Abstract
In this work, we present the result of an electric coagulation process with iron and aluminum electrodes for removal of chemical and biological oxygen demand (COD and BOD) from grey water in different car washes of Ahvaz, Iran. Nowadays, one of the important dangerous that can contaminate water resources for drinking, agriculture and industrial is Car wash effluent [1,2]. In this study, initial COD and BOD concentration, pH of the solution, voltage power and reaction time was investigated. The concentration level of remaining COD and BOD in samples was measured, using DR/5000 UV–vis HACH spectrophotometer [3,4]. The effects of contact time, initial pH, electrical potential and voltage data on removal of COD and BOD were presented. Statistical analysis of the data was carried out using Special Package for Social Sciences (SPSS 16).
Keywords: Grey water effluent, Electrocoagulation, COD removal, BOD removal
Specifications Table
| Subject area | Environment |
| More specific subject area | Chemical and biological oxygen demand |
| Type of data | Table, figure |
| How data was acquired | DR/5000 UV–vis HACH spectrophotometer |
| Data format | Raw, analyzed |
| Experimental factors |
|
| Experimental features | Electrocoagulation between many treatment processes having to be cost-effective for wastewater treatment with pollutant wide range. |
| Data source location | Ahvaz, Iran |
| Data accessibility | Data is with this article. |
Value of the data
-
•
These data describe changes in COD and BOD removal from grey water by electrocoagulation process.
-
•
Data show that electrocoagulation can be used as cost-effective for removal of other pollutant from wastewater.
-
•
Data of this study can be used to design the electrocoagulation experiments for removal of wide range of pollutant in wastewater.
-
•
Data are important for discharge environment especially resource water, aqueous and agriculture.
1. Data
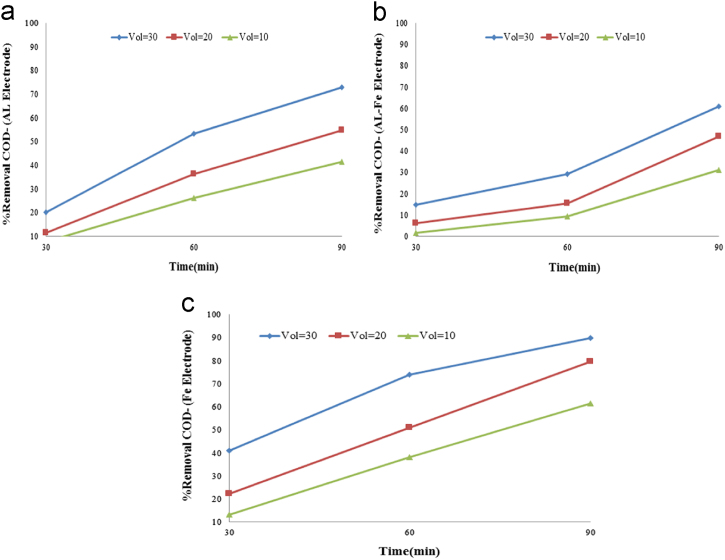
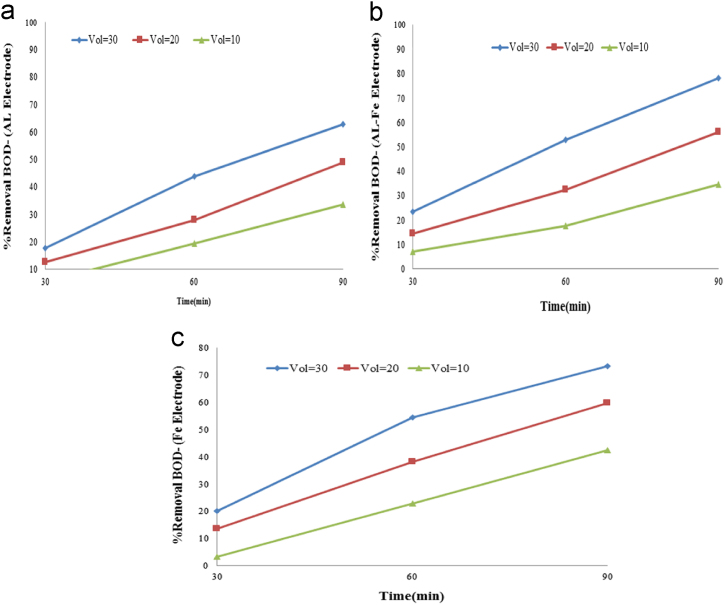
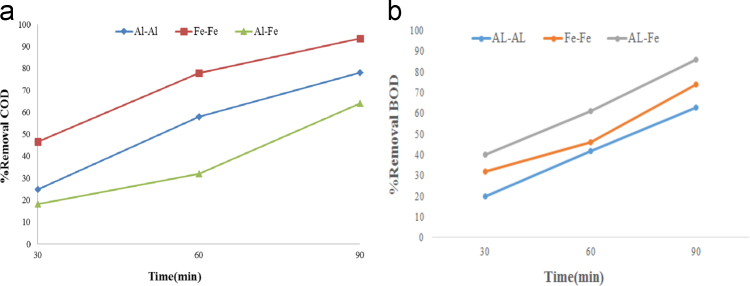
In this article the data in Table 1 present the measured parameters and characteristics of the raw grey water that used for description of experiments. Calculated values of K (1/min) and kWh/m3 in the grey water effluent are reported in Table 2. Fig. 1, Fig. 2 show data of different arrangements under optimal conditions applied in this study. The maximum removal efficiency (90.18%) of COD and BOD was obtained at optimum pH=7, level of 30 voltage, and contact time of 90 min. The effects of optimum parameters on removal efficiency of COD and BOD are shown in Fig. 3.
Table 1.
Parameters measured and characteristics of the raw carwash wastewater used for this study.
| Parameter | Range | Unit | Raw wastewater |
|---|---|---|---|
| Mean±S.D | |||
| pH | 3, 7, 11 | – | 7.08±0.03 |
| Steering time | 30, 60, 90 | min | – |
| Voltage | 10, 20, 30 | Volt | – |
| Electrode type | Al–Al, Fe–Fe, Al–Fe | – | – |
| BOD | – | (mg/L) | (102−246)±207.3 |
| COD | – | (mg/L) | (480−1560)±207.3 |
Table 2.
Electrode type, voltage, pH, K (1/min) and kWh/m3 values in the removal of COD and BOD in the present study.
| Electrode type | Voltage | pH | K (1/min) | kWh/m3 | |
|---|---|---|---|---|---|
| Fe–Fe | 30 | 7 | 14.15 | 787.5 | |
| 3 | 11.61 | 1575 | |||
| 11 | 8.73 | 2362.5 | |||
| 20 | 7 | 10.29 | 450 | ||
| 3 | 9.76 | 900 | |||
| 11 | 9.24 | 1350 | |||
| 10 | 7 | 8.53 | 189 | ||
| 3 | 4.43 | 378 | |||
| 11 | 3.62 | 567 | |||
| Al–Al | 30 | 7 | 18.24 | 675 | |
| 3 | 13.92 | 1350 | |||
| 11 | 15.10 | 2025 | |||
| 20 | 7 | 14.58 | 330 | ||
| 3 | 10.80 | 660 | |||
| 11 | 10.51 | 990 | |||
| 10 | 7 | 12.23 | 159 | ||
| 3 | 8.27 | 318 | |||
| 11 | 8.91 | 477 | |||
| Al–Fe | 30 | 7 | 13.99 | 900 | |
| 3 | 13.89 | 1800 | |||
| 11 | 11.42 | 2700 | |||
| 20 | 7 | 11.79 | 540 | ||
| 3 | 9.77 | 1080 | |||
| 11 | 11.30 | 1620 | |||
| 10 | 7 | 9.76 | 240 | ||
| 3 | 8.17 | 480 | |||
| 11 | 6.21 | 720 |
K (1/min) is the rate constant of removal (1/min) related to the removal of COD and BOD.
Fig. 1.
(a) Aluminum electrode, (b) Aluminum – Iron electrode, and (c) Iron electrode applied in the different Voltage on COD removal efficiency .
Fig. 2.
(a) Aluminum electrode, (b) Aluminum – Iron electrode, and (c) Iron electrode applied in the different Voltage on BOD removal efficiency .
Fig. 3.
(a) Aluminum electrode, (b) Aluminum – Iron electrode, and (c) Iron electrode applied in the optimum pH=7 and voltage=30 on COD and BOD removal efficiency.
2. Experimental design, materials and methods
2.1. Sample collection and analytical procedures
Our data set was obtained from All Car washes. The raw grey water was obtained along the Ahvaz in Iran. The initial concentration of samples has been tested for determination of COD and BOD. To adjust the primary pH of the solution, the sulfuric acid and one-tenth normal sodium hydroxide were used. A lab-scale reactor with diameters of 15 cm×15 cm×15 cm was used for performing experiments. Sulfuric acid (H2SO4), potassium dichromate (K2Cr2O7), mercury sulfate (HgSO4), silver sulfate (Ag2SO4), potassium hydrogen phthalate (C8H5KO4), 3-methyl-2-benzothiazoline hydrazine were used for preparing COD and BOD solutions in grey water. Steering time of 30, 60 and 90 min, voltage values of 10, 20 and 30 v were used in this study. At each experiment, removal efficiency of COD and BOD in grey water with special Al–Al, Al–Fe, Fe–Fe electrode was investigated. Spectrophotometer (DR/5000 UV–vis HACH) was used to investigate the remaining concentration level of COD and BOD in the grey water effluent [5]. Following equation was applied to calculate the electrocoagulation electrical energy consumption during experiments [4], [5].
where: U is voltage used in the process (V), I is intensity of the applied current (A), t is reaction time (min) and Vr is reactor volume (Lit).
Acknowledgments
The authors would like to thank student Research committee, Ahvaz Jundishapur University of Medical Sciences for providing financial supported by grant: (95s45) of this research.
Footnotes
Transparency data associated with this article can be found in the online version at 10.1016/j.dib.2017.03.006.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.Takdastan A., Azimi A., Jaafarzadeh N. Biological excess sludge reduction in municipal wastewater treatment by chlorine. Asian. J. Chem. 2010;22:1665–1670. [Google Scholar]
- 2.Hassani G., Babaei A.A., Takdastan A., Yousefian F., Mohammadi M.J. Occurrence and fate of 17β-estradiol in water resources and wastewater in Ahvaz. Iran, Global. Nest. J. 2016;18:855–866. [Google Scholar]
- 3.Bazrafshan E., KordMostafapoor F., Soori M.M., Mahvi A.H. Application of combined chemical coagulation and electrocoagulation process to carwash wastewater treatment. Fresen. Environ. Bull. 2012;21:2694–2701. [Google Scholar]
- 4.Al-Shannag M., Bani-Melhem K., Al-Anber Z., Al-Qodah Z. Enhancement of COD-nutrients removals and filterability of secondary clarifier municipal wastewater influent using electrocoagulation technique. Sep. Sci. Technol. 2013;48:673–680. [Google Scholar]
- 5.Kobya M., Delipinar S. Treatment of the baker׳s yeast wastewater by electrocoagulation. J. Hazard. Mater, 2008;154:1133–1140. doi: 10.1016/j.jhazmat.2007.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material