Abstract
Cytology triage has been generally recommended for human papillomavirus (HPV)-positive women, but is highly dependent on well-trained cytologists. The present study was designed to explore whether HPV E6/E7 mRNA detection in cervical exfoliated cells can be a potential triage for HPV-positive women from a clinic-based population. Both the primary HPV testing and Papanicolaou (Pap) test were performed on all eligible HPV-positive women. HPV E6/E7 mRNA was detected by QuantiVirus® HPV E6/E7 mRNA assay in cervical exfoliated cells. All HPV-positive women underwent colposcopy and further biopsy if indicated. The data were assessed by Pearson’s Chi-squared test and the receiver operating characteristic curve. A total of 404 eligible HPV-positive women were enrolled. Positive rate of E6/E7 mRNA in high-grade squamous intraepithelial lesion (HSIL) cases was higher than that in low-grade squamous intraepithelial lesion (LSIL) or normal cases. There was no statistical difference found between mRNA and cytological testing with sensitivity (89.52% vs. 86.67%, P=0.671), specificity (48.96% vs. 48.96%, P=1.000), positive predictive value (39.00% vs. 38.24%, P=1.000), and negative predictive value (92.76% vs. 90.97%, P=0.678) for detecting ≥HSIL. HPV E6/E7 mRNA detection in cervical exfoliated cells shows the same performance as Pap triage for HSIL identification for HPV-positive women. Detection of HPV E6/E7 mRNA may be used as a new triage option for HPV-positive women.
Keywords: Human papillomavirus (HPV), HPV E6/E7 mRNA, High-grade squamous intraepithelial lesion (HSIL)
1. Introduction
Human cervical cancer is the fourth most common cancer in women worldwide, and most cervical cancer cases arise in less developed countries (Torre et al., 2015). Oncogenic human papillomavirus (HPV) infection is a prerequisite for the development of cervical cancer and its precursor lesions (Walboomers et al., 1999; Clifford et al., 2003). More than 150 serotypes of HPV have been identified to date, of which about 40 types can infect the cervix. These HPV types are divided into high-and low-risk groups (Schiffman et al., 2011). Persistent high-risk HPV (HR-HPV) infection is a major cause of cervical cancer. Supported by clinical trials (Condel et al., 2002; Wright and Schiffman, 2003; Kitchener et al., 2009; Siebers et al., 2009), and taking the advantage of high sensitivity while identifying high-grade squamous intraepithelial lesion and worse lesions (HSIL+), HPV testing has been recommended as a primary screening for cervical cancer in many countries (Zappacosta et al., 2013). The introduction of cytological screening has remarkably reduced the incidence and mortality of cervical cancer, and the cytology test has been a most widely accepted triage for patients with positive primary HPV test (Zappacosta et al., 2013). However, many developing countries, including China, are very lacking in well-trained cytologists, and the cytology test cannot be carried out effectively. Therefore, other triage options should be considered in these countries.
The oncogenic potential of the HR-HPV depends on the increased expressions of the E6 and E7 genes (Sotlar et al., 2004; Cuschieri and Wentzensen, 2008). The E6/E7 oncogene transcripts are usually expressed at low levels during transient HPV infection (Benevolo et al., 2011). When the viral genome has integrated into the host genome, the oncogene transcripts are over-expressed, leading to the development of cervical cancer (Castle et al., 2007; Cuschieri and Wentzensen, 2008). E6 and E7 proteins bind to the tumor suppressor proteins p53 and pRb, respectively (Scheffner et al., 1990; Chellappan et al., 1992), and drive cervical cell proliferation and transformation (Benevolo et al., 2011). It has been reported that HPV E6/E7 mRNA expression level is highly correlated with the severity of cervical lesions (Ho et al., 2010). HPV E6/E7 mRNA may be useful as a marker for potentially progressive HR-HPV infections and may constitute a useful tool for screening and/or patient management (Jeantet et al., 2009; Sorbye et al., 2011; Giorgi Ross et al., 2013).
In this study, E6/E7 mRNAs of 14 HR-HPV types (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) were detected in cervical exfoliated cell samples, and the performance of HPV E6/E7 mRNA detection as an optional triage for HPV-positive women was evaluated.
2. Materials and methods
2.1. Patient recruitment and sample collection
Female patients who visited the Women’s Hospital, School of Medicine, Zhejiang University (Hangzhou, China), between July 2014 and December 2014, with positive HPV test results were prospectively included.
Women were recruited in the study based on the following criteria: (1) aged 24 years or older, (2) without previous cervical cancer or precancerous lesions, (3) no history of therapeutic procedure of cervix, (4) HPV DNA positive by Hybrid Capture 2 assay, and (5) non-pregnant.
Women were excluded from the study according to the following criteria: (1) younger than 24 years, (2) previously confirmed cervical cancer, (3) therapeutic procedure to cervix, and (4) pregnancy. Cervical exfoliated cell samples, used for both cytology test and HPV E6/E7 mRNA detection, were collected by sample brush from the cervical surface. This study was approved by the Ethics Committee of the Women’s Hospital, School of Medicine, Zhejiang University. All patients signed the consent forms and were informed regarding the purpose of the proposed study.
2.2. HPV testing
HR-HPV DNA was detected by Hybrid Capture 2 assay (HC2, Digene, Gaithersburg, MD, USA), according to the manufacturer’s instructions. At least 1 pg/ml HPV DNA or more was required to be identified as positive.
2.3. Cytology test
The technology of liquid-based cytology (LBC) was used for the cytology test. Thin-layer slides were prepared using the Thin Prep 2000 Processor (Cytyc Corporation, Marlborough, MA, USA) according to the manufacturer’s instructions. The prepared slides were stained by the Papanicolaou (Pap) method and assessed by cytologists of the hospital according to the criteria set out in the Bethesda System 2001 guidelines. A diagnosis was assigned to each case as being negative for intraepithelial lesion or malignancy (NILM) or having any epithelial cell abnormalities, such as atypical squamous cells of undetermined significance (ASC-US), atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion (ASC-H), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), and squamous cell carcinoma (SCC) (Liu et al., 2014).
2.4. HPV E6/E7 mRNA detection
E6/E7 mRNAs of 14 HR-HPV types (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) were detected in cervical exfoliated cell samples by QuantiVirus® HPV E6/E7 mRNA assay (Kodia, Xinxiang, China) according to the manufacturer’s instructions. The QuantiVirus® HPV E6/E7 mRNA assay is a sandwich nucleic acid hybridization procedure, based on branched DNA (bDNA) technology (DiaCarta, CA, USA).
Briefly, each sample was incubated with 600 μl lysis mixture and 5 μl proteinase at 65 °C for 1 h to release viral RNA. A total of 50 μl specimen and 50 μl working probe solution were mixed in 96-well plates and incubated at 55 °C for 3.5 h to capture target RNA (Discacciati et al., 2014). After the samples were washed, 100 μl of ThinPrep-amplifier probe working reagent was added to the samples. Each well of the capture plate was incubated at 55 °C for 40 min. Samples were then washed and 100 μl label probe working reagent was added to each well; the capture plate was then incubated at 50 °C for 40 min. Samples were washed again and 100 μl substrate working reagent was added to each well. The capture plate was incubated at 46 °C for 20 min. After reaction with substrate working reagent, the plate was cooled to room temperature for 10 min and read immediately (within 1 min) with the Kodia QuantiVirus® Benchtop Luminometer. Light emission was related directly to the amount of HPV mRNA present in each sample and results were recorded as relative light units by the luminometer system. If the signal was greater than or equal to 1.0, the QuantiVirus® HPV assay result for the patient was positive. Otherwise, the result was negative (Liu et al., 2014).
2.5. Colposcopy and histological diagnosis
All HPV-positive women were examined by colposcopy and underwent cervical biopsy. Histological diagnosis was made by pathologists of the hospital according to previous descriptions (Tavassoéli and Devilee, 2003).
2.6. Statistical analysis
All statistical analyses were performed using SPSS statistics software Version 13.0 (Chicago, IL, USA). Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and their 95% confidence intervals (CIs) of HPV E/E7 mRNA detection and cytology test were calculated for detecting HSIL+. The χ 2 test was used to compare the performance parameters of HPV E/E7 mRNA detection and the cytology test. Statistical significance was defined by a P-value of less than 0.05. The predictability was measured via the area under the receiver operating characteristic (ROC) curve.
3. Results
3.1. Histological and cytological diagnoses
In total, 404 women were enrolled during the study period, and their ages ranged from 25 to 71 years (mean age 42.3 years).
All the 404 women underwent biopsy under colposcopy, of which 11 women were excluded: 10 were diagnosed as Grade 1 vaginal intraepithelial neoplasia, and one was diagnosed as Grade 2 vaginal intraepithelial neoplasia. Cytology test results of the remaining 393 women consisted of, 155 (39.44%) NILM, 80 (20.36%) ASC-US, 89 (22.65%) LSIL, 28 (7.12%) ASC-H, and 41 (10.43%) HSIL (Table 1). Their histological diagnosis consisted of 227 normal, 61 LSIL, 101 HSIL, and 4 cancers.
Table 1.
Cytological and histological diagnoses
| Group | Histological diagnosis | Cytological diagnosis |
||||
| NILM | ASC-US | LSIL | ASC-H | HSIL | ||
| Normal | 227 | 127 | 51 | 40 | 5 | 4 |
| LSIL | 61 | 14 | 14 | 25 | 5 | 3 |
| HSIL | 101 | 14 | 15 | 24 | 18 | 30 |
| Cancer | 4 | 0 | 0 | 0 | 0 | 4 |
NILM: negative for intraepithelial lesion or malignancy; ASC-US: atypical squamous cells of undetermined significance; LSIL: low-grade squamous intraepithelial lesion; ASC-H: high-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion
3.2. Positive rate of HPV E6/E7 mRNA assay by histological diagnosis
HPV E6/E7 mRNA was positive in 241 (61.32%) of 393 total cases. The positive rate in each histologic group is shown in Table 2. The mRNA test showed a higher positive rate of E6/E7 mRNA in high-grade lesion cases than in low-grade lesion or normal cases. Positive rates were 100.00%, 89.11%, 77.05%, and 44.05% for cancer, HSIL, LSIL, and normal cases, respectively. The positive rate of HPV E6/E7 mRNA in HSIL+ (HSIL or worse) group was 89.52% (94/105), which was significantly higher than that in LSIL−(LSIL or better) group (51.04%, 147/288) (P=0.000). Increasing severity of histological lesions was associated with higher positive rates of HPV E6/E7 mRNA.
Table 2.
HPV E6/E7 mRNA detection results in different histological groups
| Group | HPV E6/E7 mRNA |
Positive rate(%) | |
| Positive | Negative | ||
| Normal | 100 | 127 | 44.05 |
| LSIL | 47 | 14 | 77.05 |
| HSIL | 90 | 11 | 89.11 |
| Cancer | 4 | 0 | 100.00 |
ROC curve analysis was also used to assess the assays for detecting HSIL+. The clinically relevant portion of the area under the curve is 0.721 (95% CI, 0.667–0.775; Fig. 1).
Fig. 1.
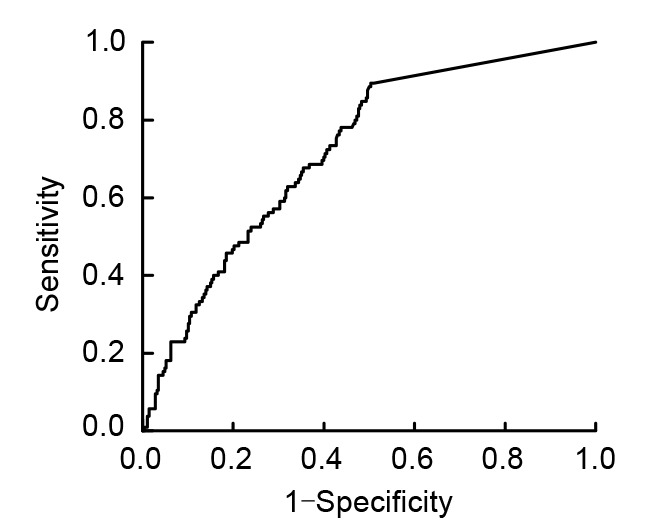
Receiver operating characteristic (ROC) curve of E6/E7 mRNA for detecting HSIL+
3.3. Concordance between HPV E6/E7 mRNA assay and cytological test
To better investigate the performance of E6/E7 mRNA and LBC for detection of samples, the concordance between these two methods was analyzed. Concordance rates between HPV E6/E7 mRNA assay and the cytology test were 81.90% (86/105) and 61.11% (176/288) in HSIL+ and LSIL−groups, respectively (Table 3).
Table 3.
Concordance between HPV E6/E7 mRNA assay and cytological test
| Group | E6/E7+/ASC-US+ | E6/E7−/NILM |
| LSIL− | 91/288 | 85/288 |
| HSIL+ | 83/105 | 3/105 |
HPV: human papillomavirus; ASC-US: atypical squamous cells of undetermined significance; NILM: negative for intraepithelial lesion or malignancy; LSIL: low-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion
3.4. Correlation of E6/E7 mRNA and cytology with histological diagnoses
The sensitivity, specificity, PPV, and NPV of the HPV E6/E7 mRNA test for detecting HSIL+ were 89.52% (94/105; 95% CI, 81.64%–94.40%), 48.96% (141/288; 95% CI, 43.07%–54.88%), 39.00% (94/241; 95% CI, 32.87%–45.50%), and 92.76% (141/152; 95% CI, 87.11%–96.15%), respectively. The sensitivity, specificity, PPV, and NPV of the cytology test for detecting HSIL+ were 86.67% (91/105; 95% CI, 78.31%–92.26%), 48.96% (141/288; 95% CI, 43.07%–54.88%), 38.24% (91/238; 95% CI, 32.09%–44.76%), and 90.97% (141/155; 95% CI, 85.03%–94.70%), respectively. No statistical difference performance parameters were found between the HPV E6/E7 mRNA test and the cytology test (Table 4).
Table 4.
Correlation of E6/E7 mRNA and cytology with histological diagnoses*
| Diagnosis | LSIL− | HSIL+ | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| E6/E7 mRNA assay | ||||||
| Negative | 141 | 11 | 89.52 (81.64–94.40) | 48.96 (43.07–54.88) | 39.00 (32.87–45.50) | 92.76 (87.11–96.15) |
| Positive | 147 | 94 | ||||
| Cytological test | ||||||
| NILM | 141 | 14 | 86.67 (78.31–92.26) | 48.96 (43.07–54.88) | 38.24 (32.09–44.76) | 90.97 (85.03–94.70) |
| ≥ASC-US | 147 | 91 | ||||
|
| ||||||
| P | 0.671 | 1.000 | 1.000 | 0.678 | ||
* Data are expressed as percentage (95% CI). LSIL: low grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion; PPV: positive predictive value; NPV: negative predictive value; NILM: negative for intraepithelial lesion or malignancy; ASC-US: atypical squamous cells of undetermined significance
4. Discussion
The two primary oncogenic transcriptions E6 and E7 mRNA are expressed by HR-HPV in the early stage of the viral life cycle. E6/E7 mRNA expressions are negatively regulated by high E2 levels. When HR-HPV DNA integrates into the host genome by disruption of E2 open reading frame, the expression of E2 decreases, preventing E2 repression on E6/E7 mRNA, and the increased E6/E7 mRNA expression in the host cell promotes cellular proliferation and malignant transformation by inactivating two tumor suppressor proteins, p53 (inactivated by E6) and pRb (inactivated by E7) (Scheffner et al., 1990; Scheurer et al., 2005). HPV E6/E7 mRNA was found to be significantly associated with the severity of cervical lesions (Cuschieri and Wentzensen, 2008). According to Liu et al. (2014), 25.7% of LSIL−cases had positive E6/E7 mRNA tests, while 71.9% of HSIL+ cases had positive E6/E7 mRNA tests. Pierry et al. (2012) found that in women under 30 years of age, 6% of benign cases, 22% of cervical intraepithelial neoplasia grade 1 (CIN1), 83% of CIN2, and 93% of CIN3 had positive E6/E7 mRNA. In our study, the positive rate of HPV E6/E7 mRNA increased as the severity of cervical lesions increased, and it was statistically higher in HSIL+ cases than that in LSIL−cases. Our results were consistent with the findings of other studies (Coquillard et al., 2011; Pierry et al., 2012; Liu et al., 2014), indicating the potential utility of HPV E6/E7 mRNA detection in cervical cancer screening.
Screening programs play a pivotal role in cervical cancer prevention, for both non-vaccinated and vaccinated women (Arbyn et al., 2012; Moyer, 2012). Accumulated evidence shows that HPV testing has advantages such as higher sensitivity (Mayrand et al., 2007; Kitchener et al., 2009; Ronco et al., 2010), prolonged screening interval (Naucler et al., 2007), and independence of cytologists in cervical cancer screening, and has been recommended as the primary cervical cancer screening in many countries (Franceschi et al., 2011). However, more than 90% women will experience HPV infection in their lifetime, of which most have a transient infection with nearly 90% eliminated spontaneously within two years and will not cause any cervical lesion (de Sanjose et al., 2007). Therefore, HPV testing alone may increase the psychological burden and may cause over referral to colposcopy. A proper triage is indispensable for HPV-positive women, and the cytology test (Pap smear and LBC) has become a widely recommended triage for HPV-positive women (Franceschi et al., 2011). Though some disadvantages of HPV testing can be overcome by cytology triage, cytology triage has some limitations. Its performance is heavily dependent on the availability of trained cytologists, and many less developed countries are lacking well-trained cytologists, and even in developed countries, cytology is flawed by poor interobserver agreement, and the interpretations may differ substantially among personnel (Condel et al., 2002).
It is very important to explore triage options which are cytologist-independent and with fewer subjective man-made factors for HPV-positive women. In our study, the performance of HPV E6/E7 mRNA detection in cervical exfoliated cells as a triage for HPV-positive women was evaluated, and some promising results were found. As a triage option for HPV-positive women, the HPV E6/E7 mRNA test achieved comparable performance with the cytology test. The results of the HPV E6/E7 mRNA test were highly concordant with cytology test results, and both triages resulted in similar sensitivity, specificity, PPV, and NPV while identifying HSIL+ lesions. Compared with cytology triage, the HPV E6/E7 mRNA test is cytologist-independent, and the result may be more objective. The HPV E6/E7 mRNA test may take the place of the cytology test of the triage program in those countries lacking cytologists.
In conclusion, our findings from a clinic-based population demonstrated that, for HPV-positive women, HPV E6/E7 mRNA detection and cytology triages have comparable performance. HPV E6/E7 mRNA detection may be an optional triage for HPV-positive women, especially in areas which lack well-trained cytologists. However, the conclusion may be somewhat limited due to the small sample size and because the present study is a hospital-based one. The conclusion cannot be generalized to the general patient population.
Footnotes
Project supported by the Special Fund for Scientific Research in the Public Interest from the National Health and Family Planning Commission of the People’s Republic of China (No. 201402010), the National Key Technology R & D Program of China (No. 2016YFC1302900), the Zhejiang Provincial Educational Project (No. Y201328819), the Zhejiang Provincial Medical & Hygienic Science and Technology Project (Nos. 2013KYB147 and 2013KYA104), and the Zhejiang Provincial Natural Science Foundation (No. LQ14H160007), China
Compliance with ethics guidelines: Ye-li YAO, Qi-fang TIAN, Bei CHENG, Yi-fan CHENG, Jing YE, and Wei-guo LU declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
- 1.Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(5):F88–F99. doi: 10.1016/j.vaccine.2012.06.095. (Available from: http://dx.doi.org/10.1016/j.vaccine.2012.06.095) [DOI] [PubMed] [Google Scholar]
- 2.Benevolo M, Vocaturo A, Caraceni D, et al. Sensitivity, specificity, and clinical value of human papillomavirus (HPV) E6/E7 mRNA assay as a triage test for cervical cytology and HPV DNA test. J Clin Microbiol. 2011;49(7):2643–2650. doi: 10.1128/JCM.02570-10. (Available from: http://dx.doi.org/10.1128/JCM.02570-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castle PE, Dockter J, Giachetti C, et al. A cross-sectional study of a prototype carcinogenic human papillomavirus E6/E7 messenger RNA assay for detection of cervical precancer and cancer. Clin Cancer Res. 2007;13(9):2599–2605. doi: 10.1158/1078-0432.CCR-06-2881. (Available from: http://dx.doi.org/10.1158/1078-0432.CCR-06-2881) [DOI] [PubMed] [Google Scholar]
- 4.Chellappan S, Kraus VB, Kroger B, et al. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. PNAS. 1992;89(10):4549–4553. doi: 10.1073/pnas.89.10.4549. (Available from: http://dx.doi.org/10.1073/pnas.89.10.4549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifford GM, Smith JS, Aguado T, et al. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003;89(1):101–105. doi: 10.1038/sj.bjc.6601024. (Available from: http://dx.doi.org/10.1038/sj.bjc.6601024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Condel JL, Mahood LK, Grzybicki DM, et al. Papanicolaou tests diagnosed as atypical by a cytotechnologist and downgraded to benign by a pathologist: a measure of laboratory quality. Am J Clin Pathol. 2002;117(4):534–540. doi: 10.1309/WVW0-48TJ-E7VA-DM8R. (Available from: http://dx.doi.org/10.1309/WVW0-48TJ-E7VA-DM8R) [DOI] [PubMed] [Google Scholar]
- 7.Coquillard G, Palao B, Patterson BK. Quantification of intracellular HPV E6/E7 mRNA expression increases the specificity and positive predictive value of cervical cancer screening compared to HPV DNA. Gynecol Oncol. 2011;120(1):89–93. doi: 10.1016/j.ygyno.2010.09.013. (Available from: http://dx.doi.org/10.1016/j.ygyno.2010.09.013) [DOI] [PubMed] [Google Scholar]
- 8.Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2536–2545. doi: 10.1158/1055-9965.EPI-08-0306. (Available from: http://dx.doi.org/10.1158/1055-9965.EPI-08-0306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453–459. doi: 10.1016/S1473-3099(07)70158-5. (Available from: http://dx.doi.org/10.1016/S1473-3099(07)70158-5) [DOI] [PubMed] [Google Scholar]
- 10.Discacciati MG, Da SI, Villa LL, et al. Prognostic value of DNA and mRNA E6/E7 of human papillomavirus in the evolution of cervical intraepithelial neoplasia grade 2. Biomark Insights. 2014;9:15–22. doi: 10.4137/BMI.S14296. (Available from: http://dx.doi.org/10.4137/BMI.S14296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franceschi S, Denny L, Irwin KL, et al. Eurogin 2010 roadmap on cervical cancer prevention. Int J Cancer. 2011;128(12):2765–2774. doi: 10.1002/ijc.25915. (Available from: http://dx.doi.org/10.1002/ijc.25915) [DOI] [PubMed] [Google Scholar]
- 12.Giorgi Rossi P, Benevolo M, Vocaturo A, et al. Prognostic value of HPV E6/E7 mRNA assay in women with negative colposcopy or CIN1 histology result: a follow-up study. PLoS ONE. 2013;8(2):e57600. doi: 10.1371/journal.pone.0057600. (Available from: http://dx.doi.org/10.1371/journal.pone.0057600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho CM, Lee BH, Chang SF, et al. Type-specific human papillomavirus oncogene messenger RNA levels correlate with the severity of cervical neoplasia. Int J Cancer. 2010;127(3):622–632. doi: 10.1002/ijc.25078. (Available from: http://dx.doi.org/10.1002/ijc.25078) [DOI] [PubMed] [Google Scholar]
- 14.Jeantet D, Schwarzmann F, Tromp J, et al. NucliSENS EasyQ HPV v1 test–testing for oncogenic activity of human papillomaviruses. J Clin Virol. 2009;45(1):S29–S37. doi: 10.1016/S1386-6532(09)70006-X. (Available from: http://dx.doi.org/10.1016/S1386-6532(09)70006-X) [DOI] [PubMed] [Google Scholar]
- 15.Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10(7):672–682. doi: 10.1016/S1470-2045(09)70156-1. (Available from: http://dx.doi.org/10.1016/S1470-2045(09)70156-1) [DOI] [PubMed] [Google Scholar]
- 16.Liu TY, Xie R, Luo L, et al. Diagnostic validity of human papillomavirus E6/E7 mRNA test in cervical cytological samples. J Virol Methods. 2014;196:120–125. doi: 10.1016/j.jviromet.2013.10.032. (Available from: http://dx.doi.org/10.1016/j.jviromet.2013.10.032) [DOI] [PubMed] [Google Scholar]
- 17.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588. doi: 10.1056/NEJMoa071430. (Available from: http://dx.doi.org/10.1056/NEJMoa071430) [DOI] [PubMed] [Google Scholar]
- 18.Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. (Available from: http://dx.doi.org/10.7326/0003-4819-156-12-201206190-00424) [DOI] [PubMed] [Google Scholar]
- 19.Naucler P, Ryd W, Tornberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357(16):1589–1597. doi: 10.1056/NEJMoa073204. (Available from: http://dx.doi.org/10.1056/NEJMoa073204) [DOI] [PubMed] [Google Scholar]
- 20.Pierry D, Weiss G, Lack B, et al. Intracellular human papillomavirus E6, E7 mRNA quantification predicts CIN 2+ in cervical biopsies better than Papanicolaou screening for women regardless of age. Arch Pathol Lab Med. 2012;136(8):956–960. doi: 10.5858/arpa.2011-0180-OA. (Available from: http://dx.doi.org/10.5858/arpa.2011-0180-OA) [DOI] [PubMed] [Google Scholar]
- 21.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11(3):249–257. doi: 10.1016/S1470-2045(09)70360-2. (Available from: http://dx.doi.org/10.1016/S1470-2045(09)70360-2) [DOI] [PubMed] [Google Scholar]
- 22.Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. (Available from: http://dx.doi.org/10.1016/0092-8674(90)90409-8) [DOI] [PubMed] [Google Scholar]
- 23.Scheurer ME, Tortolero-Luna G, Adler-Storthz K. Human papillomavirus infection: biology, epidemiology, and prevention. Int J Gynecol Cancer. 2005;15(5):727–746. doi: 10.1111/j.1525-1438.2005.00246.x. (Available from: http://dx.doi.org/10.1111/j.1525-1438.2005.00246.x) [DOI] [PubMed] [Google Scholar]
- 24.Schiffman M, Wentzensen N, Wacholder S, et al. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103(5):368–383. doi: 10.1093/jnci/djq562. (Available from: http://dx.doi.org/10.1093/jnci/djq562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siebers AG, Klinkhamer PJ, Grefte JM, et al. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors: a randomized controlled trial. JAMA. 2009;302(16):1757–1764. doi: 10.1001/jama.2009.1569. (Available from: http://dx.doi.org/10.1001/jama.2009.1569) [DOI] [PubMed] [Google Scholar]
- 26.Sorbye SW, Arbyn M, Fismen S, et al. HPV E6/E7 mRNA testing is more specific than cytology in post-colposcopy follow-up of women with negative cervical biopsy. PLoS ONE. 2011;6(10):e26022. doi: 10.1371/journal.pone.0026022. (Available from: http://dx.doi.org/10.1371/journal.pone.0026022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sotlar K, Stubner A, Diemer D, et al. Detection of high-risk human papillomavirus E6 and E7 oncogene transcripts in cervical scrapes by nested RT-polymerase chain reaction. J Med Virol. 2004;74(1):107–116. doi: 10.1002/jmv.20153. (Available from: http://dx.doi.org/10.1002/jmv.20153) [DOI] [PubMed] [Google Scholar]
- 28.Tavassoéli FA, Devilee P. Pathology and Genetics Tumours of the Breast and Female Genital Organs. Lyon, France: IARC Press; 2003. [Google Scholar]
- 29.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. (Available from: http://dx.doi.org/10.3322/caac.21262) [DOI] [PubMed] [Google Scholar]
- 30.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. (Available from: http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1) [DOI] [PubMed] [Google Scholar]
- 31.Wright TJ, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med. 2003;348(6):489–490. doi: 10.1056/NEJMp020178. (Available from: http://dx.doi.org/10.1056/NEJMp020178) [DOI] [PubMed] [Google Scholar]
- 32.Zappacosta R, Caraceni D, Ciccocioppo L, et al. Implementing specificity of HPV-DNA primary screening in a successful organised cervical cancer prevention programme. Gynecol Oncol. 2013;128(3):427–432. doi: 10.1016/j.ygyno.2012.11.030. (Available from: http://dx.doi.org/10.1016/j.ygyno.2012.11.030) [DOI] [PubMed] [Google Scholar]


