Abstract
Primary immunodeficiency disorders (PIDs) represent a range of genetically determined diseases that typically have increased susceptibility to infections and in many also have evidence of immune dysregulation that often presents as autoimmunity. Most recently, the concept of gain of function (GOF) mutations associated with PIDs has become well recognized and adds a new dimension to the understanding of this group of disorders moving beyond the more commonly seen loss of function mutations. The rapidly expanding genetic defects that have been identified in previously uncharacterized PIDs has opened up the potential for targeted therapy directed at the specific disease-causing abnormality. This has been driven by linking PID specific genetic defects to the associated unique abnormalities in cellular signaling pathways amenable to directed therapies. These include agents that either block over active or enhance under responsive cellular pathways. Selected primary immunodeficiences were chosen, whose genetic defects have been recently characterized and are amenable to targeted therapy, as a reflection of the power of precision medicine forward.
Keywords: personalized medicine, primary immunodeficiency disorders, immune dysregulation, autoimmunity, therapy, mutation
Introduction
The number of genetically defined primary immunodeficiencies (PIDs) has increased significantly over the past 10–15 years1 related to the availability of positional cloning and more recently massively parallel (next generation) sequencing. Investigations of previously uncharacterized patients using the latter technology is dramatically increasing our understanding of the genetic basis of PIDs well beyond purely developmental abnormalities to include a range of defects impacting specific aspects of immune signaling and also moving from exclusively loss of function mutations to include gain of function (GOF) mutations. Associated with this expanding understanding of immunologic disorders is the recognition that many of the more recently characterized PIDs include significant immune dysregulation often manifesting as autoimmunity in addition to increased susceptibility to infection. The capacity to precisely identify the molecular basis of an immunologic disorder has also opened the door to targeted therapy focused on either enhancing or inhibiting the consequences of an individual defect. This approach represents one of the central components of precision medicine, i.e. therapy directed at the specific causative defect of a particular disorder rather than applying a non-specific therapeutic approach. It is highly likely that the identification of new genetic defects associated with immune dysfunction will lead to additional examples of targeted therapeutic approaches for optimal clinical management of the patients affected with immune disorders. In this paper, we present the clinical phenotype of a number of more recently characterized PIDs and introduce specific therapeutic approaches that have emerged based on the current understanding of the molecular defect linked to each disorder. Owing the space limitations, this focused discussion does not address the obvious importance of identifying the PID genotype when considering possible gene therapy or the potential contribution of the SCID genotype in the optimal approach for immune reconstitution associated with hematopoietic stem cell transplantation.
Activated Phosphoinositide-3 Kinase (3)K) Delta Syndrome
PIK3CD gain of function mutations result in an immunologic disorder that causes the accumulation of senescent T cells, lymphadenopathy, immunodeficiency and autoimmunity. This disease is referred to either as Activated PI(3)K Delta Syndrome (APDS) or p110 delta activating mutation causing the accumulation of senescent T cells, lymphadenopathy, immunodeficiency (PASLI)2–4. To date there have been four different heterozygous mutations defined producing GOF mutations that are associated with the following amino acid changes: E1021K, N334K, E525K and C416R with E1021K being by far the most common. Constitutive activation of PI3K delta may also result from heterozygous splice site mutations of the PIK3R1 gene, encoding for the p85α subunit of the molecule5, 6. By removing the p110δ-binding site, these splice site mutations release p110δ from the inhibitory control mediated by the p85α subunit. This condition is also referred to as activated PI(3)K delta syndrome type 2 (APDS2). The clinical presentation of APDS/PASLI and APDS2 typically begins with recurrent sinopulmonary infections in virtually all patients7, 8. The onset of infections is typically in childhood ranging from infancy until early school years. The pulmonary infections are associated with a variety of bacterial pathogens but most commonly involve S. pneumoniae and H. influenza. In studies to date, bronchiectasis has been found commonly owing to the frequency and chronicity of the pulmonary infections4, 7, 8. Recurrent or persistent Herpesviridae family virus infections is also seen in about half of these patients including EBV, CMV, HSV and VZV7. Non-infectious complications include non-neoplastic lymphadenopathy, splenomegaly and/or hepatomegaly in the majority of patients as well as autoimmune disease (~40%), nodular mucosal lymphoid hyperplasia (~30%) and enteropathy (~25%)7, 8. The most serious complication of this disorder is the markedly increased frequency of lymphoma (particularly B cell lymphoma), a development that represents one of the major causes of mortality7, 8. As noted one unique feature of this disease that is seen in about 1/3 of patients is the presence of nodular mucosal lymphoid aggregates involving the pulmonary and/or gastrointestinal mucosa that have the appearance of cobblestones7. Neurodevelopmental delay has been reported in approximately 20–30% of the patients, and may represent a direct effect of dysregulation of PI(3)K activity in the central nervous system7, 8. Growth retardation has been noticed in 45% of APDS2 patients, but not in patients with PIK3CD mutations, and may reflect dysregulated activity of p110α and p110β subunits8.
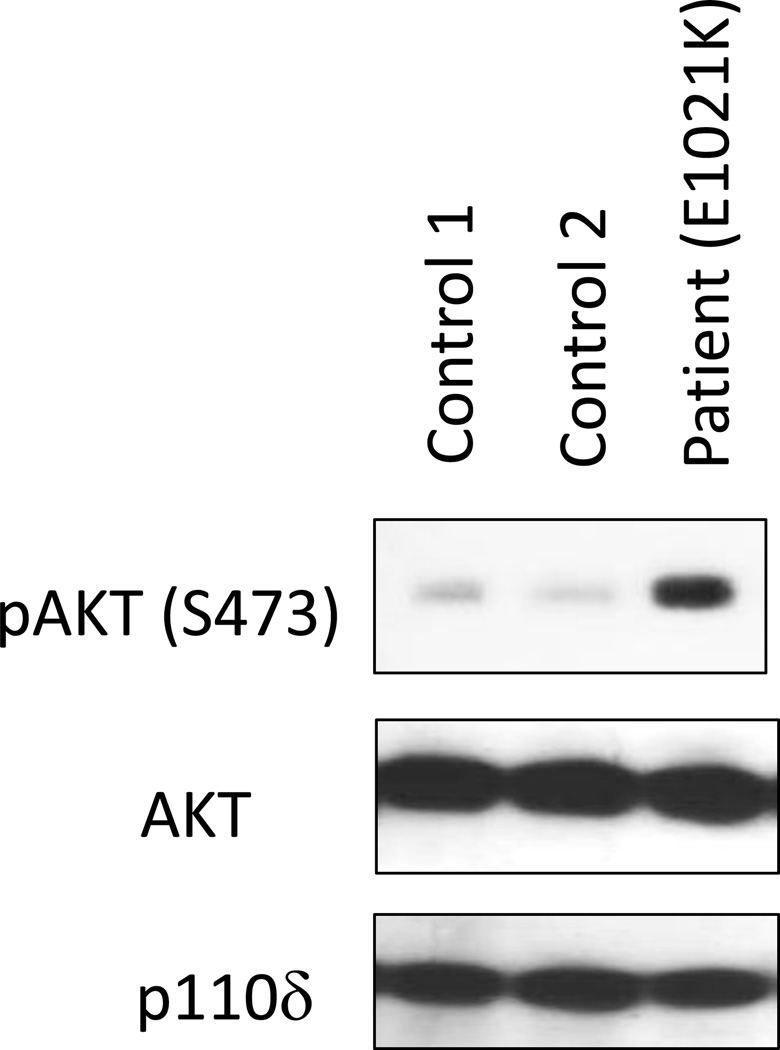
Immunologic findings include varied degrees of lymphopenia with decreased CD4 T cells, reduced naïve T cells, increased senescent T cells (CD3+CD8+CD57+) that do not proliferate normally and increased effector memory CD8 T cells in most patients7. Furthermore, T cell blasts from the patients show increased activation-induced cell death5. The B cell compartment demonstrates increased circulating transitional B cells, decreased switched memory B cells in many, increased IgM levels (~80%) with varied deficiency of IgG and IgA and importantly impaired antibody responses7, 8. Based on the genetic findings, functional testing demonstrated increased AKT phosphorylation (Figure 1) as well as increased S6 phosphorylation as a result of augmented mTOR signaling2, 3, 5, 6. Therefore, the mutations in the PI(3)K catalytic subunit p110δ clearly produce a GOF. However, T cell antigen receptor induced calcium flux and NF-κB nuclear translocation were found to be normal3. This suggested that targeting mTOR signaling potentially could control the endogenous cellular activation resulting from the GOF mutation in the PI(3)K catalytic subunit p110δ. The potential to diminish the increased level of activation was studied in vitro with experiments using rapamycin as an inhibitor of mTOR, and these produced diminished S6 phosphorylation3, 6. Furthermore, in vitro addition of a small molecule inhibitor of p110δ (Leniolisib/CDZ173) to patients’ T cells reduced intracellular levels of phosphatidylinositol-tris-phosphate (PIP3)2 and diminished AKT and S6 phosphorylation5, 6. Altogether, these data suggest that rapamycin and small molecule inhibitors of p110δ could prove effective in managing these patients.
Figure 1.
Immunoblot of phospho-AKT (serine 473), AKT and PI(3)K delta (110δ) from two control subjects and one patient with APDS/PASLI (E1021K)
The clinical outcome in patients with APDS/ PASLI or with APDS2 is often severe, due to increased risk of infections and lymphoma. Accordingly, directed therapy could prove to be very valuable. Hematopoietic stem cells transplantation (HSCT) has been attempted in a small number of patients. According to a recent report, 9 of 11 patients who have received HSCT were alive at ≥8 months post-transplant9. However, based on in vitro data, a recent trial has been initiated using the small molecule inhibitor of PI(3)K catalytic subunit p110δ, Leniolisib/CDZ173, with the result of treating the first six patients having been presented recently demonstrating marked decrease in lymphadenopathy, reduction in senescent CD4 and CD8 T cells, decrease in transitional B cells and normalization of naïve B cells. This was accompanied by a dose dependent reduction in AKT and S6 phosphorylation10. These encouraging results are being followed up with a larger phase II trial but suggest that a targeted therapy focused on the specific GOF mutation impacting PI(3)K catalytic subunit p110δ could prove to be a relatively non-toxic directed therapy in this condition. However, until additional patients are enrolled and long term outcome is determined, this remains to be proven.
CTLA4 haploinsufficiency
Cytotoxic lymphocyte antigen 4 (CTLA4), also referred to as CD152, is an immune checkpoint receptor that turns down the immune response. CTLA4 has a high affinity for the ligands, CD80 and CD86, expressed on antigen presenting cells (APC)11. This surface protein is constitutively expressed on T regulatory cells (Tregs) and is expressed on other T cells following activation. Binding to CD80/86 by T cells expressing CTLA4 yields an inhibitory signal that prevents cell cycle progression and T cell activation. This contrasts with CD80/86 binding to CD28 that provides the costimulatory signal necessary for T cell receptor (TCR) based T cell activation. Understanding the importance of the CTLA4 checkpoint in modulating immune responses lead to the generation of a fusion molecule consisting of the extracellular domain of CTLA4 fused to the Fc region of IgG1 (abatacept) that functions in vivo as an inhibitor of T cell activation and this biologic is now FDA approved for the treatment of refractory rheumatoid arthritis12. The primary side effects associated with abatacept include increased susceptibility to infections and increased risk for malignancy.
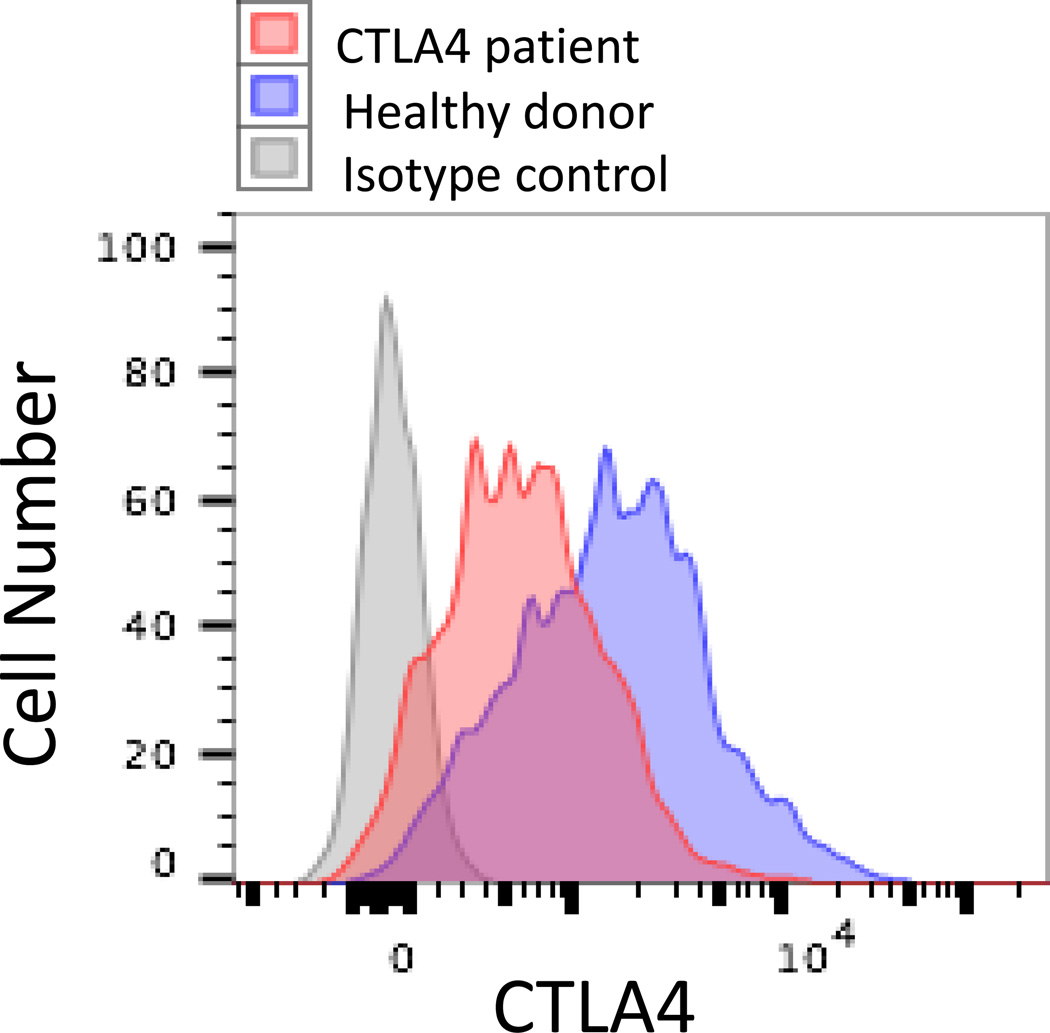
In 2014, two publications reported on CTLA4 mutations in patients with recurrent sinopulmonary and viral infections as well as significant autoimmunity13, 14. In both reports, affected patients had clinical and laboratory features that were consistent with common variable immunodeficiency (CVID), including infectious complications and hypogammaglobulinemia, with the additional findings of significant infiltrative autoimmunity primarily affecting the lung, gastrointestinal tract and nervous system as well as autoimmune cytopenias. Whole exome sequencing revealed heterozygous mutations in CTLA4 producing a loss of function associated with the mutant allele. Functional testing demonstrated that effector T cells were hyper-activated and FOXP3+ Treg cells had diminished CTLA4 expression (Figure 2) as well as decreased suppressor function. Treg cells also demonstrated a diminished capacity to transendocytose CD80 ex vivo as a reflection of another component contributing to the diminished regulatory capacity of Treg cells14. In addition, patients demonstrated diminished CTLA4 expression following activation of conventional T cells suggesting impairment of this check point that controls immune responses, including those involving self-reactive T cells that escaped central deletion. Patients with CTLA4 haploinsufficiency also have a progressive loss of B cells associated with an increase in the autoreactive CD21lo B cell subset13. Importantly, certain family members with the same CTLA4 mutation had limited or no clinical findings suggesting variable penetrance and/or expressivity of disease associated with the heterozygous CTLA4 mutations13, 14. Taken together, these data suggest CTLA4 haploinsufficiency results in diminished CTLA4 expression and this produces a combination of immune deficiency and immune dysregulation. Initially, patients were treated with rapamycin (sirolimus) in an attempt to suppress the hyperactivity of the T cells with clinical response. However, the experimental findings suggested that ex vivo addition of the CTLA4 mimetic abatacept could suppress T cell hyperproliferation and raised the potential of this as a therapeutic option for these patients13.
Figure 2.
Flow cytometry for intracellular CTLA4 expression by CD3+/CD4+/CD25+/FOXP3+ T regs from a patient with CTLA4 haploinsufficiency and a healthy donor.
The clinical effectiveness of abatacept in this disorder has been documented in a recent case report describing the treatment of a patient with CTLA4 haploinsufficiency15. The patient was a 14 year-old girl who on whole exome sequencing was found to have a novel variant in CTLA4 that was associated with enhanced inflammatory cytokine production. The clinical phenotype consisted of severe infiltrative GI disease causing chronic diarrhea, autoimmune hemolytic anemia, autoimmune hepatitis and severe growth failure. Attempts to control the autoimmunity had failed using a variety of different immunosuppressive agents. Initiation of abatacept therapy diminished the diarrhea, controlled the autoimmune hemolytic anemia and eliminated the need for additional immunosuppressive agents. There is also a recent case report of a patient with CTLA4 haploinsufficiency who had clinical features of this disorder as well as severe autoimmune enterocolitis that was unresponsive to standard immunosuppressive therapy. The difficulty in managing the colitis lead to a successful trial of vedoluzimab, an α4β7 integrin specific humanized monoclonal antibody that targets T cell homing to the gastrointestinal tract16. However, the hypogammaglobulinemia and pure red cell aplasia did not respond to vedolizumab necessitating the addition of cyclosporine that appeared to induce remission in the red cell aplasia16.
A recent study has reported on the outcome of hematopoietic stem cell transplantation (HSCT) in eight patients with CTLA4 haploinsufficiency associated with life-threatening, treatment-resistant manifestations of immune dysregulation17. One of the patients also had a history of Hodgkin lymphoma. All patients received matched unrelated donor HSCT with reduced intensity conditioning. Six of the eight patients were reported to be alive and well with stable mixed (n=2) or full (n=4) donor chimerism. Two patients died of severe acute graft versus host disease, or diabetic ketoacidosis, respectively.
It remains to be determined if the use of the CTLA4 agonist, abatacept, will prove effective in controlling autoimmunity in the majority of patients with CTLA4 haploinsufficiency and if this approach will alter the increased susceptibility to infection. However, at this juncture it seems that abatacept may be the most appropriate first line treatment option with or without other appropriate immunosuppressive agents, along with immunoglobulin replacement therapy in those patients with hypogammaglobulinemia. On the other hand, the encouraging initial experience with HSCT in patients with CTLA4 haploinsufficiency suggests that this may represent a valid therapeutic option for patients with severe clinical manifestations that are refractory to treatment with immunomodulatory drugs.
LRBA deficiency
Lipopolysaccharide-responsive and beige-like anchor (LRBA) protein deficiency is an autosomal recessive disorder, and was initially identified in patients presenting early in life with enteropathy, recurrent infections, autoimmune manifestations, and hypogammalobulinemia18. More recently, it has been shown that the clinical phenotype of the disease is more variable. Immune dysregulation has been reported in 95% of the patients, and its most common manifestations are represented by enteropathy, autoimmune hemolytic anemia and idiopathic thrombocytopenia, but type 1 diabetes, autoimmune hepatitis, myasthenia gravis, thyroid disease, uveitis, alopecia, and polyarthritis also have been described19–21. Some patients may present with enteropathy and polyendocrinopathy, a phenotype that mimics the immune dysregulation, polyendocrinopathy, X-linked (IPEX) syndrome22. Lymphoproliferative disease, with splenomegaly and lymphadenopathy, occurs in approximately 65% of the patients. Respiratory infections of viral and bacterial origin are also common, and more than 50% of the patients develop lung infiltrates, bronchiectasis, and granulomas19, 21. Neurological complications have been reported in up to 23% of the patients, and include brain atrophy, granulomas and demyelinating lesions21. The severity and chronicity of the clinical manifestations of the disease are often the cause of growth failure19, 21.
The immunological phenotype of the disease includes hypogammaglobulinemia, and a markedly reduced proportion of switched memory B cells18, 19, 21. Some patients with a clinical phenotype suggestive of autoimmune lymphoproliferative syndrome (ALPS) have an increased proportion of double negative T cells23. In most cases, the level of Treg cells is decreased22, and Treg cells express reduced levels of FOXP3, CD25 and CTLA4 proteins22, 24, all of which play a critical role in Treg function.
A breakthrough in the characterization of the pathophysiology of the immune dysregulation of LRBA deficiency came with the demonstration that LRBA co-localizes with CTLA4 in recycling endosomes; in the absence of LRBA, the CTLA4 protein is targeted to lysosomal degradation, resulting in reduced CTLA4 levels in both Treg cells and activated conventional T cells24. These observations have provided a mechanistic explanation for the clinical similarities between CTLA4 haploinsufficiency and LRBA deficiency, and have prompted a novel therapeutic approach based on the administration of abatacept24. This treatment may induce rapid improvement of the lymphocytic interstitial lung disease and may also ameliorate autoimmune manifestations of the disease. Concurrently, levels of soluble CD25 (a biomarker of T cell activation) often decrease, while there is an increase of the ratio of naïve to effector T cells24. For unclear reasons, the intestinal manifestations of the disease are less responsive to treatment with abatacept, and may benefit by simultaneous use of sirolimus or possibly another immunosuppressant.
In vitro studies have shown that chloroquine, an inhibitor of lysosomal degradation, may also prevent CTLA4 loss in patients with LRBA deficiency24, raising the interesting possibility that administration of chloroquine and hydroxychloroquine (both known for their immunomodulatory properties) may also be beneficial in the treatment of the disease. Finally, HSCT might represent a definitive cure for LRBA deficiency, but there is only limited experience with transplantation, and the outcome remains uncertain25.
STAT1 GOF mutations
Type I and type II cytokines signal through Janus-Associated Kinase (JAK) and Signal Transducers and Activator of Transcription (STAT) proteins26. In humans, there are four JAK proteins (JAK1, JAK2, JAK3, and TYK2) and seven STAT proteins (STAT1-4, STAT5a, STAT5b, and STAT6). Although this relative small number of JAK and STAT proteins implies that redundancy and pleiotropy exist in cytokine-mediated signaling, nonetheless mutations in individual JAK and STAT genes are associated with distinctive features, with some overlap27. In particular, the STAT1 protein is activated in response to engagement of various cytokine receptors, including (but not limited to) IL-6, IL-21, IL-22, IFN-α/β, and IFN-γ28. Biallelic loss of function (LOF) mutations of STAT1 cause severe susceptibility to viral and mycobacterial infections29, whereas heterozygous, dominant negative mutations of the same gene are responsible for one form of Mendelian susceptibility to mycobacterial disease30. By contrast, heterozygous STAT1 GOF mutations were initially discovered to be the most frequent cause of chronic mucocutaneous candidiasis (CMC)31, 32. However, it has been subsequently demonstrated that the clinical spectrum of STAT1 GOF is broader. In a multicenter study of 274 patients, 98% had a history of CMC with a median age at onset of 1 year33. However, bacterial infections, mainly due to S. aureus and predominantly affecting the skin and the respiratory tract, were reported in 74% of the patients. Viral infections, especially due to Herpesviridae, and mycobacterial disease were observed in 38% and in 6% of the patients, respectively33. Fungal infections are not restricted to CMC, but may also include invasive candidiasis33, mucormycosis34, aspergillosis35, coccidioidomycosis, and histoplamosis36. Autoimmune complications have been observed in more than one third of the patients, and may even represent the predominant clinical phenotype35. Hypothyroidism, type 1 diabetes, cytopenias, and systemic lupus erythemathosus are more frequent, but inflammatory bowel disease, arthritis, and multiple sclerosis have been also reported33. Cerebral aneurysms and malignancies (especially squamous cell carcinoma) have been reported in less than 10% of the patients, but their occurrence represents a major risk factor of poor outcome33.
The disease is caused by heterozygous missense mutations that affect predominantly the coiled-coil and the DNA-binding domains of the protein31, 33, and cause increased and/or persistent STAT1 phosphorylation upon in vitro stimulation with appropriate cytokines (IFN-α, IFN-γ, IL-27)35, 37. Unbalances in the levels of STAT3 and STAT1 phosphorylation have been implied to play a key role in the pathophysiology of the disease. In particular, IL-6, IL-21 and IL-23 induce IL-17 producing cells via a STAT3-dependent mechanism, which is antagonized by STAT1. The proportion of IL-17 and IL-22-producing T cells is markedly decreased in patients with STAT1 GOF mutations31, 35, and this defect is associated with reduced transcription of STAT3-inducible genes38. By contrast, the number of TH1 cells is increased39. Finally, patients with STAT1 GOF mutations often have a reduced number of memory B cells, and their circulating T follicular helper (TFH) cells from patients with STAT1 GOF mutations have a skewed phenotype, with reduced proportion of CCR6+ cells (representing proficient B-helper TFH cells), and overexpression of IFN-γ and of programmed death 1 (PD1) protein39. Since PDL-1 is aberrantly expressed on the surface of CD4+ cells in these patients40, it has been speculated that PD1-PDL-1 interaction might promote an autocrine mechanism that constrains TFH function39. From a pathophysiology standpoint, the defect of TH17 cells in patients with STAT1 GOF mutations is presumably responsible for their CMC phenotype. On the other hand, the mechanisms accounting for the autoimmunity that is frequently observed in this condition remain poorly defined, although it is possible that enhanced response to type I IFN may play a role.
Treatment includes long-term systemic anti-fungal agents, but many patients develop resistance to azole group of antifungals, requiring second or third line treatments. Anti-bacterial and anti-viral medications are often needed, depending on the type of infections. Patients with autoimmune disease require immunosuppressive medications. In spite of this, clinical outcome is poor. Mortality rate by 60 years of age is 13% in patients without invasive infection, cancer, and/or symptomatic aneurysms, but is as high as 69% in those with one or more of these complications33. HSCT has been attempted in a small number of patients with complicated disease, with controversial results33, 41.
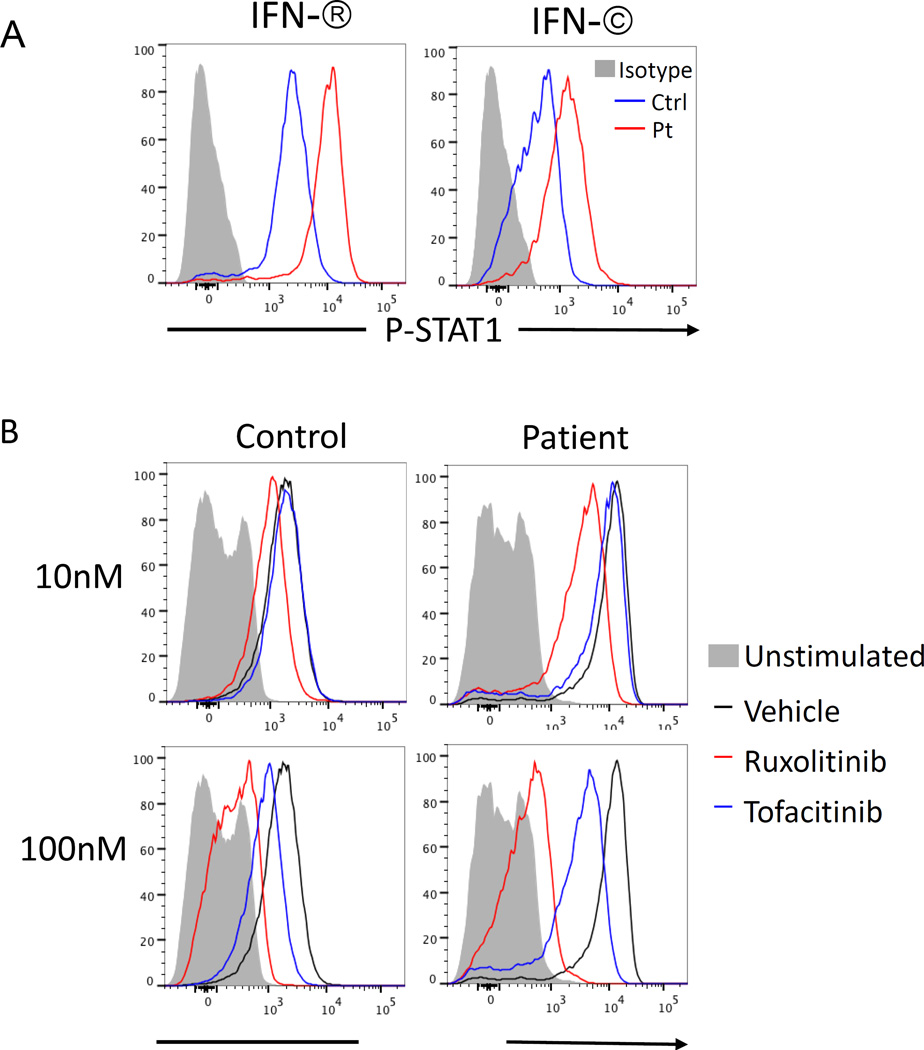
Recognition that STAT1 phosphorylation is controlled by JAKs, has prompted novel therapeutic perspectives based on the use of JAK inhibitors (also known as Jakinibs). This new class of drugs includes pan-JAK inhibitors (such as to tofacitinib), more selective JAK inhibitors, such as ruxolitinib and baricitinib (both of which are more directed at JAK1/JAK2 inhibition), and highly selective inhibitors, such as filgotinib (a JAK1 inhibitor) and decernotinib (a JAK3 inhibitor)42, 43. The choice of individual Jakinibs depends on the underlying nature of the disease and on the side effects that are anticipated. In the case of STAT1 GOF mutation, therapeutic efficacy of ruxolitinib has been reported in a young woman with alopecia and CMC, allowing resolution of the candidiasis and re-growth of hair44. In another child, ruxolitinib allowed resolution of severe Evans syndrome and demonstrated ex vivo activity inhibiting the increased level of STAT1 phosphorylation (Figure 3)43. In this patient, clinical improvement was also associated with normalization of the proportion of TH1, TH17, and TFH cells45.
Figure 3.
A) Phospho-STAT1 expression in CD4+ T cells stimulated with IFN-β (20 ng/mL) and IFN-γ (20 ng/mL) in a patient with STAT1 GOF mutation and in a healthy control. B) Phospho-STAT1 expression upon IFN-β (20ng/mL) stimulation in CD4+ T cells from control and STAT1 (E545K) patient pretreated for 4 hours with 10nM and 100nM concentrations of ruxolitinib (red line histogram) and tofacitinib (blue line histogram) or vehicle (DMSO, black lie histogram). Gray solid histogram corresponds to unstimulated cells (from ref. 43).
STAT3 GOF mutations
Heterozygous STAT3 GOF mutations were initially identified in individuals with early-onset (diagnosis at <5 years) polyautoimmunity or with isolated type 1 diabetes diagnosed in the first six months of life46. The immunological phenotype of these patients included a low number of Treg cells and skewing of CD4+ T cells to TH1 phenotype. The STAT3 mutations affected highly conserved residues within the SH2, transactivation, or DNA-binding domains of STAT3. In a luciferase reporter system, transfection of mutant STAT3 into HEK293T cells under non stimulated conditions resulted in increased activity as compared to transfection of wild-type STAT346. Shortly thereafter, several other patients with STAT3 GOF mutations were identified, whose clinical phenotype was not restricted to autoimmunity, but also included lymphoproliferation, severe and/or recurrent infections (due to viruses, bacteria, mycobacteria, or fungi) and postnatal short stature47–49. Moreover, one of the patients developed large granular lymphocytic (LGL) leukemia47, a condition that is frequently associated with somatic STAT3 GOF mutations50. Importantly, some family members with STAT3 GOF mutations presented with a milder clinical phenotype or were asymptomatic, suggesting incomplete penetrance48.
A more detailed analysis of the immunological phenotype of patients with germline STAT3 GOF mutations revealed that these patients also manifest hypogammaglobulinemia with terminal B cell maturation arrest, a variable degree of T cell lymphopenia, increased proportion of double negative TCRαβ+ T cells, and decrease number of circulating dendritic cells, eosinophils, TH17 cells, and NK cells47, 48. Biochemical studies in these larger cohorts of patients demonstrated that the STAT3 GOF mutations lead to enhanced transcriptional activity and delayed kinetics of STAT3 dephosphorylation, but not constitutive phosphorylation of STAT348. On the other hand, STAT1 and STAT5 phosphorylation are impaired48, a phenomenon that may reflect negative regulation by suppressor of cytokine signaling 3 (SOCS3, a known STAT3 target)51. Reduced STAT5 phosphorylation may account for the postnatal growth failure observed in this condition.
Overall, the clinical and laboratory phenotype of patients with STAT3 GOF mutations is distinct from that associated with dominant negative STAT3 mutations causing the autosomal dominant hyper-IgE syndrome52. Distinct missense mutations at the same position of the STAT3 protein may cause either loss or gain of function53, indicating it is the nature of the amino acid change, and not the location, that determines distinctive biochemical consequences and ultimately affects the clinical and immunological phenotype.
The profound immune dysregulation associated with STAT3 GOF mutations is consistent with the known role of STAT3 signaling in promoting inflammation and TH17 cell differentiation, and inhibiting Treg cells54, 55. Treatment of the disease is mostly based on use of immunosuppressive agents. IL-6 is one of the major cytokines that signals through STAT3. In one patient with STAT3 GOF mutation who manifested severe arthritis and scleroderma-like disease that were refractory to multiple immunosuppressive agents, treatment with tocilizumab (an anti-IL6R monoclonal antibody) led to significant clinical improvement. The proportion of TH17 cells, that was initially elevated, also normalized with treatment48. Whether a similar approach may be beneficial in other patients with STAT3 GOF mutations remains to be assessed. Finally, HSCT has been attempted in two patients with refractory autoimmunity, one of whom died with disseminated adenovirus infection post-transplant, while the other was reported to be alive and in remission48.
WHIM syndrome
Warts, Hypogammaglobulinemia, Infections, Myelokathexis (WHIM) syndrome is a rare autosomal dominant immunodeficiency due to heterozygous mutations at the carboxy-terminus region of the chemokine receptor CXCR456. The mutations disrupt negative regulatory elements, thereby causing exaggerated cellular responses to CXCL12, the natural ligand of CXCR457. CXCL12/CXCR4 interaction promotes homing and retention of hematopoietic stem cells (HSCs) and neutrophils in the bone marrow58, and regulates trafficking of lymphocytes in the bone marrow and in lymphoid organs59. Accordingly, WHIM is characterized by severe neutropenia and lymphopenia, with accumulation of mature neutrophils with hyper-segmented nuclei in the bone marrow (myelokathexis)60. Some increase of the absolute neutrophil count may be observed during acute infections. In addition, patients have a reduced number of memory B cells, whereas circulating T cells have a predominant effector memory phenotype with a restricted T cell repertoire61.
Clinical manifestations of the disease include recurrent bacterial infections and cutaneous HPV infection (warts) that may evolve into carcinoma. There is an increased risk of EBV-associated lymphoproliferative disease60. Administration of human recombinant granulocyte-colony stimulating factor (hrG-CSF) may increase the neutrophil count, and immunoglobulin replacement therapy may reduce the risk and severity of infections. The potential benefit of recombinant human papillomavirus vaccine in preventing warts and reducing the risk of cancer has not been formally tested. Chromothripsis (extensive genomic rearrangements) involving the mutated CXCR4 allele on chromosome 2 resulting in CXCR4 haploinsufficiency led to spontaneous resolution of the disease in a female patient with WHIM62, suggesting that disruption of the mutant allele by gene editing may represent an interesting therapeutic approach.
Plerixafor (AMD3100) is a small molecule inhibitor of the binding of CXCR4 to CXCL1263, and is widely used to mobilize HSCs in preparation of donors for HSCT and patients for gene therapy64. It also represents an attractive therapeutic agent to counterbalance the increased cellular response to CXCL12 that characterizes WHIM syndrome. After an initial study had shown that single subcutaneous doses of plerixafor Plerixafor administered at 2–4 day intervals resulted in an increase of neutrophil and lymphocyte count in 6 patients with WHIM65, a phase 1, open-label, dose-escalation clinical trial was conducted, in which 3 adult pan-leukopenic WHIM patients received subcutaneous injections of plerixafor twice daily for 6 months66, 67. A durable increase of leukocyte count was observed, associated with fewer infections; however, no changes of immunoglobulin levels and antibody responses were detected67. More recently, a cross-over, double blind study comparing plerixafor versus hrG-CSF for preventing infections in patients with WHIM has been started at the National Institutes of Health (https://clinicaltrials.gov/ct2/show/NCT02231879?term=WHIM&rank=1).
Discussion
The identification of an ever increasing number of PIDs associated with specific genetic defects in which the clinical phenotype includes both increased susceptibility to infections as well as immune dysregulation have proven to be therapeutic challenges. The link between these various genetic changes and very specific functional abnormalities has opened the pathway to the potential for applying recently developed biologics and small molecule modulators to directly target the disease causing molecular mechanism. This approach represents one of the central components of precision medicine and although the number of PID patients treated in this manner currently is small, with ongoing clinical trials and focused disease registries the information gathered will help fine tune therapeutic approaches for a variety of immunologic disorders. These new therapeutic approaches serve as a testament to the power of defining diseases based on genetic defects together with the associated functional abnormalities. The latter based on ex vivo testing provides a platform to evaluate the potential effectiveness of specific therapeutic agents and has already been applied to patients with APDS/PASLI, CTLA4 haploinsufficiency, STAT1 GOF and STAT3 GOF disorders. What remains to be defined is whether a specifically directed biological agent can remedy the wide range of symptoms associated with these complex disorders. It is very possible that these new therapeutic agents will prove useful in controlling disease associated immune dysregulation or immunodeficiency but not both. Alternatively, it is also a possibility that efficacy could even be more narrow addressing a limited number of disease complications. This is particularly true in the more recently described immunologic disorders where expressivity and penetrance vary widely even among family members with the same specific mutation. These observations suggest that there are likely other genetic, epigenetic and/or environmental factors that contribute to the clinical disease phenotype. Regardless of the outcome of current and future clinical trials, it is a particularly exciting time in the management of PIDs with the potential to provide very specifically directed therapy based on the underlying genetic defect for an individual patient. This approach not only has the real opportunity to benefit PID patients but it may also increase understanding of the immunopathogenesis of a variety of PIDs. The field has already moved forward at a rapid rate with regard to identifying new genetic causes for immunodeficiency and immune dysregulation disorders opening up the real potential for the development of a host of new therapies that are specifically tailored to manage the unique features of an individual patient’s disorder. As has been the case for a number of therapeutic advances in human disease management that include HSCT and gene therapy, PIDs represent a unique group of disorders that have been and will continue to be in the forefront of defining new and targeted immunomodulatory therapies and help define unique therapeutic approaches in the evolution of precision medicine.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases and the NIH Clinical Center
Abbreviations
- APDS
Activated PI(3)K Delta Syndrome
- CMC
chronic mucocutaneous candidiasis
- CTLA4
Cytotoxic Lymphocyte Antigen 4
- GOF
gain of function
- HSCT
hematopoietic stem cell transplantation
- hrG-CSF
human recombinant granulocyte colony stimulating factor
- IPEX
Immune Dysregulation, Polyendocrinopathy X-linked
- JAK
Janus-associated kinase
- Jakinib
JAK inhibitors
- LAD
leukocyte adhesion deficiency
- LGL
large granular lymphocyte
- LOF
loss of function
- LRBA
Lipolysaccharide-Responsive and Beige-like Anchor
- PASLI
p110 delta activating mutation causing the accumulation of senescent T cells, lymphadenopathy, immunodeficiency
- PI(3)K
Phosphoinositide 3 kinase
- SOCS
suppressor of cytokine secretion
- STAT
signal transducer and activator of transcription
- TCR
T cell receptor
- TFH
T follicular helper
- TH
T helper
- WHIM
Warts, Hypogammaglobulinemia, Infections, Myelokathexis
References
- 1.Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, et al. Primary Immunodeficiency Diseases: an Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015;35:696–726. doi: 10.1007/s10875-015-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, et al. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–871. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15:88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elgizouli M, Lowe DM, Speckmann C, Schubert D, Hulsdunker J, Eskandarian Z, et al. Activating PI3Kdelta mutations in a cohort of 669 patients with primary immunodeficiency. Clin Exp Immunol. 2016;183:221–229. doi: 10.1111/cei.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deau MC, Heurtier L, Frange P, Suarez F, Bole-Feysot C, Nitschke P, et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Invest. 2014;124:3923–3928. doi: 10.1172/JCI75746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas CL, Zhang Y, Venida A, Wang Y, Hughes J, McElwee J, et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J Exp Med. 2014;211:2537–2547. doi: 10.1084/jem.20141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coulter TI, Chandra A, Bacon CM, Babar J, Curtis J, Screaton N, et al. Clinical spectrum and features of activated phosphoinositide 3-kinase delta syndrome: A large patient cohort study. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkaim E, Neven B, Bruneau J, Mitsui-Sekinaka K, Stanislas A, Heurtier L, et al. Clinical and immunologic phenotype associated with activated phosphoinositide 3-kinase delta syndrome 2: A cohort study. J Allergy Clin Immunol. 2016;138:210.e9–218.e9. doi: 10.1016/j.jaci.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Nademi Z, Slatter MA, Dvorak CC, Neven B, Fischer A, Suarez F, et al. Hematopoietic Stem Cell Transplant in Patients with Activated PI3K Delta Syndrome. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 10.Rao VKCA, Su H, et al. Successful clinical study of Leniolisib (CDZ173), a small molecule PI3K-delta inhibitor in patients with APDS/PASLI. ESID 17th Biennial Meeting; Barcelona, Spain. 2016. [Google Scholar]
- 11.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrero-Beaumont G, Martinez Calatrava MJ, Castaneda S. Abatacept mechanism of action: concordance with its clinical profile. Reumatol Clin. 2012;8:78–83. doi: 10.1016/j.reuma.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20:1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Moon JS, Lee CR, Kim HE, Baek SM, Hwang S, et al. Abatacept alleviates severe autoimmune symptoms in a patient carrying a de novo variant in CTLA-4. J Allergy Clin Immunol. 2016;137:327–330. doi: 10.1016/j.jaci.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Navarini AA, Hruz P, Berger CT, Hou TZ, Schwab C, Gabrysch A, et al. Vedolizumab as a successful treatment of CTLA-4-associated autoimmune enterocolitis. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.08.042. [DOI] [PubMed] [Google Scholar]
- 17.Slatter MA, Engelhardt KR, Burroughs LM, Arkwright PD, Nademi Z, Skoda-Smith S, et al. Hematopoietic stem cell transplantation for CTLA4 deficiency. J Allergy Clin Immunol. 2016;138:615.e1–619.e1. doi: 10.1016/j.jaci.2016.01.045. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Herrera G, Tampella G, Pan-Hammarstrom Q, Herholz P, Trujillo-Vargas CM, Phadwal K, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012;90:986–1001. doi: 10.1016/j.ajhg.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamez-Diaz L, August D, Stepensky P, Revel-Vilk S, Seidel MG, Noriko M, et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol. 2016;137:223–230. doi: 10.1016/j.jaci.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Levy E, Stolzenberg MC, Bruneau J, Breton S, Neven B, Sauvion S, et al. LRBA deficiency with autoimmunity and early onset chronic erosive polyarthritis. Clin Immunol. 2016;168:88–93. doi: 10.1016/j.clim.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Alkhairy OK, Abolhassani H, Rezaei N, Fang M, Andersen KK, Chavoshzadeh Z, et al. Spectrum of Phenotypes Associated with Mutations in LRBA. J Clin Immunol. 2016;36:33–45. doi: 10.1007/s10875-015-0224-7. [DOI] [PubMed] [Google Scholar]
- 22.Charbonnier LM, Janssen E, Chou J, Ohsumi TK, Keles S, Hsu JT, et al. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immunol. 2015;135:217–227. doi: 10.1016/j.jaci.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Revel-Vilk S, Fischer U, Keller B, Nabhani S, Gamez-Diaz L, Rensing-Ehl A, et al. Autoimmune lymphoproliferative syndrome-like disease in patients with LRBA mutation. Clin Immunol. 2015;159:84–92. doi: 10.1016/j.clim.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Lo B, Zhang K, Lu W, Zheng L, Zhang Q, Kanellopoulou C, et al. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349:436–440. doi: 10.1126/science.aaa1663. [DOI] [PubMed] [Google Scholar]
- 25.Seidel MG, Hirschmugl T, Gamez-Diaz L, Schwinger W, Serwas N, Deutschmann A, et al. Long-term remission after allogeneic hematopoietic stem cell transplantation in LPS-responsive beige-like anchor (LRBA) deficiency. J Allergy Clin Immunol. 2015;135:1384–1390. e1–e8. doi: 10.1016/j.jaci.2014.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36:515–528. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz DM, Bonelli M, Gadina M, O'Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016;12:25–36. doi: 10.1038/nrrheum.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 30.Dupuis S, Dargemont C, Fieschi C, Thomassin N, Rosenzweig S, Harris J, et al. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science. 2001;293:300–303. doi: 10.1126/science.1061154. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 33.Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. 2016;127:3154–3164. doi: 10.1182/blood-2015-11-679902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar N, Hanks ME, Chandrasekaran P, Davis BC, Hsu AP, Van Wagoner NJ, et al. Gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation-related primary immunodeficiency is associated with disseminated mucormycosis. J Allergy Clin Immunol. 2014;134:236–239. doi: 10.1016/j.jaci.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uzel G, Sampaio EP, Lawrence MG, Hsu AP, Hackett M, Dorsey MJ, et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol. 2013;131:1611–1623. doi: 10.1016/j.jaci.2012.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sampaio EP, Hsu AP, Pechacek J, Bax HI, Dias DL, Paulson ML, et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol. 2013;131:1624–1634. doi: 10.1016/j.jaci.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Depner M, Fuchs S, Raabe J, Frede N, Glocker C, Doffinger R, et al. The Extended Clinical Phenotype of 26 Patients with Chronic Mucocutaneous Candidiasis due to Gain-of-Function Mutations in STAT1. J Clin Immunol. 2016;36:73–84. doi: 10.1007/s10875-015-0214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng J, van de Veerdonk FL, Crossland KL, Smeekens SP, Chan CM, Al Shehri T, et al. Gain-of-function STAT1 mutations impair STAT3 activity in patients with chronic mucocutaneous candidiasis (CMC) Eur J Immunol. 2015;45:2834–2846. doi: 10.1002/eji.201445344. [DOI] [PubMed] [Google Scholar]
- 39.Ma CS, Wong N, Rao G, Avery DT, Torpy J, Hambridge T, et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol. 2015;136:993.e1–1006.e1. doi: 10.1016/j.jaci.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romberg N, Morbach H, Lawrence MG, Kim S, Kang I, Holland SM, et al. Gain-of-function STAT1 mutations are associated with PD-L1 overexpression and a defect in B-cell survival. J Allergy Clin Immunol. 2013;131:1691–1693. doi: 10.1016/j.jaci.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aldave JC, Cachay E, Nunez L, Chunga A, Murillo S, Cypowyj S, et al. A 1-year-old girl with a gain-of-function STAT1 mutation treated with hematopoietic stem cell transplantation. J Clin Immunol. 2013;33:1273–1275. doi: 10.1007/s10875-013-9947-5. [DOI] [PubMed] [Google Scholar]
- 42.Hirahara K, Schwartz D, Gadina M, Kanno Y, O'Shea JJ. Targeting cytokine signaling in autoimmunity: back to the future and beyond. Curr Opin Immunol. 2016;43:89–97. doi: 10.1016/j.coi.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Roskoski R., Jr Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol Res. 2016;111:784–803. doi: 10.1016/j.phrs.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 44.Higgins E, Al Shehri T, McAleer MA, Conlon N, Feighery C, Lilic D, et al. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation. J Allergy Clin Immunol. 2015;135:551–553. doi: 10.1016/j.jaci.2014.12.1867. [DOI] [PubMed] [Google Scholar]
- 45.Weinacht KGCL-M, Alroqi F, Plant A, Qiao Q, Wu H, Ma C, Torgerson T, Rosenzweig SD, Fleisher TA, Notarangelo LD, Hanson IC, Forbes L, Chatila TA. Ruxolitinib reverses Dysregulated T Helper Cell Responses and controls Autoimmunity caused by a Novel STAT1 Gain of Function Mutation. Journal of Allergy and Clinical Immunology. 2016 doi: 10.1016/j.jaci.2016.11.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Lango Allen H, De Franco E, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014;46:812–814. doi: 10.1038/ng.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haapaniemi EM, Kaustio M, Rajala HL, van Adrichem AJ, Kainulainen L, Glumoff V, et al. Autoimmunity, hypogammaglobulinemia, lymphoproliferation, and mycobacterial disease in patients with activating mutations in STAT3. Blood. 2015;125:639–648. doi: 10.1182/blood-2014-04-570101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125:591–599. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haddad E. STAT3: too much may be worse than not enough! Blood. 2015;125:583–584. doi: 10.1182/blood-2014-11-610592. [DOI] [PubMed] [Google Scholar]
- 50.Koskela HL, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmaki H, Andersson EI, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366:1905–1913. doi: 10.1056/NEJMoa1114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer DC, Restifo NP. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 2009;30:592–602. doi: 10.1016/j.it.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeman AF, Holland SM. Clinical manifestations of hyper IgE syndromes. Dis Markers. 2010;29:123–130. doi: 10.3233/DMA-2010-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandrasekaran P, Zimmerman O, Paulson M, Sampaio EP, Freeman AF, Sowerwine KJ, et al. Distinct mutations at the same positions of STAT3 cause either loss or gain of function. J Allergy Clin Immunol. 2016;138:1222.e2–1224.e2. doi: 10.1016/j.jaci.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camporeale A, Poli V. IL-6, IL-17 and STAT3: a holy trinity in auto-immunity? Front Biosci (Landmark Ed) 2012;17:2306–2326. doi: 10.2741/4054. [DOI] [PubMed] [Google Scholar]
- 55.Kane A, Deenick EK, Ma CS, Cook MC, Uzel G, Tangye SG. STAT3 is a central regulator of lymphocyte differentiation and function. Curr Opin Immunol. 2014;28:49–57. doi: 10.1016/j.coi.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 56.Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70–74. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- 57.McCormick PJ, Segarra M, Gasperini P, Gulino AV, Tosato G. Impaired recruitment of Grk6 and beta-Arrestin 2 causes delayed internalization and desensitization of a WHIM syndrome-associated CXCR4 mutant receptor. PLoS One. 2009;4:e8102. doi: 10.1371/journal.pone.0008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 60.Kawai T, Malech HL. WHIM syndrome: congenital immune deficiency disease. Curr Opin Hematol. 2009;16:20–26. doi: 10.1097/MOH.0b013e32831ac557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gulino AV, Moratto D, Sozzani S, Cavadini P, Otero K, Tassone L, et al. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood. 2004;104:444–452. doi: 10.1182/blood-2003-10-3532. [DOI] [PubMed] [Google Scholar]
- 62.McDermott DH, Gao JL, Liu Q, Siwicki M, Martens C, Jacobs P, et al. Chromothriptic cure of WHIM syndrome. Cell. 2015;160:686–699. doi: 10.1016/j.cell.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDermott DH, Lopez J, Deng F, Liu Q, Ojode T, Chen H, et al. AMD3100 is a potent antagonist at CXCR4(R334X), a hyperfunctional mutant chemokine receptor and cause of WHIM syndrome. J Cell Mol Med. 2011;15:2071–2081. doi: 10.1111/j.1582-4934.2010.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bilgin YM, de Greef GE. Plerixafor for stem cell mobilization: the current status. Curr Opin Hematol. 2016;23:67–71. doi: 10.1097/MOH.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 65.Dale DC, Bolyard AA, Kelley ML, Westrup EC, Makaryan V, Aprikyan A, et al. The CXCR4 antagonist plerixafor is a potential therapy for myelokathexis, WHIM syndrome. Blood. 2011;118:4963–4966. doi: 10.1182/blood-2011-06-360586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDermott DH, Liu Q, Ulrick J, Kwatemaa N, Anaya-O'Brien S, Penzak SR, et al. The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood. 2011;118:4957–4962. doi: 10.1182/blood-2011-07-368084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDermott DH, Liu Q, Velez D, Lopez L, Anaya-O'Brien S, Ulrick J, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308–2316. doi: 10.1182/blood-2013-09-527226. [DOI] [PMC free article] [PubMed] [Google Scholar]