robinson CV. Twenty Years of Gas Phase Structural Biology. Structure. 2013;21:1541–1550. doi: 10.1016/j.str.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Chen LH, Kenyon GL, Curtin F, Harayama S, Bembenek ME, Hajipour G, Whitman CP. 4-Oxalocrotonate tautomerase, an enzyme composed of 62 amino acid residues per monomer. J Biol Chem. 1992;267:17716–17721. [PubMed] [Google Scholar]
- 17.Roper DI, Subramanya HS, Shingler V, Wigley DB. Preliminary crystallographic analysis of 4-oxalocrotonate tautomerase reveals the oligomeric structure of the enzyme. J Mol Biol. 1994;243:799–801. doi: 10.1016/0022-2836(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald MC, Chernushevich I, Standing KG, Whitman CP, Kent SB. Probing the Oligomeric Structure of an Enzyme by Electrospray Ionization Time-Of-Flight Mass Spectrometry. Proc Natl Acad Sci U S A. 1996;93:6851–6856. doi: 10.1073/pnas.93.14.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith RD, Light-Wahl KJ. The observation of non-covalent interactions in solution by electrospray ionization mass spectrometry: Promise, pitfalls and prognosis. Biol Mass Spectrom. 1993;22:493–501. [Google Scholar]
- 20.Heck AJR, van den Heuvel RH. Investigation of intact protein complexes by mass spectrometry. Mass Spectrom Rev. 2004;23:368–389. doi: 10.1002/mas.10081. [DOI] [PubMed] [Google Scholar]
- 21.Brange J, Andersen L, Laursen ED, Meyn G. Rasmussen, E. Toward Understanding Insulin Fibrillation. J Pharm Sci. 1997;86:517–525. doi: 10.1021/js960297s. [DOI] [PubMed] [Google Scholar]
- 22.Nettleton EJ, Tito P, Sunde M, Bouchard M, Dobson CM, Robinson CV. Characterization of the oligomeric states of insulin in self-assembly and amyloid fibril formation by mass spectrometry. Biophys J. 2000;79:1053–1065. doi: 10.1016/S0006-3495(00)76359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scarff CA, Thalassinos K, Hilton GR, Scrivens JH. Travelling wave ion mobility mass spectrometry studies of protein structure: biological significance and comparison with X-ray crystallography and nuclear magnetic resonance spectroscopy measurements. Rapid Commun Mass Spectrom. 2008;22:3297–3304. doi: 10.1002/rcm.3737. [DOI] [PubMed] [Google Scholar]
- 24.Wang SC, Argyris P, Bartolo ND, Bavro VN, Tucker SJ, Booth PJ, Barrera NP, Robinson CV. Ion Mobility Mass Spectrometry of Two Tetrameric Membrane Protein Complexes Reveals Compact Structures and Differences in Stability and Packing. J Am Chem Soc. 2010;132:15468–15470. doi: 10.1021/ja104312e. [DOI] [PubMed] [Google Scholar]
- 25.Lanucara F, Holman SW, Gray CJ, Eyers CE. The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nat Chem. 2014;6:281–294. doi: 10.1038/nchem.1889. [DOI] [PubMed] [Google Scholar]
- 26.Hall Z, Politis A, Robinson CV. Structural Modeling of Heteromeric Protein Complexes from Disassembly Pathways and Ion Mobility-Mass Spectrometry. Structure. 2012;20:1596–1609. doi: 10.1016/j.str.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Ruotolo BT, Benesch JP, Sandercock AM, Suk-Joon Hyung S, Robinson CV. Ion mobility-mass spectrometry analysis of large protein complexes. Nat Protocols. 2008;3:1139–1152. doi: 10.1038/nprot.2008.78. [DOI] [PubMed] [Google Scholar]
- 28.Michaelevski I, Kirshenbaum N, Sharon M. T-wave ion mobility-mass spectrometry: basic experimental procedures for protein complex analysis. J Vis Exp. 2010;41 doi: 10.3791/1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kebarle P, Tang L. From ions in solution to ions in the gas phase – the mechanism of electrospray mass spectrometry. Analytical Chemistry. Anal Chem. 1993;65:972A–986A. [Google Scholar]
- 31.Rayleigh L. XX. On the equilibrium of liquid conducting masses charged with electricity. Philos Mag. 1882;14:184–186. [Google Scholar]
- 32.Boulay C, Wilson A, D’Haene S, Kirilovsky D. Identification of a protein required for recovery of full antenna capacity in OCP-related photoprotective mechanism in cyanobacteria. Proc Natl Acad Sci U S A. 2010;107:11620–11625. doi: 10.1073/pnas.1002912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong P, Koza S, Bouvier ES. A review size-exclusion chromatography for the analysis of protein biotherapeutics and their aggregates. J liq Chromatogr Relat Technol. 2012;35:2923–2950. doi: 10.1080/10826076.2012.743724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S, Guttman M, Leverenz RL, Zhumadilova K, Pawlowski EG, Petzold CJ, Lee KK, Ralston CY, Kerfeld CA. Local and global structural drivers for the photoactivation of the orange carotenoid protein. Proc Natl Acad Sci U S A. 2015;112:5567–5574. doi: 10.1073/pnas.1512240112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Liu H, Niedzwiedzki DM, Prado M, Jiang J, Gross ML, Blankenship RE. Molecular mechanism of photoactivation and structural location of the cyanobacterial orange carotenoid protein. Biochemistry. 2014;53:13–19. doi: 10.1021/bi401539w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mason EA, McDaniel W. Transport properties of ions in gases. Wiley; 1988. [Google Scholar]
- 37.Mack E. Average cross-sectional areas of molecules by gaseous diffusion methods. J Am Chem Soc. 1925;47:2468–2482. [Google Scholar]
- 38.Shvartsburg AA, Jarrold MF. An exact hard-spheres scattering model for the mobilities of polyatomic ions. Chem Phys Lett. 1996;261:86–91. [Google Scholar]
- 39.Hall Z, Politis A, Bush MF, Smith LJ, Robinson CV. Charge-State Dependent Compaction and Dissociation of Protein Complexes: Insights from Ion Mobility and Molecular Dynamics. J Am Chem Soc. 2012;134:3429–3438. doi: 10.1021/ja2096859. [DOI] [PubMed] [Google Scholar]
- 40.Benesch JLP, Ruotolo BT. Mass spectrometry: come of age for structural and dynamical biology. Curr Opin Struct Biol. 2011;21:641–649. doi: 10.1016/j.sbi.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valentine SJ, Clemmer DE. Temperature-dependent H/D exchange of compact and elongated cytochrome c ions in the gas phase. J Am Mass Spectrom. 2002;13:506–517. doi: 10.1016/S1044-0305(02)00372-0. [DOI] [PubMed] [Google Scholar]
- 42.Clemmer DE, Hudgins RR, Jarrold MF. Naked Protein Conformations: Cytochrome c in the Gas Phase. J Am Chem Soc. 1995;117:10141–10142. [Google Scholar]
- 43.Hall Z, Robinson CV. Do Charge State Signatures Guarantee Protein Conformations? J Am Mass Spectrom. 2012;23:1161–1168. doi: 10.1007/s13361-012-0393-z. [DOI] [PubMed] [Google Scholar]
- 44.Ruotolo BT, Hyung SJ, Robinson PM, Giles K, Bateman RH, Robinson CV. Ion Mobility–Mass Spectrometry Reveals Long-Lived, Unfolded Intermediates in the Dissociation of Protein Complexes. Angew Chem Int Ed. 2007;46:8001–8004. doi: 10.1002/anie.200702161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


