Abstract
Introduction and objective
The left ventricular pseudoaneurysm (LVP) is rare, the surgical experience is limited and its surgical treatment remains still a challenge with an elevated mortality. Herein, it is presented a retrospective analysis of our experience with acquired post infarct LVP over a10-year period.
Materials and methods
Between January 2006 through August 2016, a total of 13 patients underwent operation for post infarct pseudoaneurysm of the left ventricle. There were 10 men and 3 women and the mean age was 61 ± 7.6 years. 4 patients presented acute LVP. Two patients had preoperative intraortic balloon pump implantation.
Results
Various surgical techniques were used to obliterate the pseudoaneurysm such as direct pledgeted sutures buttressed by polytetrafluoroethylene felt, a Gore-Tex or Dacron patch, transatrial closure of LVP neck in submitral pseudoaneurysm, or linear closure in cases presenting associated postinfarct ventricular septal defect. Concomitant coronary artery bypasses were performed for significant stenoses in 12 patients, ventricular septal defect closure in 4 patients, mitral valve replacement in 3 and aortic valve replacement in 1 patient. Operative mortality was 30.8% (4 patients). Three of them were acute LVP. Three patients required the continuous hemodyalisis and 8 patients required intra-aortic balloon pump. At follow-up two deaths occurred at 1 and 3 years after surgery.
Conclusion
In conclusion, this study revealed that surgical repair of post infarct left ventricular pseudoaneurysm was associated with an acceptable surgical mortality rate, that cardiac rupture did not occur in surgically treated patients.
Keywords: Left ventricular pseudoaneurysm, Myocardial infarction, Rupture free wall
Highlights
-
•
Objective: Our experience with post-infarction left ventricular pseudoaneurysms (LVP) and surgical techniques in 13 patients.
-
•
Various techniques: 1) direct pledgeted sutures; 2) single patch; 3) double-patch; 4) pericardial patch through the left atrium.
-
•
Hospital mortality 4 (30.7%). Literature review: 306 patients with LVP undergoing surgery with 21.2% (65 deaths) mortality.
-
•
In conclusion, this study revealed that surgical repair of LVP was associated with an acceptable surgical mortality rate.
-
•
Cardiac rupture did not occur. Various techniques are available and should be considered according to the case presentation.
1. Introduction
Acquired pseudoaneurysm of the left ventricle is a rare disorder that usually occurs after transmural myocardial infarction or after cardiac surgery [1], [2], [3]. Myocardial infarction is the most common cause of false aneurysms of the left ventricle, followed by cardiac surgery, trauma [6], and infection [7]. Rupture of the free wall of the left ventricle due to myocardial infarction occurs in almost 4% of patients with infarcts and in 23% of those dying to myocardial infarction [4], [5]. Acute pseudoaneurysms, a variant of myocardial rupture, are extremely unstable and bound to fatal rupture. Chronic pseudoaneurysms are usually detected because of symptoms, less often incidentally [1], [3]. Pseudoaneurysms develop when cardiac rupture is contained by pericardial adhesions.
Pseudoaneurysms of the left ventricle have a strong tendency to rupture, leading to death if it is left surgically untreated. In particular, asymptomatic pseudoaneurysms that occur a few days after acute myocardial infarction are extremely unstable and tend to rupture [8]. The left ventricular pseudoaneurysms may be associated with other mechanical complications of the myocardial infarction such as ischemic mitral valve regurgitation [9] or interventricular septal defect [10]. The purpose of this article is to present a retrospective analysis of our surgical experience with post-infarction pseudoaneurysms.
2. Materials and methods
During the period from January 2006 through August 2016, a total of 13 consecutive patients underwent operation for pseudoaneurysm of the left ventricle in our universitary institutions. They were evaluated retrospectively. All patients with post infarction free-wall rupture and left ventricular true aneurysms were excluded from the study.
The study has been registered in Research registry under the number researchregistry2246 under the accordance of the declaration of Helsinki. Also the work has been reported in line with the PROCESS [11].
There were 10 men and 3 women and the mean age was 61 ± 7.6years. All of the left ventricular pseudoaneurysms were discovered after transmural myocardial infarction. The diameters of the pseudoaneurysms were calculated by using transthoracic echocardiography in all patients. The mean maximum diameter of the pseudoaneurysms was 4.2 ± 0.7 cm. They were categorized as acute when discovered within 2 weeks of myocardial infarction and as chronic when discovered more than 2 weeks after the event. In the chronic forms, the mean interval between myocardial infarction and diagnosis was 6.2 ± 3.3months. In acute forms, the mean interval between myocardial infarction and diagnosis was 4.8 ± 3.3days and immediate surgery was undertaken upon diagnosis was made. Two patients had preoperative intraortic balloon pump implantation. Preoperative clinical features of the patients are presented in Table 1, Table 3.
Table 1.
Preoperative characteristics of patients with left ventricular pseudoaneurysm.
| Variables | Nr/% |
|---|---|
| Mean age (years) | 61 ± 7.6 |
| Male | 10 (77%) |
| Mean NYHA functional class | 3.1 ± 1.2 |
| Left main trunk | 5 (38.5%) |
| Mean LVEF (%) | 37.7 ± 8.5 |
| Mitral regurgitation grade | 2.2 ± 1.3 |
| LVEDD (mm) | 68 ± 24 |
| LVESD (mm) | 44.5 ± 19 |
| Preoperative PAP | 49 ± 18 |
| Mean maximal diameter of the LVP(cm) | 4.2 ± 0.7 |
| Acute pseudoaneurysm | 4 (30.8%) |
| Diabetes mellitus | 8 (61.5%) |
| Hypertension | 10 (77%) |
| Smoking | 11 (85%) |
| COPD | 9 (69%) |
| Peripheral vascular disease | 2 (15.4%) |
| CVD | 1 (7.7%) |
| Preoperative IABP | 2 (15.4%) |
| Chronic renal failure | 1 (7.7%) |
Legend: NYHA-New York Heart Association, LVEF-Left Ventricular Ejection Fraction, LVEDD-Left Ventricular End-Diastolic Diameter, LVESD-Left Ventricular End-Systolic Diameter, Papa-Pulmonary Artery Pressure, LVP-Left Ventricular Pseudoaneurysm, COPD-Chronic Obstructive Pulmonary Disease, CVD-Cerebrovascular Disease, IABP-Intra Aortic Ballon Pump.
Table 3.
Clinical characteristics of patients with left ventricular pseudoaneurysm.
| Gender/Age | MP | Timing Location/LVEF | 3-vessel CD | MI | Closure technique | Assoc procedure | ICU | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1/M/70 | Angina | Chronic Inferior/40% | yes, Left main | RCx | Patch closure | CABG | IABP | Survived |
| 2/F/76 | CHF, Angina | Acute Lateral/28% | yes | Cfx | Direct suture | CABG | IABP, CVVHD | Died (MOF) |
| 3/M/56 | CHF | Acute Inferior/35% | yes, VSD | RCx | Patch&Linear clos | CABG, VSD clos | IABP | Survived |
| 4/M 53 | CHF,Angina | Chronic Inferior/30% | yes,VSD | LAD | Patch&Linear clos | VSD closure | IS | Survived |
| 5/M/60 | CHF,Angina | Chronic Lateral/38% | yes, IMR | Cfx | Int Patch closure | CABG, MVR | IS | Survived |
| 6/M/62 | CHF | Acute Inferior/30% | yes,VSD | Rcx | Patch&Linear clos | CABG/VSD clos | IABP | Died |
| 7/M/60 | CHF | Acute Inferior/30% | yes, VSD | Cfx | Double patch, ET | CABG, VSD clos | IABP, CVVHD | Died |
| 8/M/65 | Angina | Chronic Inferior/48% | yes | RCx | Direct suture | CABG | IS | Survived |
| 9/M/54 | Angina | Chronic Inferior/45% | yes | RCx | Direct suture | CABG | IABP | Survived |
| 10/F/57 | CHF | Chronic Inferior/40% | yes, IMR | Cfx | Int Patch closure | CABG, MVR | IABP | Survived |
| 11/M/58 | Angina | Chronic Inferior/50% | yes | RCx | Direct suture | CABG | IS | Survived |
| 12/F/72 | CHF | Chronic Lateral/27% | single vessel | Cfx | Patch closure, ET | CABG,AVR,MVR | IABP, CVVHD | Died (MOF) |
| 13/M/52 | CHF | Chronic Inferior/50% | yes | RCx | Patch closure, ET | CABG | IS | Survived |
Legend: MP-Mode of presentation, CHF-Congestive Heart Failure, VSD-Ventricular septal defect, iMR-Ischemic mitral regurgitation, iVSD-inferior VSD, aVSD-apical VSD, CABG-Coronary artergypass grafting, MVR-Mitral Valve replacement, AVR-Aortic valve replacement, IABP-Intraortic balloon pump, CVVHHD-continuo veno-venous ultrafiltration, IS-Inotropic support, RCx-Right coronary artery, Cfx-Circumflex coronary artery, LAD-Left anterior descending artery, LVEF-Left ventricular ejection fraction, CD-coronary disease, MI-Myocardial infarction, ICU-Intensive care unit, MOF-multi organ failure, ET-exclusion technique, clos-closure.
2.1. Diagnostic testing
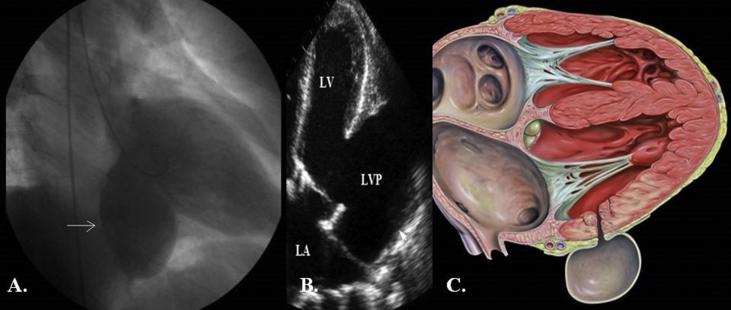
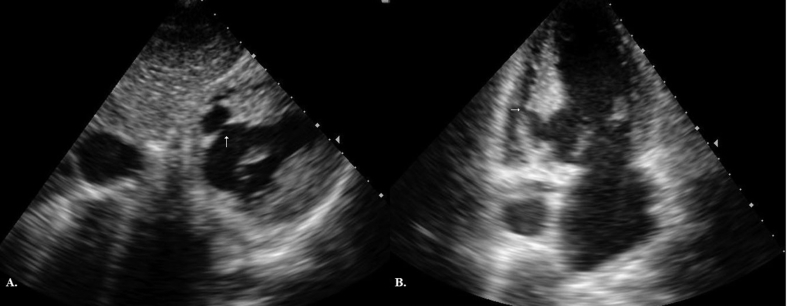
Diagnostic investigation of ventricular pseudoaneurysm included contrast ventriculography in 10 patients (77%), transesophageal echocardiography in 9 (69%), transthoracic echocardiography in 13 (100%). On cross-sectional echocardiography the diagnosis was aided by a sharp discontinuity of the endocardium at the site where the pseudoaneurysm communicated with the left ventricle. On contrast ventriculography, a paraventricular mass with a narrow neck was typically seen. However in three patients, the ventriculography demonstrated left ventricular aneurysm (Fig. 1A) as in our first patient (Table 3). The transthoracic echocardiography revealed the presence of a left ventricular pseudoaneurysm (Fig. 1B) with the respective schematic presentation (Fig. 1C), which was diagnosed intraoperatively as a chronic left ventricular pseudoaneurysm with almost total absence of the posterior wall of the left ventricle (Fig. 3A).
Fig. 1.
A. Left ventriculography demonstrating a left ventricular aneurysm. B. Transthoracic echocardiography demonstrating a left ventricular pseudoaneurysm. C. Schematic presentation. Legend: LVP-left ventricular pseudoaneurysm; LA = Left atrium; LV-Left ventricule.
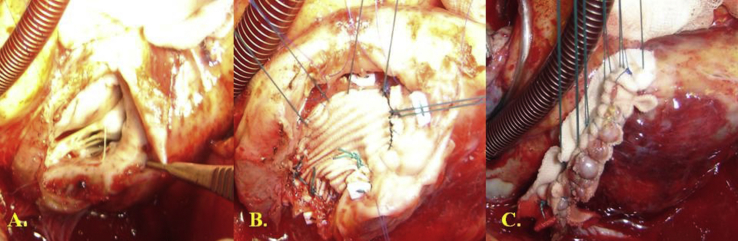
Fig. 3.
A. Posterior left ventricular pseudoaneurysm. B. Patch closure with separated sutures. C. Left ventricular wall closure above the patch.
2.2. Surgical techniques
All patients underwent midline sternotomy and cardiopulmonary bypass. Five patients underwent emergent operation because of signs of cardiovascular collapse or imminent cardiac rupture (cardiac tamponade). Cardiopulmonary bypass was instituted through the femoral vessels in 4 of them. Complete dissection of the heart was performed after cross-clamping the aorta, in an effort to avoid systemic embolization. In all other patients, ascending aorta and double venous or double-stage venous cannulation in patients undergoing interventricular septal defect or mitral valve replacement was performed. Antegrade cold blood cardioplegia was delivered after the aorta was clamped. With the anatomy identified, especially location and extent of the myocardial infarction, the decision about surgical technique was made. The dissection was initially limited to the anterior surface of the heart. Complete dissection of the heart and repair of the pseudoaneurysm was performed after cross-clamping the aorta. Ventricular fibrillation was electrically induced during repair of the pseudoaneurysm. The heart was then gently mobilized from pericardium and loose adhesions were taken down. In 4 patients the pseudoaneurysm was opened incidentally during the dissection. The bleeding site was identified and controlled by finger compression.
Various techniques were used to obliterate the pseudoaneurysm.
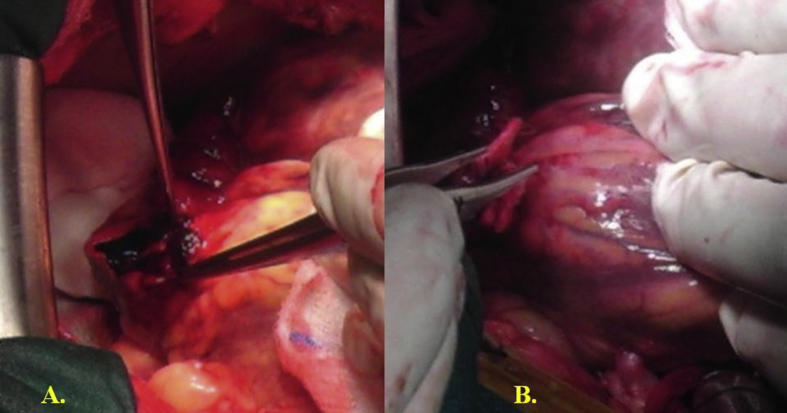
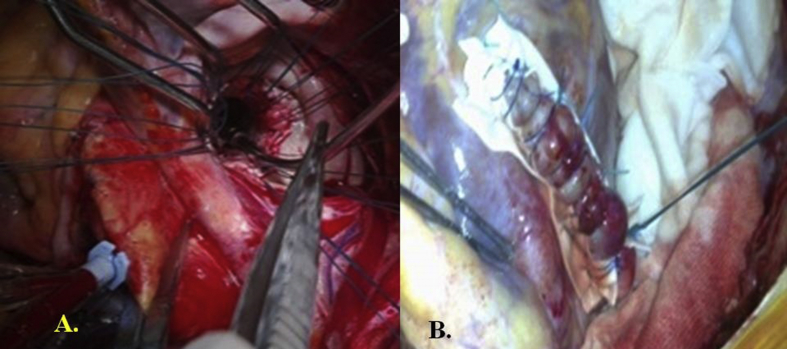
Surgical procedure 1. In 4 patients whose defect had a small neck with densely fibrotic edges, primary repair was performed using pledgeted sutures buttressed by polytetrafluoroethylene (PTFE) felt (Fig. 2).
Fig. 2.
A. Opening of the posterior left ventricular aneurysm and identification of the wall perforation. B. Closure of the left ventricular pseudoaneurysm using pledgeted sutures buttressed by polytetrafluoroethylene.
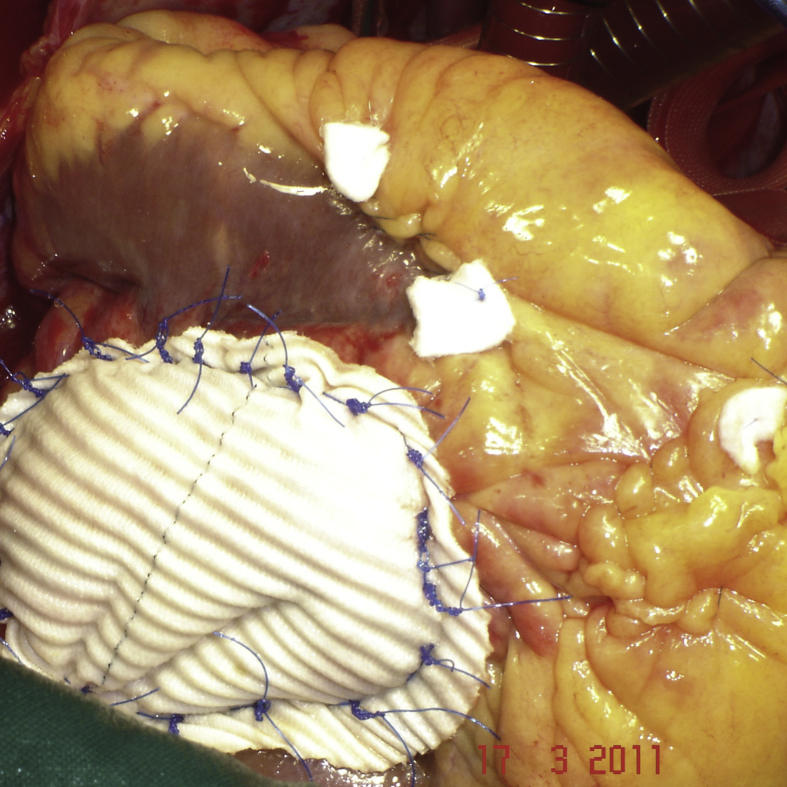
Surgical procedure 2.In 3 patients with a defect located close to the basal part of the left ventricle, the ventricular defect was closed with a patch of Gore-Tex (W.L. Gore and Associates, Flagstaff, AZ,®) to avoid potential distortion of the heart structures or excessive traction on the edges of the defect (Fig. 3). The capsule of the pseudoaneurysm was not resected but rather was used as a second layer over the patch for reinforcing the reconstructed ventricular wall and for hemostasis. The same technique consisting in patch closure of the left ventricular pseudoaneurysm without associated linear closure but only supported with Bioglue was employed in another patient (Fig. 4).
Fig. 4.
Patch closure of the left ventricular pseudoaneurysm in another patient without associated linear closure.
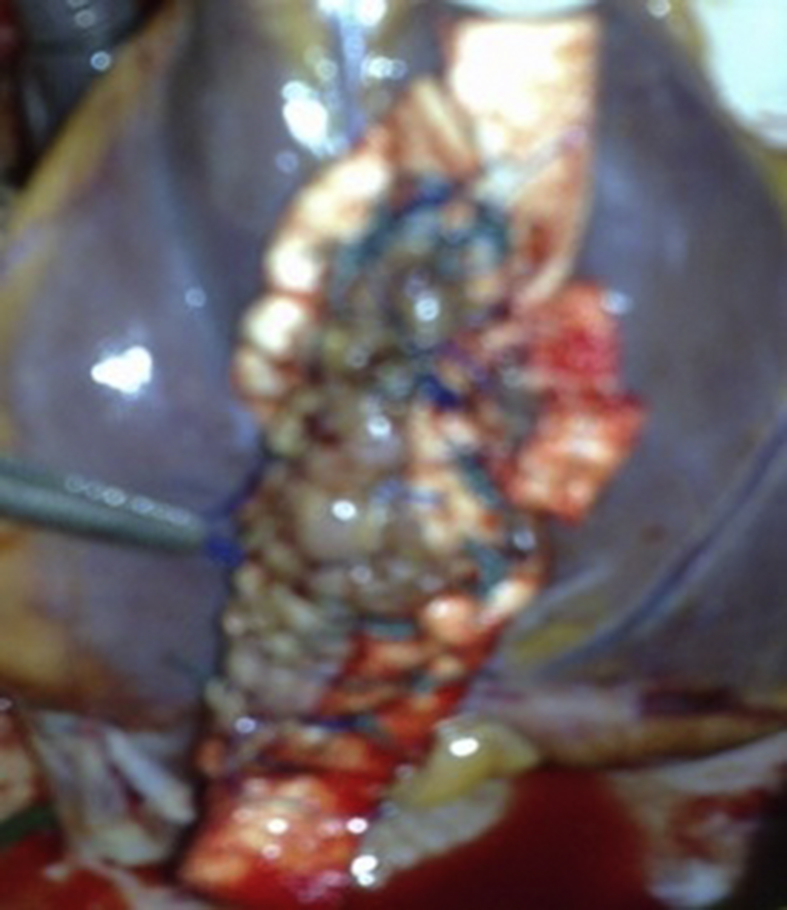
Surgical procedure 3. In 2 other patients with severe mitral valve regurgitation, the neck of the pseudoaneurysm was closed through the left atrium using a patch of autologous pericardium (Fig. 5).
Fig. 5.
A.The neck of the pseudoaneurysm is closed through the left atrium using a patch of autologous pericardium. B. The left ventricular wall repaired externally with a linear technique.
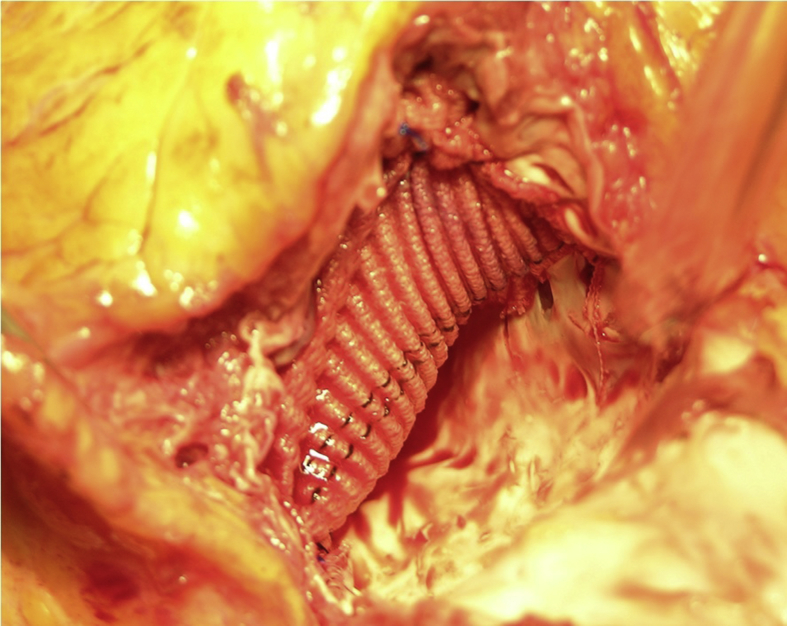
Surgical procedure 4. In another patient with post infarct interventricular septal defect and left ventricular pseudoaneurysm (Fig. 6A and B), a double patch Dacron (Terumo, Somerset, NJ,®) was employed to close the left ventricular pseudoaneurysm internally and externally the left ventricle (Fig. 7).
Fig. 6.
A. The neck of the inferior left ventricular pseudoaneurysm. B. The left ventricular pseudoaneurysm extending into the interventricular septum associated with a small septal defect.
Fig. 7.
Double patch (Dacron) was employed to close the left ventricular pseudoaneurysm internally and externally the left ventricle.
Surgical procedure 5. In two other patients with associated inferior interventricular septal defect, the postero-inferor wall of the left ventricle was opened at the bleeding site and after the defect was closed using a Dacron patch, the left ventricular wall was closed according to the linear technique, employing two strips of Teflon and continuous Prolene 3.0 suture (Fig. 8).
Fig. 8.
A. The opening of the left ventricular pseudoaneurysm and identification of the inferior ventricular septal defect. B. Closure of the septal defect using a Dacron patch. C. Left ventricular wall was closed according to the linear technique, employing two strips of Teflon and continuous Prolene 3.0 suture.
Surgical procedure 6. Another patient presented an inferior post infarction interventricular septal defect and posterior small pseudoaneurysm of the left and right ventricle very close to the posterior interventricular sulcus. The interventricular septal defect was closed with a Dacron patch which than was included between two layers of Teflon as demonstrated in Fig. 9.
Fig. 9.
Closure the interventricular septal defect with a Dacron patch which than was included between two layers of Teflon in the linear closure of the ventricular pseudoaneurysm.
Biological glue was applied in 6 patients to prevent bleeding. Concomitant coronary artery bypasses were performed for significant stenoses in 12 patients, post infarct interventricular septal defect closure in 4 patients, mitral valve replacement in 3 and aortic valve replacement in 1 patient.
3. Results
All patients underwent surgical correction. The mean cardiopulmonary bypass time was 183 ± 63 min and mean aortic cross clamping time was 146 ± 47 min. The left ventricular pseudoaneurysm was associated with post infarction interventricular septal defect which was closed in 4 (30.8%) patients. Mitral valve repair or replacement was performed in 3 (23%) patients and the aortic valve replacement in 1 (7.7%) patient. All patients underwent coronary revascularization and the mean grafts per patient was 2.8 ± 1.2, Operative and postoperative data are given Table 2, Table 3. In all patients, resection of the pseudoaneurysm and closure of the ventricular wall defect was performed without any sequela, such as embolus or mechanical complication. Four patients died in the immediate postoperative period. The overall hospital mortality was 30.8% (4 patients). Three of them died after repair of an acute pseudoaneurysm and another patient died after repair of a chronic pseudoaneurysm. Two patients died of progressive multiple organ failure; the remaining 2 died due to cardiac failure. Three patients required the employment of continuous veno-venous hemodyalisis within 3 h after surgery due to acute failure and total postoperative anuria. Six other patients required intraortic balloon pump implantation and 2 other patients that had preoperative intraortic balloon pump continued the support postoperatively. At follow-up another additional death occurred within the first year after surgery most probably due to arrhythmias. Another patient died 3 years after the operation due to cardiac failure. Another patient underwent percutaneous transluminal angioplasty 2 years after surgery.
Table 2.
Postoperative data of patients with left ventricular pseudoaneurysm.
| Variables | Nr/% |
|---|---|
| Mean mechanical ventilation (hours) | 52 ± 23 |
| Mean ICU stay (days) | 4.3 ± 1.7 |
| Hospital death | 4 (30.8%) |
| Intra-aortic balloon pump | 7 (54%) |
| Atrial fibrillation | 6 (46%) |
| Reexploration for bleeding | 1 (7.7%) |
| Mean units of blood transfusion | 3.8 ± 2.3 |
| Renal failure | 6 (46%) |
| CVVHD | 3 (23%) |
| Sepsis | 1 (7.7%) |
| Postoperative hospital stay (days) | 18 ± 7 |
Legend: CPB-Cardiopulmonary Bypass, XCL-Aortic Cross Clamp, VSD-Interventricular Setal Defect, ICU-Intensive care Unit, CVVHD-Continuous Veno-Venous Hemodyalisis.
4. Discussion
Acquired left ventricular pseudoaneurysms develop after transmural myocardial infarction (55% in reviews), surgery (33%), trauma (7%), or infection (5%) [1], [2], [3], [12], [13]. Rupture of the left ventricle after myocardial infarction usually leads to acute pericardial tamponade and immediate death. One of the mechanical complications of the myocardial infarction is the left ventricular free wall rupture which occurs in stages and progresses from endocardium to pericardium. Few patients survive due to formation of an adherent thrombus or pericardial adhesions. Pericardial adhesions may be present or may develop de novo during the rupture. Pseudoaneurysms have been reported to originate usually at the posterior basal and rarely at the apical segment of the left ventricle after occlusion of the right coronary or left anterior descending artery [14].
Left ventricular pseudoaneurysms are characterized by a small, narrow-necked channel that connects the ventricle with a larger aneurysmal sac, which contains blood and thrombus and is lined by fibrous pericardial tissue with no myocardial elements [15]. A post-infarction true aneurysm, in contrast, is caused by scar formation that results in thinning of the myocardium. Most true left ventricular aneurysms occur anteriorly, consequent to occlusion of the left anterior descending artery. However in some chronic left ventricular pseudoaneurysms, it might be difficult to find the typical narrow-neck, but a large communication between the left ventricle and aneurysmal sac might be present, misdiagnosing the pseudoaneurysm with a true aneurysm. In such cases the large communication is due to a significant rupture of the left ventricular free wall as it was identified in three cases in our series. It has been suggested that a posterior location is indicative of pseudoaneurysm rather than true aneurysm. Extensive infarction in the posterior region involves the posterior papillary muscle, which usually results in severe mitral regurgitation and death; these patients never go on to develop true aneurysm [9]. Three patients in our series had severe mitral valve regurgitation.
Left ventricular pseudoaneurysms are often asymptomatic and are discovered incidentally upon investigation of some other condition, most commonly angina pectoris or congestive heart failure. Routine echocardiography may detect pseudoaneurysm in an asymptomatic patient who is recovering from acute myocardial infarction. Diagnosis can be made preoperatively by several imaging techniques, including computed tomography, echocardiography, and magnetic resonance imaging; however, contrast ventriculography and coronary angiography seem to be necessary in evaluating the location and anatomy of the aneurysm and the state of the coronary arteries. Although the distinction of pseudoaneurysm from a true aneurysm can be difficult, the presence of a narrow neck on either color-flow Doppler echocardiography or ventriculography is strongly suggestive of pseudoaneurysm.
Because of its rarity, the natural history of pseudoaneurysm of the left ventricle is not well established. Congestive heart failure is the most common presentation, followed by angina, ventricular arrhythmias, and embolization [16]. When the diagnosis is established, surgical correction is usually mandatory. Surgery is urgently recommended when a pseudoaneurysm is discovered within the first 2–3 months after myocardial infarction, because onset of rupture is unpredictable [1], [2], [3]. However, when diagnosis is made years after myocardial infarction, the urgency and even the need for operation is determined by symptoms rather than by risk of rupture. Embolization of thrombotic material, induced by stagnant blood flow, has also been reported with large pseudoaneurysms [12], [13].
The surgical treatment of left ventricular post-infarction pseudoaneurysms raises few problems. If thrombotic material within the pseudoaneurysm has been detected by echocardiography, dissection of the heart should initially be limited to the anterior surface, to enable placement of cannulas and institution of cardiopulmonary bypass. The left ventricle should be dissected free from the pericardium after the aorta has been cross-clamped. Dissection of the heart should be done gently because of the danger of systemic embolization if the pseudoaneurysm has thrombotic material. Different techniques such as direct closure, patch closure employing synthetic, autologous or bovine pericardiac patch, sutureless technique [17] have been successfully employed. The neck of the pseudoaneurysm can be closed directly in chronic cases because of its fibrotic edges.
Primary repair carries a higher risk of arrhythmias because pressure is exerted on the left ventricle. In acute cases, closure of the freshly necrotic myocardium with synthetic or pericardial patches is effective. The sutureless technique has been recently employed successfully in acute forms of left ventricular pseudoaneurysms, however further evidence is required [17].
When the defect is large or located near the base of the heart, a patch may be preferable to avoid excessive traction on the myocardium. In these cases, reconstructing the left ventricle with a patch is preferable, to avoid distortion of the mitral valve apparatus or excessive traction on the ventricular edges.
In cases requiring mitral valve surgery, the neck of the left ventricular pseudoaneurysm can be closed through the left atrium using an autologous pericardial patch. In this approach, the left atrium is opened, the mitral valve temporarily removed, and the neck of the pseudoaneurysm directly closed or covered with a patch [18]. This approach is indicated in sub mitral pseudoaneurysm [19] but also in other cases can be tempted [20].
Also, in cases representing associated post myocardial infarction interventricular septal defect, the left ventricle might be opened at the neck of the left ventricular pseudoaneurysm and then can be closed as a normal left ventricular true aneurysm, according to the linear technique. In such cases concomitant myocardial revascularization and correction of associated mitral valve insufficiency should be performed.
A literature review was performed. All the series of patients with left ventricular pseudoaneuryms due to myocardial infarction were included in this review. The overall early mortality rate for the post-infarction pseudoaneurysm was 23% in Frances' review [12]; 20% in Atik series [3], in Komeda's series with 12 patients, it was 25% [16]; in Prêtre's study [1] it was 30% and in Eren series [3] was 35.7%; and in our series was 30.8% (Table 4). A total of 306 patients were included and the overall hospital mortality was 21.2% (65 patients). In our experience, death was not associated with technical difficulties, but mainly with poor left ventricular function, associated post infarct interventricular septal defect or valve surgery and acute form.
Table 4.
Series of reported left ventricular pseudoaneurysm surgically treated.
| Author | Ref Nr/Year | Total | Chronic | Asspro | Patch/direct Mortality | |
|---|---|---|---|---|---|---|
| Komeda M | 16/1993 | 12 | 12 | MV x 3 | 8/4 | 3 (25%) |
| Mackenzie JW | 21/1994 | 14 | 14 | – | 0/14 | 3 (21.4%) |
| Csapo K, | 14/1997 | 5 | 3 | MVx 1 and VSD x 1 | 0/6 | 2 (40%) |
| Yeo TC, | 22/1998 | 22 | 18 | – | – | 3 (13.6%) |
| Frances C | 12/1998 | 107 | 87 | – | – | 25 (23%) |
| Prêtre R | 1/2000 | 7 | 3 | 4/3 | 2 (29%) | |
| Perek B | 23/2004 | 8 | 6 | VSD x 1 | 8 | – |
| Lafci B | 24/2006 | 8 | – | – | 8 | 1 (12.5%) |
| Eren E | 2/2007 | 14 | 12 | – | 8/6 | 5 (35.7%) |
| Atik FA | 3/2007 | 30 | 22 | MV x 8 | 5/25 | 6 (20%) |
| Golbasi I | 25/2008 | 7 | 3 | – | 6/1 | 2 (28.6%) |
| Sakaguchi G | 26/2008 | 32 | – | – | sutureless technique | 5 (15.6%) |
| Narin C | 27/2008 | 5 | 5 | – | 5 | – |
| Fedakar a | 28/2010 | 22 | 19 | 13/9 | 6 (27.3%) | |
| Prifti E |
13 |
9 |
MV x 3, VSD x3 |
7/7 |
4 (30.8%) |
|
| Total | 306 | 65 (21.2%) | ||||
In conclusion, this study revealed that surgical repair of post infarct left ventricular pseudoaneurysm was associated with an acceptable surgical mortality rate and a low risk of spontaneous rupture. Various surgical techniques are available and should be considered according to the case presentation.
Ethical approval
Yes, IRB approvale.
Sources of funding
None.
Author contribution
All authors contributed on paper writing. EP, MB, AV, GG and AB did surgery, MZ and EB were the anaesthesiologists and AD and ERR the cardiologists.
Conflicts of interest
None.
Guarantor
Edvin Prifti.
Research registration unique identifying number (UIN)
researchregistry1715.
References
- 1.Pretre R., Linka A., Jenni R., Turina M.I. Surgical treatment of acquired left ventricular pseudoaneurysms. Ann. Thorac. Surg. 2000;70:553–557. doi: 10.1016/s0003-4975(00)01412-0. [DOI] [PubMed] [Google Scholar]
- 2.Eren E., Bozbuga N., Toker M.E. Surgical treatment of post-infarction left ventricular pseudoaneurysm. A two-decade experience. Tex Heart Inst. J. 2007;34:47–51. [PMC free article] [PubMed] [Google Scholar]
- 3.Atik F.A., Navia J.L., Vega P.R. Surgical treatment of postinfarction left ventricular pseudoaneurysm. Ann. Thorac. Surg. 2007;83:526–531. doi: 10.1016/j.athoracsur.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 4.Singh S., Puri A., Narain V., Sahni J. Post-traumatic left ventricular pseudoaneurysm. Interact. Cardiovasc. Thorac. Surg. 2012 Mar;14(3):359–361. doi: 10.1093/icvts/ivr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair V.V., Malankar D., Kothari S.S., Das S., Gulati G.S., Airan B. Unusual left ventricular pseudoaneurysm in a child after disseminated bacterial infection. World J. Pediatr. Congenit. Heart Surg. 2014 Jan 1;5(1):121–123. doi: 10.1177/2150135113499379. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda N., Yasu T., Kubo N. Effect of reperfusion therapy on cardiac rupture after myocardial infarction. Circ. J. 2004;68:422–426. doi: 10.1253/circj.68.422. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 7.Pollak H., Nobis H., Mlczoch J. Frequency of left ventricular free wall rupture complicating acute myocardial infarction since the advent of thrombolysis. Am. J. Cardiol. 1994;74:184–186. doi: 10.1016/0002-9149(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 8.Villanueva C., Milder D., Manganas C. Ruptured left ventricular false aneurysm following acute myocardial infarction: case report and review of the literature. Heart, Lung Circ. 2014;23:e261–e263. doi: 10.1016/j.hlc.2014.07.069. [DOI] [PubMed] [Google Scholar]
- 9.Kansiz E., Hatemi A.C., Tongut A. Surgical treatment of a giant postero-inferior left ventricular pseudoaneurysm causing severe mitral insufficiency and congestive heart failure. Ann. Thorac. Cardiovasc. Surg. 2012;18:151–155. doi: 10.5761/atcs.cr.11.01674. [DOI] [PubMed] [Google Scholar]
- 10.Rentoukas E.I., Lazaros G.A., Kaoukis A.P., Matsakas E.P. Double rupture of interventricular septum and free wall of the left ventricle, as a mechanical complication of acute myocardial infarction: a case report. J. Med. Case Rep. 2008;2:85. doi: 10.1186/1752-1947-2-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agha R.A., Fowler A.J., Rammohan S., Barai I. Orgill DP and the PROCESS group. The PROCESS statement: preferred reporting of case series in surgery. Int. J. Surg. 2016;36(Pt A):319–323. doi: 10.1016/j.ijsu.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Frances C., Romero A., Grady D. Left ventricular pseudoaneurysm. J. Am. Coll. Cardiol. 1998;32:557–561. doi: 10.1016/s0735-1097(98)00290-3. [DOI] [PubMed] [Google Scholar]
- 13.Yeo T.C., Malouf J.F., Oh J.K., Seward J.B. Clinical profile and outcome in 52 patients with cardiac pseudoaneurysm. Ann. Intern. Med. 1998;128:299–305. doi: 10.7326/0003-4819-128-4-199802150-00010. [DOI] [PubMed] [Google Scholar]
- 14.Csapo K., Voith L., Szuk T., Edes I., Kereiakes D.J. Postinfarction left ventricular pseudoaneurysm. Clin. Cardiol. 1997;20:898–903. doi: 10.1002/clc.4960201021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies M.J. Ischaemic ventricular aneurysms: true or false? Br. Heart J. 1988;60:95–97. doi: 10.1136/hrt.60.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komeda M., David T.E. Surgical treatment of postinfarction false aneurysm of the left ventricle. J. Thorac. Cardiovasc. Surg. 1993;106:1189–1191. [PubMed] [Google Scholar]
- 17.Sakaguchi G., Komiya T., Tamura N., Kobayashi T. Surgical treatment for postinfarction left ventricular free wall rupture. Ann. Thorac. Surg. 2008 Apr;85(4):1344–1346. doi: 10.1016/j.athoracsur.2007.12.073. [DOI] [PubMed] [Google Scholar]
- 18.Sutter F.P., Goldman S.M., Werthman P.E., Moghadam A.N. Circumflex artery ventricular fistula and pseudoaneurysm after mitral reoperation. Ann. Thorac. Surg. 1990;50:826–827. doi: 10.1016/0003-4975(90)90700-g. [DOI] [PubMed] [Google Scholar]
- 19.Jahangiri M., Sarkar D., Quinton P., Ward D.E. Submitral left ventricular pseudoaneurysm. Ann. Thorac. Surg. 2005 Mar;79(3):1031–1032. doi: 10.1016/j.athoracsur.2003.09.134. [DOI] [PubMed] [Google Scholar]
- 20.Da Col U., Di Bella I., Ramoni E., Affronti A., Rossi A., Ragni T. Repair of posterior left ventricular aneurysm through transatrial approach. J. Card. Surg. 2010 Jan-Feb;25(1):23–25. doi: 10.1111/j.1540-8191.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- 21.Mackenzie J.W., Lemole G.M. Pseudoaneurysm of the left ventricle. Tex Heart Inst. J. 1994;21(4):296–301. [PMC free article] [PubMed] [Google Scholar]
- 22.Yeo T.C., Malouf J.F., Oh J.K., Seward J.B. Clinical profile and outcome in 52 patients with cardiac pseudoaneurysm. Ann. Intern. Med. 1998 Feb 15;128(4):299–305. doi: 10.7326/0003-4819-128-4-199802150-00010. [DOI] [PubMed] [Google Scholar]
- 23.Perek B., Jemielity M., Dyszkiewicz W. Clinical profile and outcome of patients with chronic postinfarction left ventricular false aneurysm treated surgically. Heart Surg. Forum. 2004 Apr 1;7(2):E132–E135. doi: 10.1532/HSF98.200348618. [DOI] [PubMed] [Google Scholar]
- 24.Lafci B., Ozsöyler I., Emrecan B. Surgical treatment of postinfarction left ventricular pseudoaneurysms. Heart Surg. Forum. 2006;9(6):E876–E879. doi: 10.1532/HSF98.20061042. [DOI] [PubMed] [Google Scholar]
- 25.Golbasi I., Atahan E., Turkay C. Surgical treatment in postinfarction left ventricular pseudoaneurysm. Miner. Chir. 2007 Jun;62(3):173–177. [PubMed] [Google Scholar]
- 26.Sakaguchi G., Komiya T., Tamura N., Kobayashi T. Surgical treatment for postinfarction left ventricular free wall rupture. Ann. Thorac. Surg. 2008 Apr;85(4):1344–1346. doi: 10.1016/j.athoracsur.2007.12.073. [DOI] [PubMed] [Google Scholar]
- 27.Narin C., Ege E., Ozkara A. Surgical treatment of postinfarction pseudoaneurysms of the left ventricle. J. Card. Surg. 2008 Jul-Aug;23(4):294–298. doi: 10.1111/j.1540-8191.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- 28.Fedakar A., Bugra O., Onk A., Mataraci I., Eren E., Zeybek R. Repair of left ventricular pseudoaneurysms. Asian Cardiovasc. Thorac. Ann. 2010 Feb;18(1):39–43. doi: 10.1177/0218492309353988. [DOI] [PubMed] [Google Scholar]