Abstract
Histological low-grade squamous intraepithelial lesion/cervical intraepithelial neoplasia grade 1 (LSIL/CIN1) preceded by normal or mildly abnormal cytology is recommended for conservative follow-up, with no separated management. In this study, we assessed the triage value of human papillomavirus (HPV) 16/18 genotyping in 273 patients with LSIL/CIN1. HPV16/18 genotyping was performed at baseline and follow-up was at 6-monthly intervals for up to 2 years. At each follow-up, women positive for cytology or high-risk HPV (hrHPV) were referred for colposcopy. Enrollment cytology, HPV16/18 genotyping, and questionnaire-obtained factors were linked to the 2-year cumulative progression rate. Univariate and multivariate analyses were performed taking into account time-to-event with Cox proportional hazard regression. The results showed that 190 cases (69.6%) regressed, 37 (13.6%) persisted, and 46 (16.8%) progressed. HPV16/18 positivity (hazard ratio (HR), 2.708; 95% confidence interval (CI), 1.432–5.121; P=0.002) is significantly associated with higher 2-year cumulative progression rate. Sub-analysis by enrollment cytology and age restricted the positive association among patients preceded by mildly abnormal cytology and aged 30 years or older. Immediate treatment is a rational recommendation for the high-risk subgroup, when good compliance is not assured.
Keywords: Low-grade squamous intraepithelial lesion (LSIL), Cervical intraepithelial neoplasia grade 1 (CIN1), Human papillomavirus (HPV), HPV16/18 genotyping, Prognostic value, Prospective study
1. Introduction
Histological low-grade squamous intraepithelial lesion (LSIL), also termed cervical intraepithelial neoplasia grade 1 (CIN1) in the prior edition of three-tier terminology, is limited to the basal one-third of the squamous epithelium. Up to 70%–80% of LSIL/CIN1 will regress spontaneously, while a subset is associated with residual risk for future precancerous lesion (Schiffman et al., 2007; Martin and O'Leary, 2011). No reliable biomarker, other than cytology, has been used to predict the evolution of LSIL/CIN1. Kaiser Permanente Northern California (KPNC) data showed a relatively low 5-year progression rate of LSIL/CIN1 preceded by normal cytology (negative for intraepithelial lesion or malignancy (NILM)) or mildly abnormal cytology (atypical squamous cells of undetermined significance (ASC-US) and LSIL), but a substantially higher progressive risk with more severe abnormalities (high-grade squamous intraepithelial lesion (HSIL), atypical squamous cells—cannot rule out HSIL (ASC-H), and atypical glandular cells (AGC)) (Katki et al., 2013). Accordingly, LSIL/CIN1 preceded by normal or mildly abnormal cytology is considered low-risk and recommended for conservative follow-up only (Massad et al., 2013), with no triage management available. Yet up to 10% of progression was reported for these patients (Katki et al., 2013). Previous studies have explored the values of immunohistochemical markers (such as p16INK4A and cytokeratin 7) in predicting the behavior of LSIL/CIN1. However, microscopically interpretive variability confounded such studies, and results have been inconsistent (Liao et al., 2014; Mills et al., 2015; Huang et al., 2016; Paquette et al., 2016; Sagasta et al., 2016).
Human papillomavirus (HPV) genotypes have different carcinogenicities, leading us to wonder whether HPV genotyping could be of clinical value in refining the management of LSIL/CIN1. HPV16/18 genotyping is the most approved genotyping strategy. Progressive risk is elevated for women with positive HPV16/18, even when cytology is normal (Khan et al., 2005). Thus, HPV16/18 positivity alone is an indication for colposcopy referral among women aged 30 years or older, according to the current American Society for Colposcopy and Cervical Pathology (ASCCP) management guidelines (Massad et al., 2013). Yet no prospective study has addressed the prognostic value of HPV16/18 genotyping in LSIL/CIN1 preceded by non-severe cytology abnormalities. We conducted this hospital-based longitudinal study to evaluate the performance of HPV16/18 genotyping as a triage for LSIL/CIN1 preceded by normal or mildly abnormal cytology.
2. Materials and methods
2.1. Patient recruitment
Women with LSIL/CIN1 diagnosed by colposcopy-guided biopsy (colposcopy is adequate and endocervical curettage is negative) were prospectively recruited in the Women’s Hospital, School of Medicine, Zhejiang University, China during June 2012 to December 2013. The diagnostic criterion was intraepithelial lesion limited to the basal one-third of the squamous epithelium, exclusive of flat condyloma, koilocytotic atypia, and koilocytosis. Women were excluded from this study according to the following criteria: (1) preceded by cytological HSIL, ASC-H, or AGC; (2) surgically or ablatively treated cervix; (3) previously confirmed cervical cancer or precursor, or other malignancies; (4) with immunosuppressive diseases; and, (5) pregnancy. All eligible patients underwent HPV16/18 genotyping at baseline. Information on socio-demographic characteristics, reproductive history, contraception, menstrual status, and sexual behavior was collected via an interviewer-administered structured questionnaire.
2.2. Ethics approval and consent to participate
This study was approved by the Human Research Ethical Committee of the Women’s Hospital, School of Medicine, Zhejiang University, China with protocol No. 20110014. Informed consent was obtained from study participants according to institutional guidelines.
2.3. Follow-up
Patients were followed up at 6-monthly intervals for up to 2 years. At each follow-up, liquid-based cytology and HPV genotyping were performed as first-level exams. Women with positive cytology or high-risk HPV (hrHPV) were referred for colposcopy. The endpoints were defined as follows: (1) progression: histology-confirmed HSIL or more; (2) regression: cytology normal and hrHPV negative, or cytology/hrHPV positive but histology negative; and, (3) persistence: histology-confirmed LSIL/CIN1. Women reaching an endpoint would then be handled according to the ASCCP management guidelines (Massad et al., 2013).
2.4. Cytology and HPV genotyping
Hospital cytologists performed cytological diagnosis according to the 2001 Bethesda System (Solomon et al., 2002). HPV genotyping was carried out with HPV GenoArray test kit (Hybribio, Hong Kong, China), which was described in our previous studies (Ye et al., 2010a; 2010b). Briefly, this test kit can identify 14 hrHPVs (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) separately.
2.5. Colposcopy and histological diagnosis
Hospital colposcopists performed colposcopy according to the standardized protocol and hospital pathologists made histological diagnoses according to the Lower Anogenital Squamous Terminology (LAST) recommendations (Darragh et al., 2012). Consensus on histodiagnosis was reached by an expert panel in the event of disagreement.
2.6. Statistical analysis
Enrollment cytology, HPV16/18 genotyping, and potential risk factors identified from the questionnaire were assessed as independent prognostic markers for 2-year cumulative progression. In both univariate and multivariate analyses, Cox proportional hazards model was used to estimate hazard ratio (HR) and 95% confidence interval (CI). Factors associated with progression at P<0.1 in univariate analysis would be included in multivariate analysis and mutually adjusted. Further, analysis was stratified by enrollment cytology (“normal cytology” (NILM) versus “mildly abnormal cytology” (ASC-US or LSIL)) and age (<30 years versus ≥30 years) to optimize the application scope of triage markers. SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA) was used. All statistical tests were two-sided. A level of 0.05 was chosen to indicate statistical significance.
3. Results
Two hundred and seventy-three patients, with a mean age of 36.5 years (range 22–62 years) at registration, completed follow-up. Fifty patients (18.3%) were HPV16/18-positive, while 191 cases (70.0%) were preceded by mildly abnormal cytology (ASC-US/LSIL) at enrollment. During the 2-year follow-up, 190 cases (69.6%) regressed, 37 cases (13.6%) persisted, and 46 cases (16.8%) progressed. Among the progression, 37.0% (17/46) occurred at the first follow-up, 30.4% (14/46) at the second, 17.4% (8/46) at the third, and 15.2% (7/46) at the end.
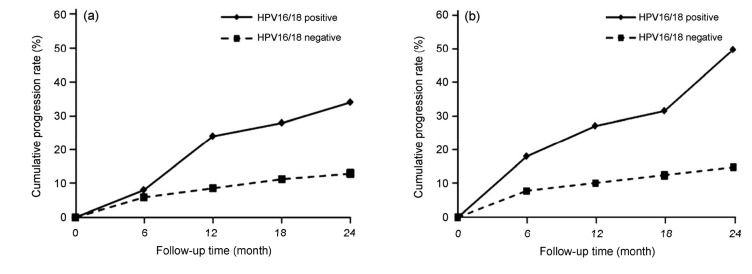
In univariate analysis, age, marital status, reproductive history, contraception, menopausal status, sexual debut, and antecedent cytology were excluded at P>0.1 (Table 1). With mutual adjustment, HPV16/18 positivity was confirmed as the independent prognostic marker for progression in the multivariate analysis (Table 1). The 2-year cumulative progression rate was 34.0% (95% CI, 20.9%–47.1%) among HPV16/18 positive patients, while 13.0% (95% CI, 8.6%–17.4%) for HPV16/18 negative patients (Fig. 1a).
Table 1.
Univariate/multivariate analysis of prognostic factors for 2-year cumulative progression rate
| Characteristics | Univariate analysis |
Multivariate analysisa
|
|||
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | |||||
| <30 years | Reference | ||||
| ≥30 years | 1.231 (0.574–2.642) | 0.594 | |||
| Marital status | |||||
| Married | Reference | ||||
| Single | 0.488 (0.118–2.020) | 0.322 | |||
| Childbearing | |||||
| 0 | Reference | ||||
| ≥1 | 2.968 (0.717–12.290) | 0.133 | |||
| Contraception | |||||
| Oral contraceptive | 0.474 (0.065–3.473) | 0.462 | |||
| Condom | 0.576 (0.282–1.175) | 0.129 | |||
| Other | Reference | ||||
| Menopausal status | |||||
| Premenopause | Reference | ||||
| Menopause | 0.608 (0.147–2.512) | 0.492 | |||
| Sexual debut | |||||
| <20 years old | 1.262 (0.495–3.216) | 0.626 | |||
| ≥20 years old | Reference | ||||
| Sexual partnerb | |||||
| 1 | Reference | ||||
| ≥2 | 0.452 (0.190–1.074) | 0.072 | 0.436 (0.183–1.039) | 0.061 | |
| Enrollment cytology | |||||
| NILM | Reference | ||||
| ASC-US/LSIL | 1.498 (0.743–3.020) | 0.259 | |||
| HPV genotyping | |||||
| HPV16/18 positive | 2.777 (1.524–5.059) | 0.001 | 2.708 (1.432–5.121) | 0.002 | |
| HPV16/18 negative | Reference | ||||
NILM: negative for intraepithelial lesion or malignancy; ASC-US: atypical squamous cells of undetermined significance; LSIL: low-grade squamous intraepithelial lesion; HPV: human papillomavirus; HR: hazard ratio; CI: confidence interval.
Factors associated with 2-year cumulative progression rate at P<0.1 in the univariate analysis were included in the multivariate analysis and mutually adjusted.
Refer to the lifetime number of sexual partners
Fig. 1.
Cumulative progression rates over 2-year period according to HPV16/18 genotyping at enrollment
(a) All patients (n=273); (b) Patients preceded by ASC-US/LSIL cytology and aged 30 years or older (n=150)
To improve the triage strategy, we stratified our analysis by cytology and age at enrollment to examine the risk in subgroups who might be managed with different clinic strategies. When separately stratified, the progressive risk associated with HPV16/18 was not significantly elevated in patients preceded by normal cytology or aged below 30 years (Table 2). Further cross stratification showed that the elevated progressive risk associated with HPV16/18 was only remarkable among cases preceded by mildly abnormal cytology (ASC-US or LSIL) and aged 30 years or older (Table 2). In this subset, the 2-year cumulative progression rate was 50.0% (95% CI, 29.1%–70.9%) among HPV16/18-positive patients, while 14.8% (95% CI, 8.6%–21.0%) for HPV16/18 negative cases (Fig. 1b).
Table 2.
HPV16/18 positivity-associated 2-year cumulative progression rate, stratified by cytology and age at enrollment
| Stratification | HR (95% CI) | P |
| Cytology | ||
| NILM | 2.757 (0.797–9.534) | 0.109 |
| ASC-US/LSIL | 3.055 (1.527–6.116) | 0.002 |
| Age | ||
| <30 years | 1.265 (0.255–6.276) | 0.774 |
| ≥30 years | 3.327 (1.733–6.386) | <0.001 |
| Cytology & age | ||
| NILM & <30 years | 1.612 (0.099–26.197) | 0.737 |
| NILM & ≥30 years | 3.650 (0.911–14.627) | 0.068 |
| ASC-US/LSIL & <30 years | 1.888 (0.220–16.241) | 0.563 |
| ASC-US/LSIL & ≥30 years | 3.314 (1.575–6.972) | 0.002 |
HPV: human papillomavirus; NILM: negative for intraepithelial lesion or malignancy; ASC-US: atypical squamous cells of undetermined significance; LSIL: low-grade squamous intraepithelial lesion; HR: hazard ratio; CI: confidence interval
4. Discussion
To our knowledge, this study is the first to assess the triage value of HPV16/18 genotyping in LSIL/CIN1 preceded by normal or mildly abnormal cytology. Among the four HPV tests approved by the US Food and Drug Administration (FDA), only the Cobas HPV test could genotype HPV16/18 separately. However, when we started our research in June 2012, the Cobas HPV test had not yet been approved by the FDA. In view of the good performance of the HPV GenoArray test in our previous studies (Ye et al., 2010a; 2010b), as well as the excellent concordance with other HPV tests such as Amplicor HPV test and Roche Linear Array (Grisaru et al., 2008; Liu et al., 2010), we adopted the HPV GenoArray test in the current research.
It is well known that HPV infection is the causal agent underlying cervical carcinogenesis (Walboomers et al., 1999). The strong etiological link between HPV infection and cervical cancer has led to the routine application of HPV testing in improving existing cytology-based cervical cancer screening, managing women with equivocal cytology following surgically or ablatively treated cervical lesions, and so on. Compared with HPV testing, HPV genotyping has not been widely applied in clinical practice.
Among HPV genotypes, HPV16 and 18 were considered to have the most potent carcinogenic potential (Dahlström et al., 2010; de Sanjose et al., 2010; Rijkaart et al., 2012; Bzhalava et al., 2013; Tjalma et al., 2013; Wheeler et al., 2014), being associated with about 70% of cervical cancers worldwide (de Sanjose et al., 2010; Li et al., 2011). The powerful carcinogenicity of HPV16/18 made them the most meaningful genotyping targets in clinical practice. In cross-sectional studies, HPV16/18 genotyping stratified HPV-positive women by their risk of prevalent high-grade cervical lesions (Castle et al., 2011; Wright et al., 2011; Lagos et al., 2015; McKenna et al., 2016). It also served as a reliable predictor of residual disease following conization for cervical precancerous lesion (Kang et al., 2016). In prospective studies, HPV16/18 positivity is linked with a higher risk of future high-grade cervical lesions (Khan et al., 2005; Bulk et al., 2007; Persson et al., 2015). Based on evidence in the research literature, ASCCP guidelines recommend that women aged 30 years or older with normal cytology but positive HPV16/18 should be referred for immediate colposcopy, because they are at particularly high risk of current or future high-grade cervical lesions (Massad et al., 2013). In this study, we have confirmed the triage value of HPV16/18 genotyping in LSIL/CIN1, which is comparable and complementary to earlier published data.
In cytology sub-analysis, HPV16/18 genotyping provided little predictive value to LSIL/CIN1 preceded by normal cytology. It is inconsistent with early data, which showed an elevated 10-year risk of high-grade cervical lesions in women with normal cytology but positive HPV16/18 (Khan et al., 2005). However, it is worth noting that this previous research had no baseline data of histodiagnosis (Khan et al., 2005), so could not exclude patients with already existing high-grade cervical lesions at enrollment. Given the high current prevalence of high-grade cervical lesions among HPV16/18-positive women with normal cytology (Wright et al., 2011), the positive follow-up association obtained without baseline histodiagnosis needs further verification. Another possible explanation for our negative relationship between HPV16/18 genotyping and LSIL/CIN1 preceded by normal cytology is low statistical power. Further study with a larger sample size and longer follow-up period is required to test this possibility.
To balance risk versus benefit, we also tailored our strategy by age. Because of maximal exposure but minimal acquired immunity, HPV prevalence is high in young women (Bosch et al., 2008; McKenna and McMenamin, 2014). However, most young women have an effective immune response to clear the acute infection within a short time (Rodriguez et al., 2008). Considering the high spontaneous regression rate among this age group, HPV testing is not recommended (Davey et al., 2014). Similarly, colposcopy associated with HPV16/18 is restricted to women aged 30 years or older (Massad et al., 2013). HPV16/18 genotyping showed no triage value under age of 30 years. Our observation supported the notion that HPV strategy should be age-related. Young women should be managed conservatively, especially for minor abnormalities (Massad et al., 2013).
5. Conclusions
HPV16/18 positivity is predictive of progression for women with LSIL/CIN1 preceded by mildly abnormal cytology, who are aged 30 years or older. Closer follow-up is required for this high-risk subset. For patients at risk of loss-to-follow-up, immediate treatment (including ablative or resectional treatment based on transformation zone type and colposcopy) is a rational recommendation.
Acknowledgments
We thank all the patients who took part in this study. We thank Dr. Hong-yun WANG (Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, Nanjing, China) for assistance with data entry.
Footnotes
Project supported by the Zhejiang Provincial Natural Science Foundation of China (No. LQ14H160007), the National Natural Science Foundation of China (No. 81402364), the Zhejiang Provincial Medical & Hygienic Science and Technology Project of China (No. 2013KYA104), the Special Fund for Scientific Research in the Public Interest from the National Health and Family Planning Commission of the People’s Republic of China (No. 2015SQ00243), and the National Key Research and Development Program of China (No. 2016YFC1302900)
Contributors: Jing YE, Xing XIE, Wei-guo LU, and Xiao-dong CHENG conceived and designed the project. Bei CHENG, Yi-fan CHENG, and Ye-li YAO participated in the recruitment and follow-up of patients. Jing YE analyzed the data and wrote the manuscript. Xing XIE, Wei-guo LU, and Xiao-dong CHENG revised the manuscript. All authors agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Compliance with ethics guidelines: Jing YE, Bei CHENG, Yi-fan CHENG, Ye-li YAO, Xing XIE, Wei-guo LU, and Xiao-dong CHENG declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
- 1.Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl. 10):K1–K16. doi: 10.1016/j.vaccine.2008.05.064. (Available from: http://dx.doi.org/10.1016/j.vaccine.2008.05.064) [DOI] [PubMed] [Google Scholar]
- 2.Bulk S, Bulkmans NW, Berkhof J, et al. Risk of high-grade cervical intra-epithelial neoplasia based on cytology and high-risk HPV testing at baseline and at 6-months. Int J Cancer. 2007;121(2):361–367. doi: 10.1002/ijc.22677. (Available from: http://dx.doi.org/10.1002/ijc.22677) [DOI] [PubMed] [Google Scholar]
- 3.Bzhalava D, Guan P, Franceschi S, et al. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology. 2013;445(1-2):224–231. doi: 10.1016/j.virol.2013.07.015. (Available from: http://dx.doi.org/10.1016/j.virol.2013.07.015) [DOI] [PubMed] [Google Scholar]
- 4.Castle PE, Stoler MH, Wright TCJr, et al. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011;12(9):880–890. doi: 10.1016/S1470-2045(11)70188-7. (Available from: http://dx.doi.org/10.1016/S1470-2045(11)70188-7) [DOI] [PubMed] [Google Scholar]
- 5.Dahlström LA, Ylitalo N, Sundström K, et al. Prospective study of human papillomavirus and risk of cervical adenocarcinoma. Int J Cancer. 2010;127(8):1923–1930. doi: 10.1002/ijc.25408. (Available from: http://dx.doi.org/10.1002/ijc.25408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136(10):1266–1297. doi: 10.5858/arpa.LGT200570. (Available from: http://dx.doi.org/10.5858/arpa.LGT200570) [DOI] [PubMed] [Google Scholar]
- 7.Davey DD, Goulart R, Nayar R. 2013 statement on human papillomavirus DNA test utilization. Am J Clin Pathol. 2014;141(4):459–461. doi: 10.1309/AJCPKXBQLWOJ4ZUB. (Available from: http://dx.doi.org/10.1309/AJCPKXBQLWOJ4ZUB) [DOI] [PubMed] [Google Scholar]
- 8.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8. (Available from: http://dx.doi.org/10.1016/S1470-2045(10)70230-8) [DOI] [PubMed] [Google Scholar]
- 9.Grisaru D, Avidor B, Niv J, et al. Pilot study of prevalence of high-risk human papillomavirus genotypes in Israeli Jewish women referred for colposcopic examination. J Clin Microbiol. 2008;46(5):1602–1605. doi: 10.1128/JCM.02483-07. (Available from: http://dx.doi.org/10.1128/JCM.02483-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang EC, Tomic MM, Hanamornroongruang S, et al. p16ink4 and cytokeratin 7 immunostaining in predicting HSIL outcome for low-grade squamous intraepithelial lesions: a case series, literature review and commentary. Mod Pathol. 2016;29(12):1501–1510. doi: 10.1038/modpathol.2016.141. (Available from: http://dx.doi.org/10.1038/modpathol.2016.141) [DOI] [PubMed] [Google Scholar]
- 11.Kang WD, Ju UC, Kim SM. A human papillomavirus (HPV)-16 or HPV-18 genotype is a reliable predictor of residual disease in a subsequent hysterectomy following a loop electrosurgical excision procedure for cervical intraepithelial neoplasia 3. J Gynecol Oncol. 2016;27(1):e2. doi: 10.3802/jgo.2016.27.e2. (Available from: http://dx.doi.org/10.3802/jgo.2016.27.e2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katki HA, Gage JC, Schiffman M, et al. Follow-up testing after colposcopy: five-year risk of CIN 2+ after a colposcopic diagnosis of CIN 1 or less. J Low Genit Tract Dis. 2013;17(5 Suppl. 1):S69–S77. doi: 10.1097/LGT.0b013e31828543b1. (Available from: http://dx.doi.org/10.1097/LGT.0b013e31828543b1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97(14):1072–1079. doi: 10.1093/jnci/dji187. (Available from: http://dx.doi.org/10.1093/jnci/dji187) [DOI] [PubMed] [Google Scholar]
- 14.Lagos M, van de Wyngard V, Poggi H, et al. HPV16/18 genotyping for the triage of HPV positive women in primary cervical cancer screening in Chile. Infect Agents Cancer. 2015;10:43. doi: 10.1186/s13027-015-0038-5. (Available from: http://dx.doi.org/10.1186/s13027-015-0038-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Franceschi S, Howell-Jones R, et al. Human papillomavirus type distribution in 30 848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128(4):927–935. doi: 10.1002/ijc.25396. (Available from: http://dx.doi.org/10.1002/ijc.25396) [DOI] [PubMed] [Google Scholar]
- 16.Liao GD, Sellors JW, Sun HK, et al. p16INK4A immunohistochemical staining and predictive value for progression of cervical intraepithelial neoplasia grade 1: a prospective study in China. Int J Cancer. 2014;134(7):1715–1724. doi: 10.1002/ijc.28485. (Available from: http://dx.doi.org/10.1002/ijc.28485) [DOI] [PubMed] [Google Scholar]
- 17.Liu SS, Leung RC, Chan KK, et al. Evaluation of a newly developed GenoArray human papillomavirus (HPV) genotyping assay and comparison with the Roche Linear Array HPV genotyping assay. J Clin Microbiol. 2010;48(3):758–764. doi: 10.1128/JCM.00989-09. (Available from: http://dx.doi.org/10.1128/JCM.00989-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin CM, O'Leary JJ. Histology of cervical intraepithelial neoplasia and the role of biomarkers. Best Pract Res Clin Obstet Gynaecol. 2011;25(5):605–615. doi: 10.1016/j.bpobgyn.2011.04.005. (Available from: http://dx.doi.org/10.1016/j.bpobgyn.2011.04.005) [DOI] [PubMed] [Google Scholar]
- 19.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4):829–846. doi: 10.1097/AOG.0b013e3182883a34. (Available from: http://dx.doi.org/10.1097/AOG.0b013e3182883a34) [DOI] [PubMed] [Google Scholar]
- 20.McKenna M, McMenamin MM. Human papillomavirus testing in young women: clinical outcomes of human papillomavirus triage in a UK cervical screening program. Cancer Cytopathol. 2014;122(9):702–710. doi: 10.1002/cncy.21444. (Available from: http://dx.doi.org/10.1002/cncy.21444) [DOI] [PubMed] [Google Scholar]
- 21.McKenna M, McMenamin M, McDowell A. HPV16 and HPV18 genotyping triage in young women with borderline cytology or mild dyskaryosis: effect of age on genotype-specific risk of high-grade CIN. Cytopathology. 2016;27(4):261–268. doi: 10.1111/cyt.12316. (Available from: http://dx.doi.org/10.1111/cyt.12316) [DOI] [PubMed] [Google Scholar]
- 22.Mills AM, Paquette C, Castle PE, et al. Risk stratification by p16 immunostaining of CIN1 biopsies: a retrospective study of patients from the quadrivalent HPV vaccine trials. Am J Surg Pathol. 2015;39(5):611–617. doi: 10.1097/PAS.0000000000000374. (Available from: http://dx.doi.org/10.1097/PAS.0000000000000374) [DOI] [PubMed] [Google Scholar]
- 23.Paquette C, Mills AM, Stoler MH. Predictive value of cytokeratin 7 immunohistochemistry in cervical low-grade squamous intraepithelial lesion as a marker for risk of progression to a high-grade lesion. Am J Surg Pathol. 2016;40(2):236–243. doi: 10.1097/PAS.0000000000000548. (Available from: http://dx.doi.org/10.1097/PAS.0000000000000548) [DOI] [PubMed] [Google Scholar]
- 24.Persson M, Elfström KM, Olsson SE, et al. Minor cytological abnormalities and up to 7-year risk for subsequent high-grade lesions by HPV type. PLoS ONE. 2015;10(6):e0127444. doi: 10.1371/journal.pone.0127444. (Available from: http://dx.doi.org/10.1371/journal.pone.0127444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13(1):78–88. doi: 10.1016/S1470-2045(11)70296-0. (Available from: http://dx.doi.org/10.1016/S1470-2045(11)70296-0) [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez AC, Schiffman M, Herrero R, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100(7):513–517. doi: 10.1093/jnci/djn044. (Available from: http://dx.doi.org/10.1093/jnci/djn044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sagasta A, Castillo P, Saco A, et al. p16 staining has limited value in predicting the outcome of histological low-grade squamous intraepithelial lesions of the cervix. Mod Pathol. 2016;29(1):51–59. doi: 10.1038/modpathol.2015.126. (Available from: http://dx.doi.org/10.1038/modpathol.2015.126) [DOI] [PubMed] [Google Scholar]
- 28.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. (Available from: http://dx.doi.org/10.1016/S0140-6736(07)61416-0) [DOI] [PubMed] [Google Scholar]
- 29.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287(16):2114–2119. doi: 10.1001/jama.287.16.2114. (Available from: http://dx.doi.org/10.1001/jama.287.16.2114) [DOI] [PubMed] [Google Scholar]
- 30.Tjalma WA, Fiander A, Reich O, et al. Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int J Cancer. 2013;132(4):854–867. doi: 10.1002/ijc.27713. (Available from: http://dx.doi.org/10.1002/ijc.27713) [DOI] [PubMed] [Google Scholar]
- 31.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. (Available from: http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- 32.Wheeler CM, Hunt WC, Cuzick J, et al. The influence of type-specific human papillomavirus infections on the detection of cervical precancer and cancer: a population-based study of opportunistic cervical screening in the United States. Int J Cancer. 2014;135(3):624–634. doi: 10.1002/ijc.28605. (Available from: http://dx.doi.org/10.1002/ijc.28605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright TCJr, Stoler MH, Sharma A, et al. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol. 2011;136(4):578–586. doi: 10.1309/AJCPTUS5EXAS6DKZ. (Available from: http://dx.doi.org/10.1309/AJCPTUS5EXAS6DKZ) [DOI] [PubMed] [Google Scholar]
- 34.Ye J, Cheng X, Chen X, et al. Prevalence and risk profile of cervical human papillomavirus infection in Zhejiang Province, southeast China: a population-based study. Virol J. 2010;7:66. doi: 10.1186/1743-422X-7-66. (Available from: http://dx.doi.org/10.1186/1743-422X-7-66) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye J, Cheng X, Chen X, et al. Short-term type-specific HPV persistence and its predictors in an asymptomatic general female population in Zhejiang, China. Int J Gynecol Obstet. 2010;110(3):217–222. doi: 10.1016/j.ijgo.2010.03.040. (Available from: http://dx.doi.org/10.1016/j.ijgo.2010.03.040) [DOI] [PubMed] [Google Scholar]