Abstract
Objective: To investigate the analgesic effects of electroacupuncture (EA) at 2 and 100 Hz on type 2 diabetic neuropathic pain (DNP) and on the expressions of the P2X3 receptor and calcitonin gene-related peptide (CGRP) in the dorsal root ganglion (DRG). Methods: Rat type 2 DNP was induced by a high calorie and high sugar diet fed for 7 weeks, plus a single intraperitoneal injection of streptozotocin (STZ) after 5 weeks. EA at 2 and 100 Hz was carried out once every day after 7 weeks for 7 consecutive days. Body weight, serum fasting insulin (FINS), fasting blood glucose (FBG), insulin sensitivity index (ISI), and paw withdrawal latency (PWL) were measured. The expressions of L4–L6 DRG P2X3 receptors and CGRP were assessed by immunofluorescence. Results: Type 2 DNP was successfully induced as shown by the increased body weight, FINS, and FBG, as well as the reduced ISI and PWL. Expressions of P2X3 receptors and CGRP in L4–L6 DRGs increased. EA at both 2 and 100 Hz relieved type 2 DNP, but the analgesic effect of EA was stronger at 2 Hz. P2X3 receptor expression decreased in L4–L6 DRGs following EA at 2 Hz and in L5 and L6 DRGs following EA at 100 Hz. EA at both 2 and 100 Hz down-regulated CGRP overexpression in L4–L6 DRGs. Conclusions: These findings indicate that EA at 2 Hz is a good option for the management of type 2 DNP. The EA effect may be related to its down-regulation of the overexpressions of the DRG P2X3 receptors and CGRP in this condition.
Keywords: Electroacupuncture, Type 2 diabetic neuropathic pain, Dorsal root ganglion, P2X3 receptor, Calcitonin gene-related peptide
1. Introduction
Diabetes mellitus (DM) is a consumptive disease that affects 8% of the global population, and type 2 DM (T2DM) accounts for probably 90% of cases (Alberti and Zimmet, 1998; Zimmet et al., 2001). Among T2DM patients, about one-half will suffer complications with a neuropathy during the course of the disease (Maser et al., 1989). Peripheral neuropathy is the most common complication, which may lead to diabetic neuropathic pain (DNP) characterized by aberrant pain sensations. Spontaneous pain, allodynia (pain with innocuous stimuli, e.g. a light touch), and hyperalgesia (severe pain with mild painful stimuli) are symptoms of peripheral neuropathy and afflict most T2DM patients with a neuropathy (Brown and Asbury, 1984; Dyck et al., 1993; Harati, 1996; Khan et al., 2002; Gooch and Podwall, 2004; Pabbidi et al., 2008). Treating painful DNP remains a significant clinical challenge, and current treatment options are very limited and only marginally effective.
Sensitization of dorsal root ganglion (DRG) neurons and their associated nerve fibers is considered to be a main cause of DNP (Khan et al., 2002; Jagodic et al., 2007). P2X receptors are cationic channels abundantly expressed in the DRG neurons, which participate in the progression and regulation of pain (Cook et al., 1997; Petruska et al., 2000; Chen and Gu, 2005). The receptors are selectively expressed mainly in the minor diameter DRG neurons in relation to nociception. Increasing evidence suggests that the expression and function of P2X3 receptors in DRGs are closely related to DNP sensitization (Migita et al., 2009; Xu et al., 2011). Calcitonin gene-related peptide (CGRP), a neuropeptide including 37 amino acids, is synthesized and released from sensory neurons and small sensory C and Aδ fibers (Bernardini et al., 2004; Price and Flores, 2007). This peptide is also thought to play a vital role in neuropathic pain sensitization (Jiang et al., 2013).
Electroacupuncture (EA) combines traditional acupuncture and modern electrotherapy, and has a good analgesic effect. Its stimulation can be altered objectively and quantitatively (Ishiko et al., 1978). EA at low or high frequencies can have distinct analgesic effects that may occur in specific neural pathways (Silva et al., 2011). Although EA has been shown to be effective in treating DNP (Manni et al., 2011), its optimal frequency has not yet been clarified. Furthermore, little is known about the mechanisms underlying EA analgesia of DNP. Given the high morbidity rate of T2DM and its DNP complication, it is essential to establish an optimal animal model of type 2 DNP to assess its treatment and explore possible mechanisms. A rat diabetic model induced by injection of a single large dose of streptozotocin (STZ) is commonly used to investigate DNP. However, the symptoms often seen in clinics are not accurately reflected by this model. In this study, a rat type 2 DNP model was induced by a period of high calorie and high sugar diet plus a single intraperitoneal injection of a small dose of STZ. The effects of EA at 2 or 100 Hz on type 2 DNP and the expressions of the DRG P2X3 receptors and CGRP were assessed to find the optimal EA frequency for type 2 DNP, and to explore differences in the analgesic mechanisms.
2. Materials and methods
2.1. Experimental rats
Male Sprague-Dawley rats (110–140 g body weight) purchased from SLAC Laboratory Animal Co. Ltd. (SCXK (hu) 2013-0016; Shanghai, China). They were housed at the Zhejiang Chinese Medical University (SYXK (zhe) 2013-0184; Hangzhou, China) at five per cage and maintained in a light-and temperature-controlled room (a 12-h light/dark cycle, (24±2) °C). All rats had feedstuff and drank water freely.
The rats were acclimatized to the housing facilities for 3 d before the start of the experiment. All animals were kept according to the regulations of the State Science and Technology Commission for the care and use of experimental animals (State Science and Technology Commission Order No. 2,1988).
2.2. Induction of type 2 DNP model
After 3 d of adaptive feeding, 52 rats were each randomly assigned to one of two groups: a normal group (n=8) or a model group (n=44). Rats in the normal group were fed a normal diet. Those in the model group were fed a high calorie and high sugar diet composed of 72.5% (mass fraction, all the same below) normal diet plus 10.0% lard, 10.0% sucrose, 2.0% cholesterol, 0.5% sodium cholate, and 5.0% yolk powder (Dang et al., 2014). After 5 weeks, rats with a reduced insulin sensitivity index (ISI) in the model group were given a single intraperitoneal injection of STZ (35 mg/kg, Sigma, USA). Rats in the normal group were injected with the same dose of citrate buffer. Rats in the model group with a fasting blood glucose (FBG) level of ≥11.1 mmol/L and paw withdrawal latency (PWL) of ≤85% of the base value after 7 weeks were adopted as type 2 DNP rats (n=26) (Brussee et al., 2008; Lin and Sun, 2010). These rats were then randomly assigned to a DNP group (n=8), DNP+2 Hz EA group (n=9), or DNP+100 Hz EA group (n=9). They were continuously fed with a high calorie and high sugar diet until the end of the experiment.
2.3. Body weight and FBG measurement
Rats were weighed at the start of the experiment and after 5 and 7 weeks. After body weight was measured, blood samples were obtained from the tail vein. FBG was detected using a compact glucometer (Roche, China).
2.4. ELISA for serum fasting insulin and ISI calculations
After fasting for 12 h and drinking water freely, 1 ml of blood was obtained from the orbital venous system. The blood samples were stored at room temperature for 30 min, and then centrifuged at 3500 r/min for 10 min at 4 °C to obtain serum. Serum fasting insulin (FINS) was measured using a rat insulin enzyme-linked immunosorbent assay (ELISA) kit (Cayman Chemical, USA) according to the manufacturer’s directions. The ISI was then calculated using the following formula: ISI=1/(fasting glucose concentration×FINS concentration) (Bai et al., 2015). This value was calculated using the natural logarithm because the data were not normally distributed (Muniyappa et al., 2008). Both FINS and ISI were assessed at the start of the experiment and after 5 and 7 weeks.
2.5. Measurement of PWL
All tests were performed by an experimenter blinded to the treatment groups. To evaluate thermal hyperalgesia, PWL was measured by a plantar tester (Ugo Basile 37370, Italy) as in our previous studies (Fang et al., 2013b; Jiang et al., 2015). After acclimatizing the rats for 30 min in a clear plastic chamber, a stimulus of radiant heat (high-intensity projector lamp bulb) was placed under the glass floor directly beneath the hind paws. The stimulus was stopped automatically and the time recorded as the PWL when the rat withdrew its hind paw. To prevent hind paw injury, a 20-s cut-off was set. The response of each animal was measured three times with an interval of 5 min between tests, and the average PWL was calculated: PWL=(PWL of left hind paw+PWL of right hind paw)/2. The PWL was measured at the start of the experiment, at 5 and 7 weeks, at 7 weeks plus 3 and 5 d, and at 8 weeks.
2.6. EA intervention
The acupoints Zusanli (ST 36) and Kunlun (BL 60) were selected for EA intervention. As the analgesic effects of these two acupoints are well documented in different types of pain models, sham acupuncture or acupuncture at other acupoints was not carried out as a control in this study (Fang et al., 2013a; Jiang et al., 2013). We focused on the effects of EA at different frequencies on DNP and the expressions of the DRG P2X3 receptor and CGRP, but not on the specificity of the acupoints. Stainless steel acupuncture needles (Huawei, China; 0.25 mm in diameter) were inserted to a depth of 5 mm. Rats were administered EA at 2 Hz in the DNP+2 Hz EA group, and at 100 Hz in the DNP+100 Hz EA group. In both groups, currents ranging from 1 to 2 mA (15 min each, total 30 min) were applied. EA was administered once daily for 7 consecutive days after 7 weeks. Animals were awake and were kept calm by placing their heads in black hoods without physical constraint during EA treatment. Rats in the normal and DNP groups were subjected to the same calming procedure.
2.7. Immunofluorescence
Rats were deeply anesthetized by an intraperitoneal injection of 10% (0.1 g/ml) chloral hydrate (3.5 ml/kg) and perfused with 0.9% (9 g/L) NaCl solution (4 °C) followed by 4% (v/v) paraformaldehyde in 0.1 mol/L phosphate-buffered saline (PBS; pH 7.4). Bilateral L4, L5, and L6 DRGs were dissected out immediately, postfixed in the same fixative for 3 h, and then consecutively immersed in 15% (0.15 g/ml) and 30% (0.3 g/ml) sucrose solutions overnight at 4 °C. Tissues were embedded in optimal cutting temperature (OCT), frozen, and then cut into 14-μ m sections. Sections were mounted on glass slides, rinsed in Tris-buffered saline with Tween 20 (TBST; pH 7.4), blocked in 10% (0.1 g/ml) goat serum with 0.3% TritonX-100 for 1 h at 37 °C, and incubated with the primary antibody, rabbit anti-rat P2X3 receptor (1:2000; Abcam) or mouse anti-rat CGRP (1:600; Abcam) overnight at 4 °C. After being washed in TBST (pH 7.4), sections were incubated with the secondary antibodies Alexa Fluor488-conjugated goat anti-rabbit IgG (1:800; Jackson) or Cy3-AffiniPure goat anti-mouse IgG (H+L) (1:800; Jackson) for 1 h at 4 °C. Sections were then washed and a coverslip applied with mounting medium. Sections from different groups were processed together in the same batches to minimize the effects of staining variability between batches. Images were obtained using a fluorescence microscope. Immunoreactivity was quantified using Image-Pro Plus 6.0 software (Thermo) under blinded conditions. The ratio of positive to negative immunoreactivity was determined as the percentage of positive DRG neurons among the total DRG neurons. Five non-consecutive sections were calculated for the average of each rat, and three rats were analyzed for each group.
2.8. Statistical analysis
The data are represented by the mean±standard error of the mean (SEM) and were analyzed using SPSS 22.0 software. Independent-sample t-tests were used for comparisons between two groups, and one-way analysis of variance (ANOVA) for comparisons among groups. A P-value of <0.05 was considered statistically significant.
3. Results
3.1. Induction of type 2 DNP
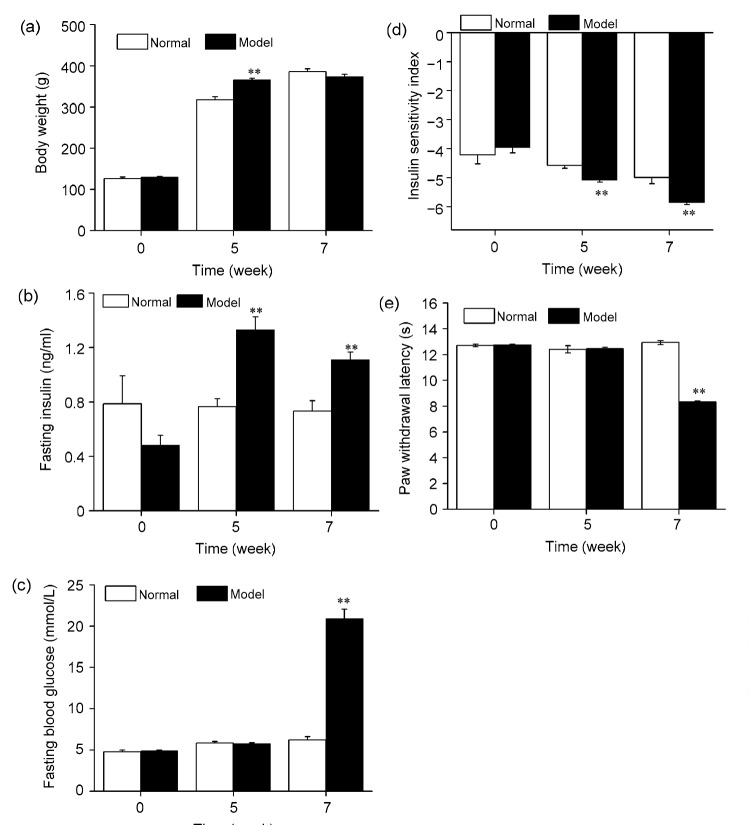
Compared with the normal group, the body weight and FINS of rats in the model group increased significantly after feeding on a high calorie and high sugar diet for 5 weeks (P<0.01; Figs. 1a and 1b). Insulin resistance was also found in these rats, as shown by the reduced ISI (P<0.01; Fig. 1d). After a single intraperitoneal injection of a small dose (35 mg/kg) of STZ at 5 weeks plus 2 weeks of the high calorie and high sugar diet, significant increases in FINS and FBG, and decreases in ISI and PWL were found (P<0.01; Figs. 1b–1e). In the model group, 26 of the 44 rats developed type 2 DNP with FBG ≥11.1 mmol/L and PWL ≤85% of the base value.
Fig. 1.
Changes in body weight (a), fasting insulin (b), fasting blood glucose (c), insulin sensitivity index (d), and paw withdrawal latency (e) of rats subjected to a high calorie and high sugar diet for 7 weeks and a single intraperitoneal injection of a small dose (35 mg/kg) of STZ at 5 weeks
Data are presented as mean±SEM (n=8 in the normal group and n=44 in the model group). The data were analyzed using an independent-sample t-test. ** P<0.01, compared with the normal group
3.2. Effects of EA at 2 or 100 Hz on DNP-induced thermal hyperalgesia
DNP rats developed thermal hyperalgesia, as shown by the drastic reduction in PWL from 7 weeks to the end of the experiment (Fig. 2). Both 2 and 100 Hz EA significantly increased the PWLs of the DNP rats (P<0.01). EA was more potent at 2 Hz than at 100 Hz in increasing the PWLs of the DNP rats (P<0.01).
Fig. 2.
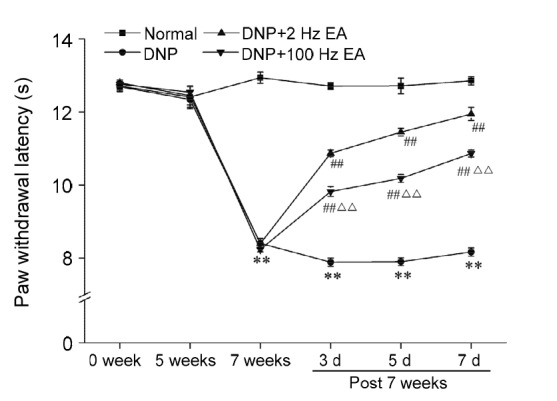
Effects of electroacupuncture (EA) at different frequencies on the paw withdrawal latency of diabetic neuropathic pain (DNP) rats
Data are presented as mean±SEM (n=8 or 9 rats per group). The data were analyzed using ANOVA. ** P<0.01, compared with the normal group; ## P<0.01, compared with the DNP group; ∆∆ P<0.01, compared with the DNP+2 Hz EA group
3.3. Effects of EA at 2 or 100 Hz on the DRG P2X3 receptors of DNP rats
Representative L4, L5, and L6 DRG sections from rats in the normal, DNP, DNP+2 Hz EA, and DNP+100 Hz EA groups are shown in Fig. 3a. Immunofluorescence visualization showed that P2X3 receptor-immunoreactive (IR) neurons in the DRG were mostly small to medium in size (20–50 μ m). The expressions of P2X3 receptor-IR neurons in L4, L5, and L6 DRGs of DNP rats had obviously increased compared to those in the normal group (P<0.01; Fig. 3b). The increased P2X3 receptor-IR expression in L4, L5, and L6 DRGs of DNP rats was significantly reduced by 2 Hz EA (P<0.01). EA at 100 Hz significantly down-regulated the increased P2X3 receptor-IR expression in L5 and L6 DRGs (P<0.05). P2X3 receptor-IR expression in L4 DRG of the DNP+100 Hz EA group was obviously higher than that of the DNP+2 Hz EA group (P<0.05).
Fig. 3.
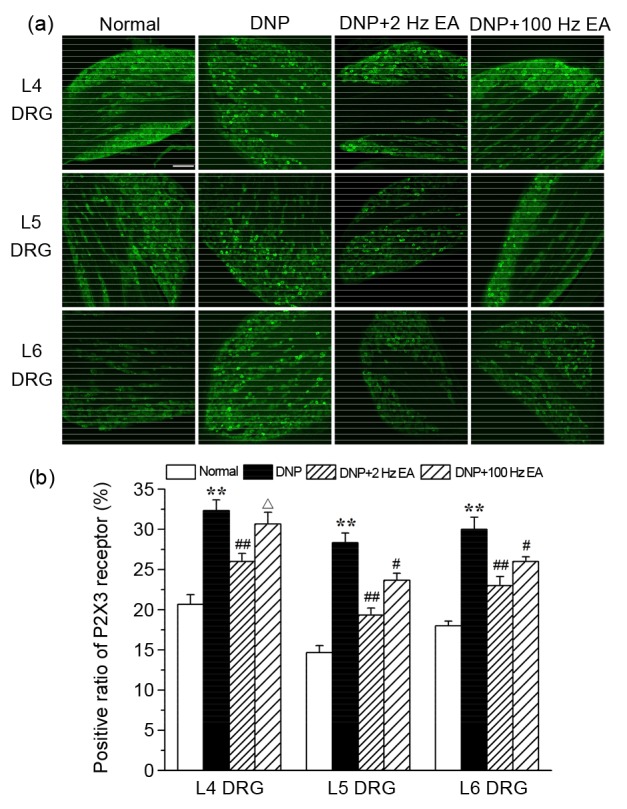
Effects of electroacupuncture (EA) at different frequencies on dorsal root ganglion (DRG) P2X3 receptors of diabetic neuropathic pain (DNP) rats
(a) Representative bright-field micrographs showing P2X3 receptor-immunoreactive (IR) neurons in L4, L5, and L6 DRGs of rats in the normal, DNP, DNP+2 Hz EA, and DNP+100 Hz EA groups. Scale bar=100 μ m. (b) Statistical analyses of L4, L5, and L6 DRG P2X3 receptor-IR neurons. Five non-consecutive sections were analyzed to obtain the average for each rat, and three rats were analyzed for each group. Data are presented as mean±SEM. The data were analyzed using ANOVA. ** P<0.01, compared with the normal group; # P<0.05, ## P<0.01, compared with the DNP group; ∆ P<0.05, compared with the DNP+2 Hz EA group
3.4. Effects of EA at 2 and 100 Hz on DRG CGRP of DNP rats
Representative L4, L5, and L6 DRG sections from rats in the normal, DNP, DNP+2 Hz EA, and DNP+100 Hz EA groups are shown in Fig. 4a. The expressions of CGRP-IR neurons in the L4, L5, and L6 DRGs of DNP rats had clearly increased compared to those in the normal group (P<0.01; Fig. 4b). The increased expressions of CGRP-IR neurons in L4, L5, and L6 DRGs were markedly reduced by EA at 2 and 100 Hz (P<0.01, P<0.01, and P<0.05, respectively).
Fig. 4.
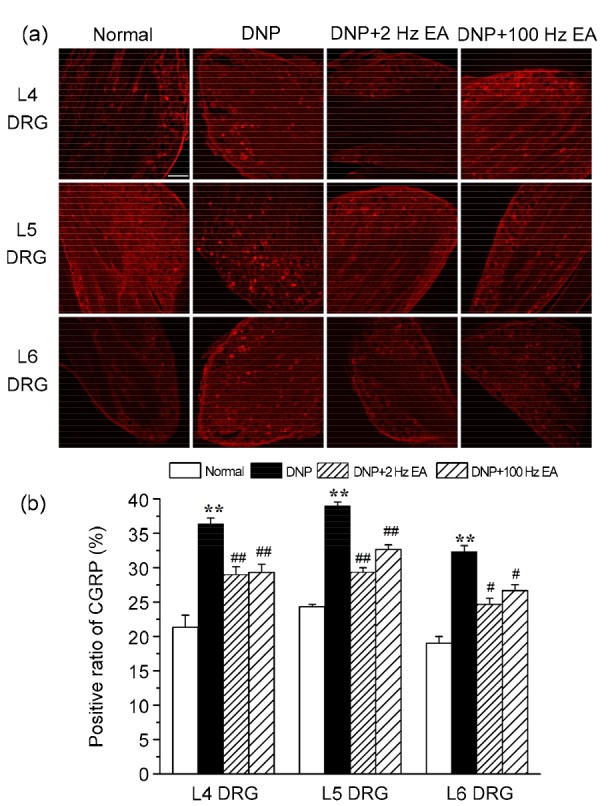
Effects of electroacupuncture (EA) at different frequencies on calcitonin gene-related peptide (CGRP) in dorsal root ganglion (DRG) of diabetic neuropathic pain (DNP) rats
(a) Representative bright-field micrographs showing CGRP-immunoreactive (IR) neurons in L4, L5, and L6 DRGs of rats in the normal, DNP, DNP+2 Hz EA, and DNP+100 Hz EA groups. Scale bar=100 μ m. (b) Statistical analyses of L4, L5, and L6 DRG CGRP-IR neurons. Five non-consecutive sections were analyzed to obtain the average for each rat, and three rats were analyzed for each group. Data are presented as mean±SEM. The data were analyzed using ANOVA. ** P<0.01, compared with the normal group; # P<0.05, ## P<0.01, compared with DNP group
4. Discussion
4.1. Type 2 DNP model
The world health organization (WHO) estimated that the number of people with diabetes will increase to 360 million by 2030. More than 90% of these patients will have T2DM, and half of those will suffer the complication of DNP (Wild et al., 2004). Currently, studies on DNP have widely adopted the rat diabetic model established by injection of a large dose of STZ due to the easy induction of hyperglycemia as a result of the destruction of pancreatic β cells (Hong et al., 2004). However, this type of model is not well suited to studies of T2DM and its complications (Hong et al., 2004; Sharma et al., 2006), including characteristic weight loss and insulin deficiency (Jiang et al., 2011). It has been reported that a T2DM model can be established in rodents by feeding high calorie and high sugar foods for a long time, but this model is not suitable for study because it is time-consuming (Weisberg et al., 2008; El-Moselhy et al., 2011). In our study, we combined a high calorie and high sugar diet with injection of a small dose of STZ to induce a type 2 DNP model. We found rat body weight and FINS concentration increased significantly, and ISI decreased significantly after the rats were fed the high calorie and high sugar diet for 5 weeks. This may have resulted from the inhibition of insulin molecular signaling pathways (Kahn, 1998). To compensate, pancreatic islets may have produced enough insulin to prevent the formation of insulin resistance, which may explain why the blood glucose level was not elevated after 5 weeks (Hayden et al., 2005). After inducing insulin resistance, STZ was injected intraperitoneally to destroy the β cells of the islet, weakening the compensatory ability of the pancreas and leading to increased FBG levels. This accurately mimics the development of human T2DM (Muoio and Newgard, 2008). Hyperglycemia was found after 7 weeks, and was accompanied by the symptoms of weight gain, hyperinsulinemia, and insulin resistance, which are the most common characteristics of T2DM in patients (Surwit et al., 1988; Kobayashi et al., 2004; Winzell and Ahrén, 2004; Gallou-Kabani et al., 2007). The PWL of diabetic rats was also significantly reduced after 7 weeks, indicating the validity of our type 2 DNP model. Our diet formula and the induction procedure may be of great value for the establishment of experimental type 2 DNP models.
4.2. Effects of acupuncture at 2 or 100 Hz on type 2 DNP
Treating painful DNP remains a significant clinical challenge. In particular, current treatment options are very limited and only marginally effective. EA has become increasingly popular and is used in the treatment of patients with chronic diseases of the nervous system, including DNP (Nori et al., 2013). EA has been demonstrated as a therapy for managing DNP safely and effectively (Nori et al., 2013), although its mechanism of action remains unclear. The analgesic effect produced by EA depends on proper prescription of specific acupoints and electrical parameters. Studies have shown that EA at the “Zusanli” and “Kunlun” acupoints can effectively elicit analgesia in rats (Fang et al., 2013b; Jiang et al., 2013). The frequency of EA is another important factor affecting its analgesic effect. Different electrical frequencies may result in distinct EA analgesia. An expected analgesic effect was not produced at 0.4 Hz, whereas a considerable analgesic effect was induced by 4 or 200 Hz (Cheng and Pomeranz, 1979). Jiang et al. (2013) showed that EA at 2 Hz could effectively relieve spinal nerve ligation-induced neuropathic pain. In this study, we found that EA at either 2 or 100 Hz at the “Zusanli” and “Kunlun” acupoints had a significant analgesic effect on DNP, but the effect was more pronounced at 2 Hz, which is consistent with the results of Hwang et al. (2011).
4.3. Effects of acupuncture at 2 or 100 Hz on DRG P2X3 receptors and CGRP
Spontaneous pain, allodynia, and hyperalgesia symptoms usually afflict patients with DNP. Changes in the phenotype of primary sensory neurons following peripheral nerve injury contribute to allodynia and hyperalgesia in neuropathic pain (Costigan et al., 2009). The P2X3 receptor is a member of P2X family channels, which are ligand-gated cation channels that generate inward current evoked by adenosine triphosphate (ATP) (Inoue, 2006). Activation of P2X3 receptors in sensory neurons results in pain perception. Migita et al. (2009) showed that the P2X receptors in the DRG were responsible for DNP. In mice, Migita et al. (2009) found that the level of P2X3 receptor mRNA in DRGs increased following STZ-induced diabetes, while P2X receptor antagonists inhibited STZ-induced mechanical allodynia. In the present study, we also found that DNP resulted in thermal hyperalgesia and a significant increase in P2X3 receptor expression in L4, L5, and L6 DRGs. These findings suggest that up-regulation of the P2X3 receptor in primary sensory neurons is crucial to DNP. The enhanced P2X3 receptor expression in L4, L5, and L6 DRGs was significantly reduced by 2 Hz EA, and partially by 100 Hz EA. The reduction in P2X3 receptor expression in DRGs may be involved in the analgesia produced by 2 and 100 Hz EA in DNP rats. The much greater reduction of DRG P2X3 receptor expression at 2 Hz may have contributed to the stronger analgesia produced by that frequency.
CGRP is a proinflammatory neuropeptide implicated in a variety of painful conditions (Jimenez-Andrade et al., 2010; Raddant and Russo, 2011). Cady et al. (2011) showed that CGRP stimulation of P2X3, mitogen-activated protein (MAP) kinases, and cyclic adenosine monophosphate (cAMP)-dependent protein kinase (PKA) could lead to increased nociceptive responses to thermal, mechanical, and chemical stimuli. Simonetti et al. (2008) found that pain-inducing substances like CGRP may increase the expression and enhance the function of P2X3 receptors and support long-lasting neuronal sensitization. In the present study, increased levels of CGRP during DNP in L4, L5, and L6 DRGs were significantly reduced by EA at 2 and 100 Hz. Jiang et al. (2013) also showed that analgesia produced by EA was accompanied by a reduction of CGRP in sensory neurons during spinal nerve ligation-induced neuropathic pain. These findings indicate that down-regulation of DRG CGRP levels contributes to EA analgesia of DNP.
5. Conclusions
In summary, our study showed that type 2 DNP can result in up-regulations of P2X3 receptors and CGRP in DRGs. EA at 2 and 100 Hz could relieve type 2 DNP in our model. This effect may be associated with the ability of EA to down-regulate the expressions of DRG P2X3 receptors and CGRP. EA at 2 Hz was more effective in relieving type 2 DNP and reducing DRG P2X3 expression than EA at 100 Hz. These findings suggest that EA at 2 Hz is a good option for the management of type 2 DNP.
Acknowledgments
We would like to thank all the rats for giving their lives. We thank Qi-yang SHOU (Zhejiang Chinese Medical University, Hangzhou, China) for technical assistance.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81303039), the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20133322120001), the Zhejiang Postdoctoral Science Foundation (No. BSH1302083), the Zhejiang Province Top Key Discipline of Chinese Medicine-Acupuncture & Tuina (No. [2012]80), and the Key Science and Technology Innovation Team of Zhejiang Province (No. 2013TD15), China
Compliance with ethics guidelines: Xiao-fen HE, Jun-jun WEI, Sheng-yun SHOU, Jian-qiao FANG, and Yong-liang JIANG declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. (Available from: http://dx.doi.org/10.1002/(SICI)1096-9136(199807)15:7<) [DOI] [PubMed] [Google Scholar]
- 2.Bai J, Zheng S, Jiang D, et al. Oxidative stress contributes to abnormal glucose metabolism and insulin sensitivity in two hyperlipidemia models. Int J Clin Exp Pathol. 2015;8(10):13193–13200. [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardini N, Neuhuber W, Reeh PW, et al. Morphological evidence for functional capsaicin receptor expression and calcitonin gene-related peptide exocytosis in isolated peripheral nerve axons of the mouse. Neuroscience. 2004;126(3):585–590. doi: 10.1016/j.neuroscience.2004.03.017. (Available from: http://dx.doi.org/10.1016/j.neuroscience.2004.03.017) [DOI] [PubMed] [Google Scholar]
- 4.Brown MJ, Asbury AK. Diabetic neuropathy. Ann Neurol. 1984;15(1):2–12. doi: 10.1002/ana.410150103. (Available from: http://dx.doi.org/10.1002/ana.410150103) [DOI] [PubMed] [Google Scholar]
- 5.Brussee V, Guo G, Dong Y, et al. Distal degenerative sensory neuropathy in a long-term type 2 diabetes rat model. Diabetes. 2008;57(6):1664–1673. doi: 10.2337/db07-1737. (Available from: http://dx.doi.org/10.2337/db07-1737) [DOI] [PubMed] [Google Scholar]
- 6.Cady RJ, Glenn JR, Smith KM, et al. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011;7:94. doi: 10.1186/1744-8069-7-94. (Available from: http://dx.doi.org/10.1186/1744-8069-7-94) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Gu JG. A P2X receptor-mediated nociceptive afferent pathway to lamina I of the spinal cord. Mol Pain. 2005;1:4. doi: 10.1186/1744-8069-1-4. (Available from: http://dx.doi.org/10.1186/1744-8069-1-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng RS, Pomeranz B. Electroacupuncture analgesia could be mediated by at least two pain-relieving mechanisms; endorphin and non-endorphin systems. Life Sci. 1979;25(23):1957–1962. doi: 10.1016/0024-3205(79)90598-8. (Available from: http://dx.doi.org/10.1016/0024-3205(79)90598-8) [DOI] [PubMed] [Google Scholar]
- 9.Cook SP, Vulchanova L, Hargreaves KM, et al. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387(6632):505–508. doi: 10.1038/387505a0. (Available from: http://dx.doi.org/10.1038/387505a0) [DOI] [PubMed] [Google Scholar]
- 10.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. (Available from: http://dx.doi.org/10.1146/annurev.neuro.051508.135531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang JK, Wu Y, Cao H, et al. Establishment of a rat model of type II diabetic neuropathic pain. Pain Med. 2014;15(4):637–646. doi: 10.1111/pme.12387_1. (Available from: http://dx.doi.org/10.1111/pme.12387_1) [DOI] [PubMed] [Google Scholar]
- 12.Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817–824. doi: 10.1212/wnl.43.4.817. (Available from: http://dx.doi.org/10.1212/WNL.43.4.817) [DOI] [PubMed] [Google Scholar]
- 13.El-Moselhy MA, Taye A, Sharkawi SS, et al. The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-α and free fatty acids. Food Chem Toxicol. 2011;49(5):1129–1140. doi: 10.1016/j.fct.2011.02.004. (Available from: http://dx.doi.org/10.1016/j.fct.2011.02.004) [DOI] [PubMed] [Google Scholar]
- 14.Fang JQ, Du JY, Liang Y, et al. Intervention of electroacupuncture on spinal p38 MAPK/ATF-2/VR-1 pathway in treating inflammatory pain induced by CFA in rats. Mol Pain. 2013;9:13. doi: 10.1186/1744-8069-9-13. (Available from: http://dx.doi.org/10.1186/1744-8069-9-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang JQ, Jiang YL, Qiu SC, et al. Involvement of peripheral beta-endorphin and mu, delta, kappa opioid receptors in electro acupuncture analgesia for prolonged inflammatory pain of rats. Eur J Inflamm. 2013;11(2):375–383. (Available from: http://dx.doi.org/10.1177/1721727X1301100208) [Google Scholar]
- 16.Gallou-Kabani C, Vigé A, Gross MS, et al. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity. 2007;15(8):1996–2005. doi: 10.1038/oby.2007.238. (Available from: http://dx.doi.org/10.1038/oby.2007.238) [DOI] [PubMed] [Google Scholar]
- 17.Gooch C, Podwall D. The diabetic neuropathies. Neurologist. 2004;10(6):311–322. doi: 10.1097/01.nrl.0000144733.61110.25. (Available from: http://dx.doi.org/10.1097/01.nrl.0000144733.61110.25) [DOI] [PubMed] [Google Scholar]
- 18.Harati Y. Diabetes and the nervous system. Endocrinol Metab Clin North Am. 1996;25(2):325–359. doi: 10.1016/s0889-8529(05)70327-3. (Available from: http://dx.doi.org/10.1016/S0889-8529(05)70327-3) [DOI] [PubMed] [Google Scholar]
- 19.Hayden MR, Tyagi SC, Kerklo MM, et al. Type 2 diabetes mellitus as a conformational disease. JOP. 2005;6(4):287–302. [PubMed] [Google Scholar]
- 20.Hong S, Morrow TJ, Paulson PE, et al. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat. J Biol Chem. 2004;279(28):29341–29350. doi: 10.1074/jbc.M404167200. (Available from: http://dx.doi.org/10.1074/jbc.M404167200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang HS, Yang EJ, Lee SM, et al. Antiallodynic effects of electroacupuncture combined with MK-801 treatment through the regulation of p35/p25 in experimental diabetic neuropathy. Exp Neurobiol. 2011;20(3):144–152. doi: 10.5607/en.2011.20.3.144. (Available from: http://dx.doi.org/10.5607/en.2011.20.3.144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109(1-2):210–226. doi: 10.1016/j.pharmthera.2005.07.001. (Available from: http://dx.doi.org/10.1016/j.pharmthera.2005.07.001) [DOI] [PubMed] [Google Scholar]
- 23.Ishiko N, Yamamoto T, Murayama N, et al. Electroacupuncture: current strength-duration relationship for initiation of hypesthesia in man. Neurosci Lett. 1978;8(4):273–276. doi: 10.1016/0304-3940(78)90135-0. [DOI] [PubMed] [Google Scholar]
- 24.Jagodic MM, Pathirathna S, Nelson MT, et al. Cell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. J Neurosci. 2007;27(12):3305–3316. doi: 10.1523/JNEUROSCI.4866-06.2007. (Available from: http://dx.doi.org/10.1523/JNEUROSCI.4866-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang YL, Ning Y, Liu YY, et al. Effects of preventive acupuncture on streptozotocin-induced hyperglycemia in rats. J Endocrinol Invest. 2011;34(10):e355–e361. doi: 10.3275/7859. (Available from: http://dx.doi.org/10.3275/7859) [DOI] [PubMed] [Google Scholar]
- 26.Jiang YL, Yin XH, Shen YF, et al. Low frequency electroacupuncture alleviated spinal nerve ligation induced mechanical allodynia by inhibiting TRPV1 upregulation in ipsilateral undamaged dorsal root ganglia in rats. Evid Based Complement Alternat Med. 2013;2013:170910. doi: 10.1155/2013/170910. (Available from: http://dx.doi.org/10.1155/2013/170910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang YL, He XF, Shen YF, et al. Analgesic roles of peripheral intrinsic met-enkephalin and dynorphin A in long-lasting inflammatory pain induced by complete Freund’s adjuvant in rats. Exp Ther Med. 2015;9(6):2344–2348. doi: 10.3892/etm.2015.2407. (Available from: http://dx.doi.org/10.3892/etm.2015.2407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez-Andrade JM, Bloom AP, Stake JI, et al. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci. 2010;30(44):14649–14656. doi: 10.1523/JNEUROSCI.3300-10.2010. (Available from: http://dx.doi.org/10.1523/JNEUROSCI.3300-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahn BB. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell. 1998;92(5):593–596. doi: 10.1016/s0092-8674(00)81125-3. (Available from: http://dx.doi.org/10.1016/S0092-8674(00)81125-3) [DOI] [PubMed] [Google Scholar]
- 30.Khan GM, Chen SR, Pan HL. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience. 2002;114(2):291–299. doi: 10.1016/s0306-4522(02)00372-x. (Available from: http://dx.doi.org/10.1016/S0306-4522(02)00372-X) [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi M, Ohno T, Tsuchiya T, et al. Characterization of diabetes-related traits in MSM and JF1 mice on high-fat diet. J Nutr Biochem. 2004;15(10):614–621. doi: 10.1016/j.jnutbio.2004.05.001. (Available from: http://dx.doi.org/10.1016/j.jnutbio.2004.05.001) [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, Sun Z. Current views on type 2 diabetes. J Endocrinol. 2010;204(1):1–11. doi: 10.1677/JOE-09-0260. (Available from: http://dx.doi.org/10.1677/JOE-09-0260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manni L, Florenzano F, Aloe L. Electroacupuncture counteracts the development of thermal hyperalgesia and the alteration of nerve growth factor and sensory neuromodulators induced by streptozotocin in adult rats. Diabetologia. 2011;54(7):1900–1908. doi: 10.1007/s00125-011-2117-5. (Available from: http://dx.doi.org/10.1007/s00125-011-2117-5) [DOI] [PubMed] [Google Scholar]
- 34.Maser RE, Steenkiste AR, Dorman JS, et al. Epidemiological correlates of diabetic neuropathy: report from Pittsburgh epidemiology of diabetes complications study. Diabetes. 1989;38(11):1456–1461. doi: 10.2337/diab.38.11.1456. (Available from: http://dx.doi.org/10.2337/diab.38.11.1456) [DOI] [PubMed] [Google Scholar]
- 35.Migita K, Moriyama T, Koguchi M, et al. Modulation of P2X receptors in dorsal root ganglion neurons of streptozotocin-induced diabetic neuropathy. Neurosci Lett. 2009;452(2):200–203. doi: 10.1016/j.neulet.2009.01.048. (Available from: http://dx.doi.org/10.1016/j.neulet.2009.01.048) [DOI] [PubMed] [Google Scholar]
- 36.Muniyappa R, Lee S, Chen H, et al. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–E26. doi: 10.1152/ajpendo.00645.2007. (Available from: http://dx.doi.org/10.1152/ajpendo.00645.2007) [DOI] [PubMed] [Google Scholar]
- 37.Muoio DM, Newgard CB. Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(3):193–205. doi: 10.1038/nrm2327. (Available from: http://dx.doi.org/10.1038/nrm2327) [DOI] [PubMed] [Google Scholar]
- 38.Nori SL, Rocco ML, Florenzano F, et al. Increased nerve growth factor signaling in sensory neurons of early diabetic rats is corrected by electroacupuncture. Evid Based Complement Alternat Med. 2013;2013(12):652735. doi: 10.1155/2013/652735. (Available from: http://dx.doi.org/10.1155/2013/652735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pabbidi RM, Cao DS, Parihar A, et al. Direct role of streptozotocin in inducing thermal hyperalgesia by enhanced expression of transient receptor potential vanilloid 1 in sensory neurons. Mol Pharmacol. 2008;73(3):995–1004. doi: 10.1124/mol.107.041707. (Available from: http://dx.doi.org/10.1124/mol.107.041707) [DOI] [PubMed] [Google Scholar]
- 40.Petruska JC, Cooper BY, Johnson RD, et al. Distribution patterns of different P2x receptor phenotypes in acutely dissociated dorsal root ganglion neurons of adult rats. Exp Brain Res. 2000;134(1):126–132. doi: 10.1007/s002210000414. (Available from: http://dx.doi.org/10.1007/s002210000414) [DOI] [PubMed] [Google Scholar]
- 41.Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8(3):263–272. doi: 10.1016/j.jpain.2006.09.005. (Available from: http://dx.doi.org/10.1016/j.jpain.2006.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med. 2011;13(201):e36. doi: 10.1017/S1462399411002067. (Available from: http://dx.doi.org/10.1017/S1462399411002067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma S, Kulkarni SK, Agrewala JN, et al. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006;536(3):256–261. doi: 10.1016/j.ejphar.2006.03.006. (Available from: http://dx.doi.org/10.1016/j.ejphar.2006.03.006) [DOI] [PubMed] [Google Scholar]
- 44.Silva JRT, Silva ML, Prado WA. Analgesia induced by 2-or 100-Hz electroacupuncture in the rat tail-flick test depends on the activation of different descending pain inhibitory mechanisms. J Pain. 2011;12(1):51–60. doi: 10.1016/j.jpain.2010.04.008. (Available from: http://dx.doi.org/10.1016/j.jpain.2010.04.008) [DOI] [PubMed] [Google Scholar]
- 45.Simonetti M, Giniatullin R, Fabbretti E. Mechanisms mediating the enhanced gene transcription of P2X3 receptor by calcitonin gene-related peptide in trigeminal sensory neurons. J Biol Chem. 2008;283(27):18743–18752. doi: 10.1074/jbc.M800296200. (Available from: http://dx.doi.org/10.1074/jbc.M800296200) [DOI] [PubMed] [Google Scholar]
- 46.Surwit RS, Kuhn CM, Cochrane C, et al. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37(9):1163–1167. doi: 10.2337/diab.37.9.1163. (Available from: http://dx.doi.org/10.2337/diab.37.9.1163) [DOI] [PubMed] [Google Scholar]
- 47.Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149(7):3549–3558. doi: 10.1210/en.2008-0262. (Available from: http://dx.doi.org/10.1210/en.2008-0262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. (Available from: http://dx.doi.org/10.2337/diacare.27.5.1047) [DOI] [PubMed] [Google Scholar]
- 49.Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl. 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. (Available from: http://dx.doi.org/10.2337/diabetes.53.suppl_3.S215) [DOI] [PubMed] [Google Scholar]
- 50.Xu GY, Li G, Liu N, et al. Mechanisms underlying purinergic P2X3 receptor-mediated mechanical allodynia induced in diabetic rats. Mol Pain. 2011;7(1):60. doi: 10.1186/1744-8069-7-60. (Available from: http://dx.doi.org/10.1186/1744-8069-7-60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. doi: 10.1038/414782a. (Available from: http://dx.doi.org/10.1038/414782a) [DOI] [PubMed] [Google Scholar]