Abstract
A general theory of mammalian sexual differentiation is proposed. All biological sex differences are the result of the inequality in effects of the sex chromosomes, which are the only factors that differ in XX vs. XY zygotes. This inequality leads to male-specific effects of the Y chromosome, including expression of the testis-determining gene Sry that causes differentiation of testes. Thus, Sry sets up lifelong sex differences in effects of gonadal hormones. Y genes also act outside of the gonads to cause male-specific effects. Differences in the number of X chromosomes between XX and XY cells causes sex differences in expression (1) of Xist, (2) of X genes that escape inactivation, and (2) of parentally imprinted X genes. Sex differences in phenotype are ultimately the result of multiple, independent sex-biasing factors, hormonal and sex chromosomal. These factors act in parallel and in combination to induce sex differences. They can also can offset each other to reduce sex differences. Other mechanisms, operating at the level of populations, cause groups of males to differ on average from groups of females. The theory has advantages for directing attention to inherent sex-biasing factors that operate in many tissues to cause sex differences, to cause sex-biased protection from disease, and to frame questions for further study.
Keywords: testosterone, estradiol, X chromosome, Y chromosome
Introduction: History and Definitions
The study of sexual differentiation has undergone dramatic changes in the last half century. Before 1980, investigators in this field had predominantly studied the most obvious phenotypic sex differences, in the gonads, external and internal genitalia, and behavior (Arnold, 2002). These investigators viewed themselves largely as reproductive biologists and psychologists, because of the function of the tissues or behaviors they studied. Earlier in the 20th century, investigators had asked the fundamental question whether phenotypic sex differences were dictated by the sex chromosomes or by gonadal secretions (Allen, 1932; Young, 1961). For the birds and mammals, the answer was that sexual development outside the gonads was controlled by gonadal hormones. Experiments showed that changing the gonadal hormones could profoundly change the sexual phenotype of reproductive tissues other than the gonads. For example, it was possible to give male hormones to genetic (XX) females to make the genitals or behavior similar to that of a male, or to take male hormones away from genetic (XY) males to make their genitals and behavior like that of females (Jost, 1947). By the 1930s, the central idea of 20th Century sexual differentiation theory was accepted, even though the most definitive experiments were done later, for example by Jost (Jost et al., 1973) and Phoenix et al. (Phoenix et al., 1959). This central idea was articulated by Lillie (Lillie, 1939), who concluded that in “higher” vertebrates such as mammals, genetic sex determination occurs first -- sex chromosome complement determines the sex of the gonads (Ford et al., 1959). Once the gonads differentiate as testes or ovaries, however, their hormonal secretions determine the sexual phenotype of the rest of the body and behavior (sexual differentiation). This simple dichotomization of the sexual differentiation process (genetic sex determination followed by hormonal sexual differentiation of non-gonadal tissues) is no longer tenable based on recent experimental results (Arnold, 2011; McCarthy and Arnold, 2011), but persists in the literature on sex determination. In the theory summarized here, all biological sex differences in gonadal and non-gonadal tissues are seen as downstream from the inherent sexual inequality in the sex chromosomes (Arnold, 2011).
Several developments have contributed to a revision of the old dogma. One is that the revolution in molecular genetics has given us a much better understanding of the genes on the sex chromosomes, their evolution, and function (Deng et al., 2014; Graves, 2006; Lahn and Page, 1997; Skaletsky et al., 2003). This new knowledge shows that the inherent inequality of X and Y genetic material in the two sexes has effects throughout the body, not just on the gonads. A second major influence has been that various experimental findings have uncovered cases in which the old theory was inadequate. These include studies of tamar wallabies, in which some non-gonadal sexual tissues develop differently in the two sexes before the gonads differentiate (Renfree and Short, 1988). Their sexual differentiation cannot be caused by sex differences in gonadal secretions. In studies of songbirds, various examples were discovered in which the sexual phenotype of non-gonadal tissues, including the brain, did not correlate with the type of gonads, but did correlate with the type of sex chromosomes (Agate et al., 2003; Wade and Arnold, 1996). The studies in songbirds, at least in our own lab, catalyzed a shift to study of mice in which the complement and number of sex chromosomes could be manipulated without changing the type of gonad (Burgoyne et al., 1995; De Vries et al., 2002). Extensive studies of mice leave no doubt that the complement of sex chromosomes has direct effects outside the gonads, including on the brain, to cause sex differences (Arnold et al., 2016; Arnold and Chen, 2009).
At the same time as these developments, the study of sex differences was expanding beyond tissues related to reproduction. Especially since 1990, there has been increasing realization that sex differences occur throughout the body. Tissues not specialized for reproduction, including non-reproductive areas of the brain, function differently in females and males, and are differentially affected by disease in the two sexes (US National Institute of Medicine Committee on Understanding the Biology of Sex and Gender Disorders, 2001). In some cases, sex differences in disease can be dramatic, as in systemic lupus erythematosus (SLE), which occurs nine times more often in women than men, or autism spectrum disorder which occurs 2–4 times more often in boys and men than in females. When striking sex differences occur, any fundamental understanding of the disease requires understanding of the causes and consequences of sex differences the disease. Put simply, one cannot understand SLE without understanding sex differences in SLE. To explain sex differences in disease, one turns to the theory of sexual differentiation, which enumerates and classifies inherent factors that differ in the two sexes, suggesting experiments that can be performed to uncover the origins for any sex difference. Moreover, a sex difference in disease means that one sex is protected from the disease more than the other. This fact provides a rationale for discovering the sex-biasing factors that are protective or harmful, as part of a strategy for discovery of novel protective factors that might be targets of therapy. If testosterone protects from a disease, for example, then understanding the downstream genes regulated by testosterone might point to previously unknown gene pathways that are protective and could be drug targets. This information is potentially useful for both sexes.
In mammals and birds, sex differences originate in the genome, at the time of conception. However, beginning at birth, the human infant is placed into a highly gendered social and physical environment. Boys and girls are expected and required to behave differently. They choose different occupations and life paths, on average, with differences in physical and emotional stress, diet, and much more (Kishi et al., 2002; Lippa, 2005). The large sex differences in environment no doubt contribute to sex differences in function of the brain, and in incidence and progression of diseases. For example, occupations chosen more often by one sex may create specific types of stress that make that sex more susceptible to certain maladies. Moreover, it is likely that different environments experienced by females and males interact with the biological sex differences in individuals. The social and biological factors can augment each other (e.g., both favoring disease in one sex) or cancel each other out (e.g., when the disease is reduced in one sex by biological factors but increased by social factors). Specific environments may cancel out sex-biasing effects of gonadal hormones more in some brain regions than in others, reducing sex differences in a brain region-specific manner (Joel et al., 2015). At this point in history, however, this is as much a reasoned statement of belief as a well-documented phenomenon. It is possible to perform studies of humans that show both the effects of biological factors and social / environmental factors, but it is often difficult or impossible to decide, especially for neural and behavioral phenotypes, the relative importance of the two factors. One reason is that biological factors typical of one sex co-vary with social factors typical of that sex, so that it can be impossible to gauge their relative importance or even independent effects on a trait. Another reason is that biological and environmental factors change each other. A knock out of the androgen receptor in XY individuals (CAIS, Complete Androgen Insensitivity Syndrome in humans) alters the body so that it looks completely like that of a female, proving that the masculine structure of many reproductive tissues requires androgen action in males. However, because the XY CAIS girl is reared as a female, one cannot easily separate the biologically and socially mediated effects of the mutation on many attributes that one might measure in CAIS women, for example their brain function or susceptibility to disease (e.g., (Hamann et al., 2014). In addition, differences in social or physical environments are expected to have lasting effects on the epigenome (DNA methylation and modifications of histones), so that the environment alters the read-out of the genome (Szyf et al., 2008). One way or another, the effects of the environment on an individual are mediated by changes in the person’s biology, making it difficult to disentangle the two sources of variation. Much of the argument, about whether social or biological factors cause sex differences in physiology and disease, may be based as much on which factors a specific author finds to be interesting or preferable, rather than on any evidence that effects of one factor can be dissociated from effects of others and found to be more important. The environmentalist and biologist are both susceptible to the mistake of overgeneralizing the importance of factors that they are trained to study or prefer. The theory of sexual differentiation, presented below, focuses exclusively on the biological factors that make females and males different from each other. This focus comes with the acknowledgement that sex-biasing factors are also found in the social and physical environments.
“Sexual differentiation” is a phrase that has had two main connotations, which have caused some confusion. Differentiation, a concept of developmental biology, suggests a change in cells and tissues during ontogeny. Cells lose pluripotency and commit irreversibly to a differentiated fate. Based on this connotation, “sexual differentiation” has sometimes referred to the irreversible processes that commit male and female tissues to different adult phenotypes. A slightly different connotation stems from the idea that any sex difference, at any life stage, can be seen as the result of sexual differentiation, even if that difference is reversible or impermanent. Historically, permanent (“organizational”) effects of testosterone, causing irreversible male-specific differentiation of male tissues, have been called “sexual differentiation”. In contrast, “activational” (reversible) hormonal effects have not been seen as differentiation, even though these actions make the two sexes different. These ideas underlie the organizational - activational dichotomy of Phoenix et al. (1959) (Arnold, 2009), who believed that testosterone acts on the brain of males to alter the substrate, just as it acts on the genital tissues to cause differentiation of a different form (a penis rather than clitoris). Later effects of testosterone were not thought to differentiate the neural substrate, but “activated” the already masculinized substrate, again by analogy to androgen effects on the adult penis. Because sex-biasing factors (androgens, estrogens, etc.) wax and wane at different times of life, some sex differences come and go. In my view, all factors that cause sex differences need to be subsumed in a theory of sexual differentiation, because even transient sex differences are sexually differentiated and controlled by inherent sex differences in the genome. Indeed, such transient effects of gonadal hormones may be the most potent proximate factors that make male and female tissues different.
Several considerations make it advantageous to articulate a general theory of sexual differentiation. (1) The theory identifies major classes of factors that cause sex differences in any tissue. This generality is useful, because studies of mechanisms of sexual differentiation in one tissue will suggest concepts to be tested in other tissues. (2) The theory invites public evaluation of its merits, and focuses efforts to test it. Current theory will likely be incomplete or false, and will be improved by future research. (3) A valid theory is itself evidence of the maturity, importance, and independence of the field of sexual differentiation. One view is that sexual differentiation is only studied within the confines of other traditional disciplines. Sexual differentiation of the brain is studied within Neuroscience, and sexual differentiation of obesity is studied within the field of Metabolism. Because common mechanisms sexually differentiate brain and adipose tissues, results in one traditional discipline illuminate the general theory of sexual differentiation applied to any tissue. The theory of sexual differentiation links studies across traditional disciplines, and forms a set of ideas that are tested within the discipline of Sexual Differentiation. The theory suggests questions that might not be framed by other disciplinary perspectives. The sexual differentiationist studying obesity asks different questions (Arnold et al., 2013) than would be asked from purely a metabolic perspective (Link et al., 2013). (4) The theory suggests methods and experimental designs that can be used repeatedly to investigate sexual differentiation at various levels of analysis (behavior, tissue, cellular, molecular). These methods include the manipulation of sex chromosomes and gonadal hormonal to discover their effects at each level. (5) The theory may provide a simplifying framework. The 20th Century dogma was that two testicular hormones (testosterone and Müllerian Inhibiting Hormone) were responsible for coordinating the masculine differentiation of all non-gonadal tissues of mammals, and that female differentiation was the “default”, which is what happens when no masculinizing hormones are present (Jost et al., 1973). Those oversimplifications were accepted in part because they were heuristically pleasing. It made sense that only two male hormones coordinate sexual development so that different parts of the body were uniformly male or female. A more accurate model, proposed here, still allows a pleasing simplification, which is that all sex differences derive from the inherent inequality in the sex chromosomes (Arnold, 2011). Although this theory is more complex, and implies that multiple, parallel-acting and interacting sex-biasing factors contribute to the sexual differentiation of tissues, it nevertheless still provides a simplifying conceptual framework for a complex set of phenomena.
A Theory of Sexual Differentiation
(1) Sex chromosome genes are the primary factors causing sexual differentiation
In species with heteromorphic sex chromosomes (mammals, birds, etc.), all sex differences arise from the genetic inequality found in the sex chromosomes of male and female zygotes. In mammals, females have two X chromosomes and males have one, and males have a Y chromosome lacking in females. The differential representation of X and Y genetic material is the sole source of all subsequent sex differences during development and adulthood, because all other factors (autosomal genes, cytoplasmic material, prenatal environment of the zygote) are thought to be equivalent, on average, between males and female zygotes.
(2) The Y chromosome has male-specific effects that make males different from females
(2A) Sry initiates sexual differentiation of the gonads
The Y-encoded Sry gene initiates masculine differentiation of the gonads, making gonadal hormones different in males compared to females, thus indirectly causing major sex differences in tissue function. Sry causes relatively undifferentiated gonadal tissue to commit to a testicular fate (Koopman, 2010). In the absence of Sry in females, X-linked or autosomal genes, which (unlike Sry) are not inherently sexually different in their representation in the genome, initiate ovarian development. Thus, Sry (present vs. absent) is the root sexual inequality leading to sexual differentiation of the gonads. Gonadal differentiation sets up life-long sex differences in the plasma levels of gonadal steroid hormones such as testosterone, estradiol, and progesterone, which act throughout the body at multiple life stages to make tissues of one sex different from the other. It is thought that these hormonal factors cause the majority of sex differences in the brain (McCarthy and Arnold, 2011)(but see below). Effects of gonadal hormones are operationally defined by their permanent (“organizational effects”) or reversible actions (“activational effects”) (Arnold, 2009; Arnold and Breedlove, 1985; Phoenix et al., 1959).
(2B) Non-gonadal effects of Y genes
The Y chromosome has cell-autonomous effects outside of the gonads that make Y-bearing cells different from those lacking a Y chromosome. Examples are as follows:Sry acts directly within the brain to make it function differently (Czech et al., 2014; Dewing et al., 2006).Other Y genes have an inherently male function because they act on germ cells in a cell-autonomous fashion and are required for spermatogenesis, a male-specific function (Burgoyne and Mitchell, 2007).Other Y genes or genetic regions also likely contribute to sex differences in autoimmune disease (Case et al., 2013; Case and Teuscher, 2015).
(3) The number of X chromosomes causes sex differences
(3A) Expression of Xist is female-specific
The presence of two (or more) X chromosomes triggers the expression of the long noncoding RNA Xist from one of the two X chromosomes, thereby making all such cells different from those with one X chromosome. Xist initiates the transcriptional silencing of that chromosome, which does not occur in any XY cells. Xist has not, however, traditionally been considered a sex-determining or sex-differentiating gene, despite it profound female-specific effects on cells. Instead, X inactivation has long been viewed as a process that reduces sex differences, because X genes are expressed from a single X chromosome in XX cells, as in XY cells. This process allows the cell to avoid higher expression of most X genes in cells with two X chromosomes instead of one. However, X-inactivation leaves the XX cell inherently different from the XY cell, and therefore ranks as one of the most important genes of sexual differentiation. At the very least, when XX and XY cells have similar function that is influenced by the level of expression of an X gene, the cellular mechanisms leading to sexual equivalence of XX and XY cells are different in the two types of cell. In addition, although it has been speculated that Xist might have female-specific effects on autosomes, such trans effects are not known.
(3B) Higher expression of X escapee genes in XX than XY cells
Sex differences in gene expression also arise because some X genes escape X inactivation and are expressed from both chromosomes in XX cells so that expression is inherently higher in XX cells than XY cells (Disteche, 2012). This higher expression is likely to cause sex differences in phenotypes.
(3C) Sexual inequality of parental imprints on X chromosome genes
XX cells have X chromosomes and imprints from both parents, but XY cells receive only the X imprint from the mother. This could cause a sex difference in the expression of the imprinted gene in either direction (Babak et al., 2015; Bonthuis et al., 2015). For example, an X gene that is silenced when inherited from the mother will be expressed higher in XX than XY, but a gene silenced when inherited from the father will be expressed higher in XY than XX tissues.
(3D) Female-specific effects of the inactive X chromosome
Some evidence suggests that the presence of a large heterochromatic X chromosome in XX but not XY cells could have indirect effects on expression of autosomal genes. For example, “heterochromatizing factors”, or factors that regulate the epigenetic status the entire genome, might be in limited supply and devote their actions to maintenance of inactivation of the X chromosome in XX cells, rather than to epigenetic regulation of autosomes which would occur more in XY cells (Wijchers and Festenstein, 2011). These putative effects in mammals extend earlier findings of sex-specific heterochromatin Drosophila, in which the large heterochromatic Y chromosome has epigenetic effects (not effects of Y gene expression), which regulates many autosomal genes, especially those involved in mitochondrial function and immune response (Lemos et al., 2010; Silkaitis and Lemos, 2014).
(4) Independent sex-biasing factors and their downstream pathways interact -- summing, amplifying or counteracting each other, producing and reducing emergent sex differences in tissue function
Recent evidence indicates that sexually differentiated mechanisms underlie phenotypes that are quite similar in the two sexes (Arnold, 2014; De Vries, 2004). In some cases, an unavoidable inherent sex difference in cells causes a sex difference that is not adaptive. New sex-specific forces evolve that counteract or reduce the effect of the maladaptive sex difference, producing greater sexual equality of phenotype. Because disease or environmental factors can increase or decrease the individual counteracting sex-biasing factors, they can change how much compensation occurs. In this manner, phenotypes that are balanced in the two sexes can diverge under specific disease or environmental conditions.
Population-based Sex-biasing Mechanisms
The main theory describes sex-biasing factors that are inherently different in XX vs. XY cells and tissues, which operate during ontogeny of all individuals to differentiate the two sexes. These ontogenetic mechanisms should be discoverable in inbred strains of laboratory animals such as mice. In addition to these mechanisms, groups of females and males can differ on average because of population-level forces that act differentially on the two sexes. The population-level differences, however, are unlikely to be modeled well in inbred strains because they require genetic heterogeneity that has been bred out of inbred strains.
1. Hemizygous exposure of X alleles
Because most males have one X chromosome, any variant of X alleles has greater impact than in females, who have two alleles whose effects are more or less averaged. A well-known example of a sex difference is red-green color blindness, caused by a variation in the X-linked opsin gene, which shifts the spectral sensitivity of cones in the retina. The shift is experienced more by males, because of hemizygous exposure of the X allele. Females carrying the mutation are less likely to experience the color blindness because they have a second X allele that does not shift the spectrum of photosensitivity, so that they can discriminate red from green. The resulting male bias in many X-linked diseases is well known and has long been appreciated, and has been suggested to contribute to the overall lower survival of males (Migeon, 2007). However, more subtle variations in X alleles, not causing disease, no doubt increase the incidence of sex differences in phenotypes via the same mechanism operating in populations of males and females.
2. Tissue mosaicism: Better protection in females against deleterious X alleles
XX tissues are inherently mosaics, because they are composed of a mixture of cells in which the active X chromosome is either maternal or paternal. The two X chromosomes differ both in their alleles (different variants inherited from the two parents) and in their parental imprints. The mosaicism could cause differences in tissue function, compared to that in males. Generally, tissues that have two different populations of cells might function differently than tissues that have only a single population. An example is that the two types of cells may be in competition during ontogeny. If one parent’s X chromosome has an inherent functional superiority over the other, cells with that X chromosome active may divide and differentiate more robustly, and take over most of a specific organ. This process has been modeled in mice with a heterozygous knock out of the X-linked Hccs gene (Drenckhahn et al., 2008). Early in embryonic life of these mice, the heart is a mixture of cells in which the active X chromosome is from either parent, with both the null or WT allele of Hccs. By the time of birth, however, the WT allele is found to be expressed in the majority of cardiac cells, indicating a competitive advantage of the WT Hccs locus. Under these conditions, the mosaicism of the XX tissues actually widens the sexual inequalitty in effects of the mutation, so that males are either mutant (dead) or not, but females can compensate developmentally for the inheritance of a weak allele and reduce its effect. If the protection is complete, with complete loss of the disease allele during development, then females never experience detrimental effects of the disease allele.
3. Sex differences in the frequencies of autosomal alleles
It is also likely that some autosomal alleles are more adaptive in one sex than the other, leading to embryonic sex-specific or sex-biased functions or diseases. For example, some alleles might not be compatible with male levels of testosterone. Such alleles would drop out of the population of males early in their lifetime (because of embryonic lethality), whereas other alleles might drop out of the population of females because of sensitivity to female-enhanced factors. Thus, populations of males and females may differ on average in their complement of autosomal alleles, which would shift the mean phenotype of males and females away from each other.
4. Sexual conflict because of sex-biased inheritance of mitochondrial DNA
Sex-biased evolutionary forces may produce a loss of viability disproportionately in the two sexes. An example is the phenomenon dubbed “mother’s curse” (Camus et al., 2012; Frank, 2012). The mitochondrial genome is inherited through the female lineage from mother to daughter. This could allow mitochondrial alleles to be selected that are beneficial to females but deleterious to males, because the disadvantage to males never results in differential fitness of the females who pass down the genes. Such variations in mitochondrial genes would give rise to greater disease or developmental defects primarily affecting males. Evidence from experimental studies of Drosophila support the existence of this phenomenon. When mitochondria from one strain were bred onto different nuclear genetic background strains, males were disproportionately affected, with male-specific decreases in viability (Innocenti et al., 2011). Such a mechanism, operating in natural populations, may increase the disease burden on males and produce population-level sex differences in disease incidence.
Applying the Theory to Whole Animal Physiology
Although there is ample evidence for effects of gonadal hormones that cause sexual differentiation, much of the theory articulated above has not been tested extensively. Many of the ideas leading to the theory come from analysis of the X and Y chromosomes and their effects on cells in vitro, where it can be demonstrated that the inequality of X and Y chromosomes causes sex differences in gene expression or other cell-level phenotypes. What is not very clear, at the present time, is how important each of the sex-biased mechanisms is, for whole-organ or organismal physiology and disease. For example, a few studies indicate that inequality in the number of X chromosomes contributes to sex differences in disease models in mice (Arnold et al., 2016). In mouse models of obesity, cardiovascular disease, and sex chromosome aneuploidy, mice with two X chromosomes have worse disease outcomes than mice with one X chromosome. However, there are no studies to date that have determined which of the four X-chromosome based mechanisms (3ABCD) discussed above account for the different effects of one vs. two X chromosomes on disease. This is an important area for future research.
The growing appreciation of the importance of sex chromosome effects in mammals, not mediated by gonadal hormones, has been possible because of experiments on a few mouse models, in which the complement of sex chromosomes (XX vs. XY) can be manipulated without changing the type of gonad. Two salient models are the Four Core Genotypes (FCG) and XY* models, which make XX, XY, or XO mice with ovaries, for example (Arnold, 2014; Arnold and Chen, 2009; De Vries et al., 2002). These models will be used increasingly to uncover further cases in which sex chromosome complement contributes to sex differences in physiology. However, these models give only information about mice, and are therefore are best suited to phenomena that can be discovered in mice. It would be advantageous to develop other animal models in which specific sex-biasing mechanisms, enumerated above, are manipulated to mimic the difference that occurs in females vs. males. For example, comparing animals with one vs. two copies of specific X genes that escape inactivation would mimic the normal male-female difference in expression, to determine the effects of that individual gene on sex differences by virtue of its escape from X inactivation. With improved methods for manipulation of the genome in diverse animal groups, this kind of experiment may become increasingly feasible in informative animal systems.
The theory articulated here involves almost no discussion of the role of epigenetic effects, long non-coding RNAs, and microRNAs, because little is known at this point in history. The theory is expected to be greatly enriched by future studies in this area. For example, both gonadal hormones and sex-biased sex chromosome genes are known regulators of DNA methylation and histone modifications (Berletch et al., 2013; Gagnidze et al., 2013; Ghahramani et al., 2014; Nugent et al., 2015), but this knowledge is only just now beginning to be integrated into sexual differentiation theory. There has been no systematic discovery of sexual differentiation of noncoding RNAs that regulate many other genes, but that situation is likely to change in the near future (Reinius et al., 2010).
Sex-biasing factors need to be understood in a larger evolutionary framework. The genes escaping X inactivation (mechanism 3B), for example, sometimes have a very closely related gene on the Y chromosome, which has very similar DNA sequence and function (Lahn and Page, 1999). These X-Y gene pairs are both derived from a common ancient precursor. As the Y chromosome lost most of its genes during evolution, a few terribly important genes were highly resistant to deletion. These Y gene were retained to balance the effects of the X paralogous genes, so that females have two copies (one on each X chromosome), and males have two copies (one X and one Y) (Bellott et al., 2014; Cortez et al., 2014). Patterns of sex chromosome evolution therefore imply that the X-Y partner genes have similar function. In contrast, recent studies suggest that the X and Y forms have at least partly diverged in their functions (Shpargel et al., 2012; Xu et al., 2008a; Xu et al., 2008b), so that having two vs. one copy of the X gene makes for functional differences between XX and XY cells. These discoveries mean that the sex-biasing effects of one vs. two copies of the X gene need to be studied within a larger context of understanding the similarities and differences in function of the X-Y gene pairs. More information is needed to resolve the importance of these genes in sexual differentiation.
Point 4 of the theory elevates the importance of interactions of diverse sex-biasing factors. Natural selection can either favor the evolution of sex-biased mechanisms to produce sex differences that are adaptive, or to offset other sex differences when they are maladaptive. Although we are just beginning to appreciate the complex interactions that require or involve such compensation, we have almost no information about which sex-biased effects interact with others. In the study of metabolism and obesity, for example, estrogens reduce body weight and body fat in female mice (Foryst-Ludwig and Kintscher, 2010), but a second X chromosome increases body weight and fat (Chen et al., 2012). In studies of sex differences in cardiovascular ischemia / reperfusion injury in mice, estrogens protect from cardiovascular damage (Murphy and Steenbergen, 2014) but a second X chromosome makes disease worse (Li et al., 2014). In both cases, no studies have yet to manipulate sex chromosome complement and hormonal status at the same time, to begin to unravel the molecular mechanisms leading to the interactions. It is possible that each factor (estradiol and an X chromosome gene) acts on the same or different molecular pathways to affect physiology and disease, or that they act on completely separate tissues, cells, or cellular functions, to influence an emergent phenotype revealed by system-level measurements of disease. In other systems, sex chromosome complement acts on two different tissues in the same disease. In mouse models of multiple sclerosis, the XX sex chromosome complement (relative to XY) appears to act within the immune system to exacerbate disease (Smith-Bouvier et al., 2008), but acts within the brain to make disease worse (Du et al., 2014). A great deal of further work is needed to clarify the separate and interacting effects of multiple sex-biasing mechanisms highlighted in the theory of sexual differentiation.
The effects of gonadal hormones have been studied much more than sex chromosome effects, for several reasons. The theory of sexual differentiation emerging from research in the 20th century pointed almost exclusively to gonadal hormones as the proximate sex-biasing factors, so any program of research on sex differences focused first on hormones. This focus was usually rewarded, because most sex differences were found to be influenced by sex hormones. The resulting large literature on sex hormone effects gives the impression that hormones dominate as the causes of sex differences. That inference may be true, but has not been tested rigorously by independent manipulation of sex chromosome complement and gonadal status in the same system. A recent study of sex differences in brain structure in mice found that sex differences in some areas of the brain were more influenced by hormones, and in others by sex chromosomes, with about the same number of sex differences caused by each (Corre et al., 2014). A study of sex differences in brain gene expression in stressed mice also found a surprising number of differences caused by sex chromosome complement rather than by hormones (Seney et al., 2013). Thus, the impression of greater importance of sex hormones may be at least partly an artifact of the number of studies of that class of sex-biasing factors.
Many studies to investigate sex chromosome effects are conducted in gonadectomized mice, in the absence of gonadal hormones, because of the need to control hormonal differences between groups as much as possible, so that group differences caused by sex chromosomes can be uncovered. That method, however, raises questions whether the sex chromosome effects also occur when hormones are present. In some cases, they do, but their relative magnitude can be altered by gonadal hormones. In the regulation of body weight, for example, mice with testes are larger than mice with ovaries, so body size depends on gonadal hormones (Chen et al., 2012). However, mice with two X chromosomes weigh more than mice with one X chromosome, a difference that is larger when gonads are absent than when they are present. In this case, a female sex chromosome complement partially compensates for the effects of female hormones on body weight, so sex chromosome effects interact with the effects of gonadal hormones. In another example, sex chromosome effects on plasma cholesterol occur whether or not the gonads are present (Link et al., 2015). Indeed, when multiple sex-biasing factors all influence the same emergent sex difference in physiology and disease, the testing conditions may determine which of the factors appears most important. In a mouse model of stroke, tests of young FCG mice found little influence of sex chromosome complement on sex differences in stroke outcome; rather, females were protected predominantly by their gonadal hormones, estrogens (Manwani et al., 2015). When a similar study was conducted on aging mice, however, the opposite result was obtained. In that case, group differences in type of gonad had little effect, but XX mice had larger infarcts than XY mice (McCullough et al., 2016). In aging mice, the group differences were not attributable to levels of gonadal hormones. Thus, the effects of ischemia on the brain are influenced by both gonadal hormones and sex chromosome complement, but the balance between effects of factors may change as a consequence of age, levels of gonadal hormones, or other factors. To date, therefore, the limited studies that have pitted sex chromosome effects against sex hormone effects suggest that both play a role, but that a lot more information is needed to understand the circumstances under which each is important.
Understanding the Sexome
To reduce the bewildering complexity of cells and tissues, we can imagine them as composed of highly interconnected networks of molecules, pulsating with activity, in which individual components increase or decrease the activity of each other to produce emergent phenotypes of the system. These networks are probably generally similar in the two sexes (Van Nas et al., 2009), because about 95% of the genome is about the same in the two sexes, and most physiological networks are predominantly regulated by autosomal genes. Some gene, protein, or molecular networks, however, are affected by the limited number of factors, enumerated above by the theory of sexual differentiation, that cause sex differences in function. These factors reach into the pulsating networks (or alter them from within), pushing them one way or another, raising or lowering their activity, creating differences in the networks in XX vs. XY cells. The aggregate of all sex-biasing influences can be conceptualized as the “sexome” (Arnold and Lusis, 2012). The theory of sexual differentiation determines how we think about the sexome, because we imagine multiple independent mechanisms that are inherently sex-biased, and which interact with each other. Their inherent nature does not imply that they are not modifiable. Rather, outside circumstances, coming from the rest of the genome, from other sex-biased and sexually equivalent factors, or from the environment, can gate, inhibit, or enhance the sex differences in physiology. Sex differences in physiology can come and go, depending on life stage, disease status, environmental conditions, and many other variables.
Despite this complexity, it is useful to attempt to understand the factors causing sex differences, for several reasons. One is the drive to understand ourselves and our position in the universe. The second is the practical advantage that understanding the system leads to the ability to control it. If we can understand which sex-biasing factors protect from disease, we may be able to manipulate them to treat diseases in both sexes. Finally, articulating a theory of sexual differentiation leads to new questions that would not otherwise be asked. For example, the theory motivates experiments to study the interaction of independent sex-biasing factors, to increase understanding of their relative effects on sex differences themselves. The discovery of organizational effects of gonadal hormones was entirely a consequence of an effort to test the existing theory of sexual differentiation and to resolve what makes males and females different (Phoenix et al., 1959). The then-existing theory was that activational effects of gonadal hormones account for many sex differences in behavior, but they were inadequate to explain all such sex differences. That tension motivated the early sexual differentiationists to find the mystery factor, which turned out to be the prenatal levels of testosterone. Today, we are at a different place, where we have expanded the theory’s list of inherent sex-biasing factors to include X and Y genes, but we have a great deal of work to do to understand when and how those factors explain the differences between females and males.
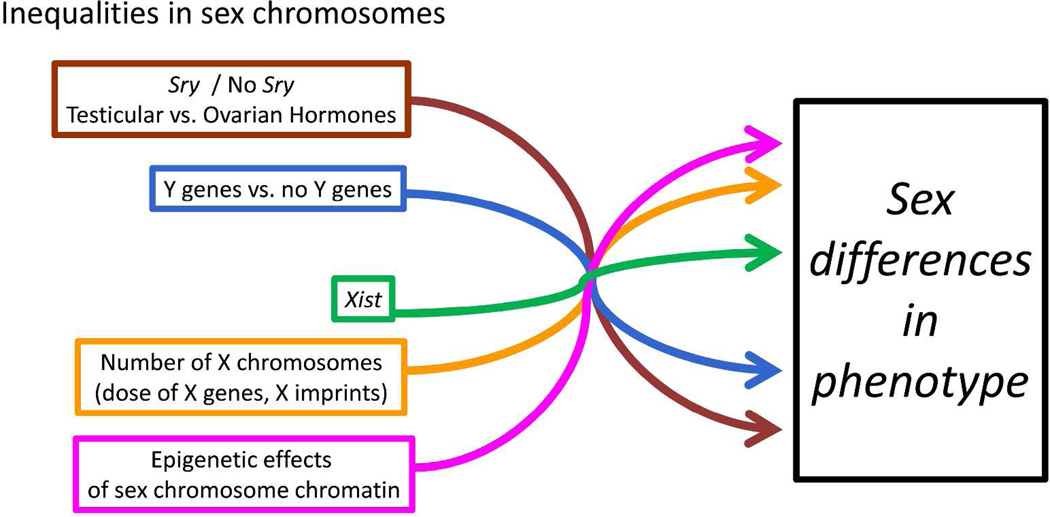
Figure 1.
Schematic diagram showing the effects of five classes of sex-biasing factors discussed here. Each factor derives from the inherent inequality in sex chromosomes when comparing XX and XY cells, tissues, or animals. The factors can act independently to contribute to sex differences in phenotype. The intersection of arrows is intended to evoke the idea that sex-biasing factors can interact, either by augmenting or opposing each other within specific cells, or by acting on different cells that each contribute to sex differences in the phenotype. The sex-biased factors therefore can produce or reduce sex differences in phenotype. Sex differences can wax or wane depending on a variety of factors such as age or disease.
Significance.
Sexual differentiation encompasses the biological processes that make males and females different from each other. Understanding sexual differentiation not only contributes to our conceptualization of the nature of females and males, it also uncovers sex-biased factors that protect from diseases that affect the two sexes differently. Here a theory is proposed to provide a comprehensive list of all of the biological factors that underlie sex differences in physiology and disease. The theory provides a focus and conceptual framework for future improvements to the theory.
Acknowledgments
Research supported by NIH grants NS043196, DK083561, HD076125, HL131182. The ideas presented here have emerged from countless discussions with colleagues who are too numerous to be listed comprehensively. I particularly thank Paul Burgoyne, Karen Reue, Rhonda Voskuhl, Eric Vilain, Mansoureh Eghbali, Geert de Vries, Christine Disteche, and Jennifer Graves.
Footnotes
The author declares no conflict of interest.
Reference List
- Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural not gonadal origin of brain sex differences in a gynandromorphic finch. Proc. Natl. Acad. Sci. U. S A. 2003;100:4873–4878. doi: 10.1073/pnas.0636925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E. Sex and Internal Secretions. Baltimore: Williams & Wilkins Co; 1932. [Google Scholar]
- Arnold AP. Concepts of genetic and hormonal induction of vertebrate sexual differentiation in the twentieth century, with special reference to the brain. In: Pfaff DW, Arnold AP, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain, and Behavior. San Diego: Academic Press; 2002. pp. 105–135. [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. The end of gonad-centric sex determination in mammals. Trends Genet. 2011;28:55–61. doi: 10.1016/j.tig.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. Conceptual frameworks and mouse models for studying sex differences in physiology and disease: Why compensation changes the game. Exp. Neurol. 2014;259:2–9. doi: 10.1016/j.expneurol.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroid hormones on vertebrate brain and behavior: a re-analysis. Horm. Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the "four core genotypes" mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X, Link JC, Itoh Y, Reue K. Cell-autonomous sex determination outside of the gonad. Dev. Dyn. 2013;242:371–379. doi: 10.1002/dvdy.23936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Lusis AJ. Understanding the sexome: measuring and reporting sex differences in gene systems. Endocrinol. 2012;153:2551–2555. doi: 10.1210/en.2011-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Reue K, Eghbali M, Vilain E, Chen X, Ghahramani N, Itoh Y, Li J, Link JC, Ngun T, Williams-Burris SM. The importance of having two X chromosomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371 doi: 10.1098/rstb.2015.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babak T, DeVeale B, Tsang EK, Zhou Y, Li X, Smith KS, Kukurba KR, Zhang R, Li JB, van der Kooy D, Montgomery SB, Fraser HB. Genetic conflict reflected in tissue-specific maps of genomic imprinting in human and mouse. Nat. Genet. 2015;47:544–549. doi: 10.1038/ng.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, Cho TJ, Koutseva N, Zaghlul S, Graves T, Rock S, Kremitzki C, Fulton RS, Dugan S, Ding Y, Morton D, Khan Z, Lewis L, Buhay C, Wang Q, Watt J, Holder M, Lee S, Nazareth L, Rozen S, Muzny DM, Warren WC, Gibbs RA, Wilson RK, Page DC. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature. 2014;508:494–499. doi: 10.1038/nature13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berletch JB, Deng X, Nguyen DK, Disteche CM. Female bias in Rhox6 and 9 regulation by the histone demethylase KDM6A. PLoS. Genet. 2013;9:e1003489. doi: 10.1371/journal.pgen.1003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthuis PJ, Huang WC, Stacher Horndli CN, Ferris E, Cheng T, Gregg C. Noncanonical Genomic Imprinting Effects in Offspring. Cell Rep. 2015;12:979–991. doi: 10.1016/j.celrep.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Mitchell MJ. The role of mouse Y chromosome genes in spermatogenesis. In: Lau YFC, Chan WY, editors. Y Chromosome and Male Germ Cell Biology. Hackensack NJ: World Scientific Publishers; 2007. pp. 27–45. [Google Scholar]
- Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse. Philos. Trans. R. Soc. Lond B Biol. Sci. 1995;350:253–260. doi: 10.1098/rstb.1995.0159. [DOI] [PubMed] [Google Scholar]
- Camus MF, Clancy DJ, Dowling DK. Mitochondria, maternal inheritance, and male aging. Curr. Biol. 2012;22:1717–1721. doi: 10.1016/j.cub.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Case LK, Teuscher C. Y genetic variation and phenotypic diversity in health and disease. Biol. Sex Differ. 2015;6:6. doi: 10.1186/s13293-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LK, Wall EH, Dragon JA, Saligrama N, Krementsov DN, Moussawi M, Zachary JF, Huber SA, Blankenhorn EP, Teuscher C. The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome Res. 2013;23:1474–1485. doi: 10.1101/gr.156703.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8:e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre C, Friedel M, Vousden DA, Metcalf A, Spring S, Qiu LR, Lerch JP, Palmert MR. Separate effects of sex hormones and sex chromosomes on brain structure and function revealed by high-resolution magnetic resonance imaging and spatial navigation assessment of the Four Core Genotype mouse model. Brain Struct. Funct. 2014;221:997–1016. doi: 10.1007/s00429-014-0952-0. [DOI] [PubMed] [Google Scholar]
- Cortez D, Marin R, Toledo-Flores D, Froidevaux L, Liechti A, Waters PD, Grutzner F, Kaessmann H. Origins and functional evolution of Y chromosomes across mammals. Nature. 2014;508:488–493. doi: 10.1038/nature13151. [DOI] [PubMed] [Google Scholar]
- Czech DP, Lee J, Correia J, Loke H, Moller EK, Harley VR. Transient neuroprotection by SRY upregulation in dopamine cells following injury in males. Endocrinol. 2014;155:2602–2612. doi: 10.1210/en.2013-2158. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinol. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Berletch JB, Nguyen DK, Disteche CM. X chromosome regulation: diverse patterns in development, tissues and disease. Nat. Rev. Genet. 2014;15:367–378. doi: 10.1038/nrg3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Chiang CWK, Sinchak K, Sim H, Fernagut PO, Kelly S, Chesselet MF, Micevych PE, Albrecht KH, Harley VR, Vilain E. Direct regulation of adult brain function by the male-specific factor SRY. Curr. Biol. 2006;16:415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Disteche CM. Dosage compensation of the sex chromosomes. Annu. Rev. Genet. 2012;46:537–560. doi: 10.1146/annurev-genet-110711-155454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn JD, Schwarz QP, Gray S, Laskowski A, Kiriazis H, Ming Z, Harvey RP, Du XJ, Thorburn DR, Cox TC. Compensatory growth of healthy cardiac cells in the presence of diseased cells restores tissue homeostasis during heart development. Dev. Cell. 2008;15:521–533. doi: 10.1016/j.devcel.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Du S, Itoh N, Askarinam S, Hill H, Arnold AP, Voskuhl RR. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 2014;111:2806–2811. doi: 10.1073/pnas.1307091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CE, Jones KW, Polani PE, de Almeida JC, Briggs JH. A sex chromosome anomaly in a case of gonadal dysgenesis (Turner's syndrome) Lancet. 1959;1:711–713. doi: 10.1016/s0140-6736(59)91893-8. [DOI] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J. Steroid Biochem. Mol. Biol. 2010;122:74–81. doi: 10.1016/j.jsbmb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Frank SA. Evolution: mitochondrial burden on male health. Curr. Biol. 2012;22:R797–R799. doi: 10.1016/j.cub.2012.07.066. [DOI] [PubMed] [Google Scholar]
- Gagnidze K, Weil ZM, Faustino LC, Schaafsma SM, Pfaff DW. Early histone modifications in the ventromedial hypothalamus and preoptic area following oestradiol administration. J. Neuroendocrinol. 2013;25:939–955. doi: 10.1111/jne.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, Muir S, Rubbi L, Arnold AP, De Vries GJ, Forger NG, Pellegrini M, Vilain E. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol. Sex Differ. 2014;5:8. doi: 10.1186/2042-6410-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves JAM. Sex chromosome specialization and degeneration in mammals. Cell. 2006;124:901–914. doi: 10.1016/j.cell.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Hamann S, Stevens J, Vick JH, Bryk K, Quigley CA, Berenbaum SA, Wallen K. Brain responses to sexual images in 46,XY women with complete androgen insensitivity syndrome are female-typical. Horm. Behav. 2014;66:724–730. doi: 10.1016/j.yhbeh.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Innocenti P, Morrow EH, Dowling DK. Experimental evidence supports a sex-specific selective sieve in mitochondrial genome evolution. Science. 2011;332:845–848. doi: 10.1126/science.1201157. [DOI] [PubMed] [Google Scholar]
- Joel D, Berman Z, Tavor I, Wexler N, Gaber O, Stein Y, Shefi N, Pool J, Urchs S, Margulies DS, Liem F, Hanggi J, Jancke L, Assaf Y. Sex beyond the genitalia: The human brain mosaic. Proc. Natl. Acad. Sci. U. S. A. 2015;112:15468–15473. doi: 10.1073/pnas.1509654112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost A. Reserches sur la différenciation sexuelle de l'embryon de lapin. Arch. Anat. Microsc. Morphol. Exp. 1947;36:271–315. [Google Scholar]
- Jost A, Vigier B, Prepin J, Perchellet JP. Studies on sex differentiation in mammals. Rec. Prog. Horm. Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- Kishi R, Kitahara T, Masuchi A, Kasai S. Work-related reproductive, musculoskeletal and mental disorders among working women--history, current issues and future research directions. Ind. Health. 2002;40:101–112. doi: 10.2486/indhealth.40.101. [DOI] [PubMed] [Google Scholar]
- Koopman P. The delicate balance between male and female sex determining pathways: potential for disruption of early steps in sexual development. Int. J. Androl. 2010;33:252–258. doi: 10.1111/j.1365-2605.2009.01001.x. [DOI] [PubMed] [Google Scholar]
- Lahn BT, Page DC. Functional coherence of the human Y chromosome. Science. 1997;278:675–680. doi: 10.1126/science.278.5338.675. [DOI] [PubMed] [Google Scholar]
- Lahn BT, Page DC. Four evolutionary strata on the human X chromosome. Science. 1999;286:964–967. doi: 10.1126/science.286.5441.964. [DOI] [PubMed] [Google Scholar]
- Lemos B, Branco AT, Hartl DL. Epigenetic effects of polymorphic Y chromosomes modulate chromatin components, immune response, and sexual conflict. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15826–15831. doi: 10.1073/pnas.1010383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen X, McClusky R, Ruiz-Sundstrom M, Itoh Y, Umar S, Arnold AP, Eghbali M. The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: one X is better than two. Cardiovasc. Res. 2014;102:375–394. doi: 10.1093/cvr/cvu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie FR. General biological introduction. In: Allen E, Danforth CH, Doisy EA, editors. Sex and Internal Secretions. Baltimore: Williams and Wilkins Co; 1939. pp. 3–14. [Google Scholar]
- Link JC, Chen X, Arnold AP, Reue K. Metabolic impact of sex chromosomes. Adipocyte. 2013;2:74–79. doi: 10.4161/adip.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link JC, Chen X, Prien C, Borja MS, Hammerson B, Oda MN, Arnold AP, Reue K. Increased High-Density Lipoprotein Cholesterol Levels in Mice With XX Versus XY Sex Chromosomes. Arterioscler. Thromb. Vasc. Biol. 2015;35:1778–1786. doi: 10.1161/ATVBAHA.115.305460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa RA. Subdomains of gender-related occupational interests: do they form a cohesive bipolar M-F dimension? J. Pers. 2005;73:693–729. doi: 10.1111/j.1467-6494.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J. Cereb. Blood Flow Metab. 2015;35:221–229. doi: 10.1038/jcbfm.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Liu F. Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. 2016 doi: 10.18632/aging.100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon BR. Females are mosaic: X inactivation and sex differences in disease. Oxford: Oxford University Press; 2007. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Estrogen regulation of protein expression and signaling pathways in the heart. Biol. Sex Differ. 2014;5:6. doi: 10.1186/2042-6410-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. Brain feminization requires active repression of masculinization via DNA methylation. Nat. Neurosci. 2015;18:690–697. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinol. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Reinius B, Shi C, Hengshuo L, Sandhu KS, Radomska KJ, Rosen GD, Lu L, Kullander K, Williams RW, Jazin E. Female-biased expression of long non-coding RNAs in domains that escape X-inactivation in mouse. BMC Genomics. 2010;11:614. doi: 10.1186/1471-2164-11-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfree MB, Short RV. Sex determination in marsupials: evidence for a marsupial-eutherian dichotomy. Philosophical Transactions of the Royal Society of London. B:Biological Sciences. 1988;322:41–53. doi: 10.1098/rstb.1988.0112. [DOI] [PubMed] [Google Scholar]
- Seney ML, Ekong KI, Ding Y, Tseng GC, Sibille E. Sex chromosome complement regulates expression of mood-related genes. Biol. Sex Differ. 2013;4:20. doi: 10.1186/2042-6410-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpargel KB, Sengoku T, Yokoyama S, Magnuson T. UTX and UTY demonstrate histone demethylase-independent function in mouse embryonic development. PLoS Genet. 2012;8:e1002964. doi: 10.1371/journal.pgen.1002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silkaitis K, Lemos B. Sex-biased chromatin and regulatory cross-talk between sex chromosomes, autosomes, and mitochondria. Biol. Sex Differ. 2014;5:2. doi: 10.1186/2042-6410-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, Repping S, Pyntikova T, Ali J, Bieri T, Chinwalla A, Delehaunty A, Delehaunty K, Du H, Fewell G, Fulton L, Fulton R, Graves T, Hou SF, Latrielle P, Leonard S, Mardis E, Maupin R, McPherson J, Miner T, Nash W, Nguyen C, Ozersky P, Pepin K, Rock S, Rohlfing T, Scott K, Schultz B, Strong C, Tin-Wollam A, Yang SP, Waterston RH, Wilson RK, Rozen S, Page DC. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–837. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR. A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environ. Mol. Mutagen. 2008;49:46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- US National Institute of Medicine Committee on Understanding the Biology of Sex and Gender Disorders. Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington D.C.: National Academy Press; 2001. [Google Scholar]
- Van Nas A, GuhaThakurta D, Wang SS, Yehya N, Horvath S, Zhang B, Ingram-Drake L, Chaudhuri G, Schadt EE, Drake TA, Arnold AP, Lusis AJ. Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinol. 2009;150:1235–1249. doi: 10.1210/en.2008-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Functional testicular tissue does not masculinize development of the zebra finch song system. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5264–5268. doi: 10.1073/pnas.93.11.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijchers PJ, Festenstein RJ. Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends Genet. 2011;27:132–140. doi: 10.1016/j.tig.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS One. 2008a;3:e2553. doi: 10.1371/journal.pone.0002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Deng X, Watkins R, Disteche CM. Sex-specific differences in expression of histone demethylases Utx and Uty in mouse brain and neurons. J. Neurosci. 2008b;28:4521–4527. doi: 10.1523/JNEUROSCI.5382-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WC. Sex and Internal Secretions. Baltimore: Williams & Wilkins Co; 1961. [Google Scholar]