Abstract
Objective
Obesity and high fat diet (HFD) consumption in rodents is associated with hypothalamic inflammation and reactive gliosis. While neuronal inflammation promotes HFD-induced metabolic dysfunction, the role of astrocyte activation in susceptibility to hypothalamic inflammation and diet-induced obesity (DIO) remains uncertain.
Methods
Metabolic phenotyping, immunohistochemical analyses, and biochemical analyses were performed on HFD-fed mice with a tamoxifen-inducible astrocyte-specific knockout of IKKβ (GfapCreERIkbkbfl/fl, IKKβ-AKO), an essential cofactor of NF-κB-mediated inflammation.
Results
IKKβ-AKO mice with tamoxifen-induced IKKβ deletion prior to HFD exposure showed equivalent HFD-induced weight gain and glucose intolerance as Ikbkbfl/fl littermate controls. In GfapCreERTdTomato marker mice treated using the same protocol, minimal Cre-mediated recombination was observed in the mediobasal hypothalamus (MBH). By contrast, mice pretreated with 6 weeks of HFD exposure prior to tamoxifen administration showed substantially increased recombination throughout the MBH. Remarkably, this treatment approach protected IKKβ-AKO mice from further weight gain through an immediate reduction of food intake and increase of energy expenditure. Astrocyte IKKβ deletion after HFD exposure—but not before—also reduced glucose intolerance and insulin resistance, likely as a consequence of lower adiposity. Finally, both hypothalamic inflammation and astrocytosis were reduced in HFD-fed IKKβ-AKO mice.
Conclusions
These data support a requirement for astrocytic inflammatory signaling in HFD-induced hyperphagia and DIO susceptibility that may provide a novel target for obesity therapeutics.
Keywords: Obesity, Astrocytes, Inflammation, Metabolism, Hypothalamus, Energy homeostasis
Abbreviations: Agrp, Agouti-related peptide; ARC, arcuate nucleus; Bdnf, brain-derived neurotrophic factor; DIO, diet-induced obesity; Ccl2, C–C motif chemokine ligand 2; Cart, cocaine- and amphetamine-regulated transcript; DMH, dorsomedial hypothalamus; GFAP, glial fibrillary acidic protein; GSIS, glucose-stimulated insulin secretion; GTT, glucose tolerance test; IKKβ, inhibitor of kappa B kinase beta; HFD, high-fat diet; Iba1, ionized calcium binding adaptor molecule 1; IHC, immunohistochemistry; ir, immunoreactivity; ITT, insulin tolerance test; Il, interleukin; LPS, lipopolysaccharide; MBH, mediobasal hypothalamus; Npy, neuropeptide Y; NF-κB, nuclear factor kappa B; Pomc, proopiomelanocortin; RER, respiratory exchange ratio; TMX, tamoxifen; Tnfa, tumor necrosis factor α; VMN, ventromedial nucleus
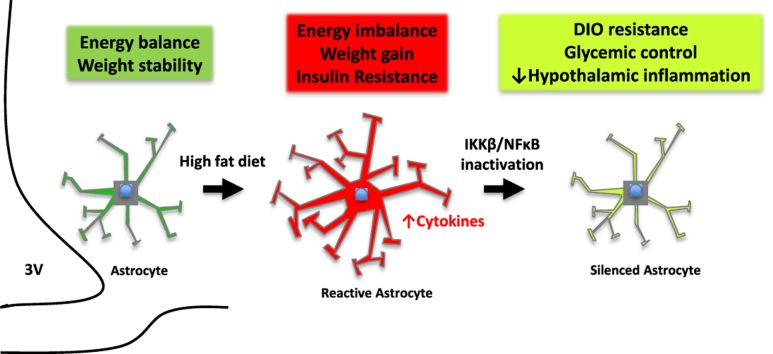
Graphical abstract
Overview of astrocyte IKKβ inactivation in chronic HFD-fed mice. Rodents exposed to HFD have increased hypothalamic inflammation and accumulation of reactive astrocytes in the mediobasal hypothalamus. Conditional deletion of IKKβ/NFκB in astrocytes of HFD-fed mice results in obesity resistance, improved glucose homeostasis, and decreased hypothalamic inflammation. 3V = third ventricle
Highlights
-
•
The first direct evidence that astrocyte inflammatory activation promotes obesity.
-
•
GfapCreER mice given tamoxifen show minimal recombination in MBH astrocytes.
-
•
GfapCreER mice given tamoxifen after 6 wks of HFD have recombination in the MBH.
-
•
Astrocyte IKKβ deletion with tamoxifen before HFD has no effect on energy balance.
-
•
Astrocyte IKKβ deletion with tamoxifen given after HFD reduces DIO susceptibility.
1. Introduction
Obesity and excessive dietary consumption promote an inflammatory state in peripheral metabolic tissues driven by ingress and activation of immune cells [1], [2]. Likewise, the central nervous system (CNS) responds to high fat and sugar-rich diets with rapid upregulation of the master inflammatory NF-κB pathway in important brain regions including the hypothalamus, a critical site of energy homeostasis regulation [3], [4], [5], [6], [7]. High-fat diet (HFD) consumption drives hypothalamic cytokine production, neuronal stress, and insulin/leptin resistance that together promote excess weight gain and food intake [4], [8], [9], [10]. Reducing neuronal inflammatory capacity through deletion or inhibition of NF-κB pathway intermediates (e.g. IKKβ, MyD88, IκBα) restores hypothalamic control of energy balance [5], [6], [11], resulting in reduced susceptibility to diet-induced obesity (DIO) and glucose intolerance. Thus, identifying mechanisms that regulate the hypothalamic inflammatory process can advance our understanding of obesity pathogenesis and assist with development of new treatment targets.
Recent evidence suggests that neuronal inflammation may be a downstream event during DIO, with the recruitment and activation of hypothalamic glial cells being a more proximal response to HFD exposure [3], [10], [12], [13], [14]. This gliosis process is characterized by the accumulation and proliferation of activated microglia and astrocytes in the region of the mediobasal hypothalamus (MBH) [10], [12], [13], [14], [15], [16], [17]. While several studies have implicated microglia in the generation of diet-induced inflammatory signals and metabolic dysfunction [17], [18], a similar role for astrocytes remains unclear. One study demonstrated a modest contribution of astrocyte inflammation to caloric intake on the first day of HFD feeding, but no analysis of DIO susceptibility was reported [19].
Astrocytes are abundant throughout the CNS and involved in many fundamental processes including synaptic transmission, neurovascular coupling, and blood–brain barrier maintenance [20]. In addition, astrocytes participate in CNS immune responses, adopting an activated phenotype with increased glial fibrillary acidic protein (GFAP) expression and release of proinflammatory cytokines that can enhance neurotoxicity and neurodegenerative disease progression [20], [21], [22]. Thus, astrocytes have the potential to impact energy homeostasis regulation in health and disease. Indeed, MBH astrocytes modulate feeding behavior when pharmacologically activated [23], [24] and show dynamic responses to circulating signals of nutrient availability such as insulin and leptin [25], [26], [27], [28]. In addition, MBH astrocytes become activated with obesity and HFD feeding in rodents and humans [10], [29], raising the possibility that astrocyte inflammation disrupts hypothalamic regulation of energy balance and promotes DIO. To address this hypothesis, we developed a mouse model with an inducible astrocyte-specific deletion of IKKβ. Using this approach, we demonstrate that reduction of astrocyte inflammatory signaling protects mice from HFD-induced hypothalamic inflammation and reduces susceptibility to DIO and glucose intolerance. These results highlight the important role of non-neuronal cells in obesity pathogenesis and suggest the possibility of new cellular targets for therapy.
2. Material and methods
2.1. Animals
All mice used in the experiments were male and on a C57BL/6J background. The reporter strain was generated by crossing GfapCreER mice (strain #012849, Jackson Laboratory) with ROSA26-stopfl/fl-tdTomato mice (Ai14, strain #007914, Jackson Laboratory). The Ikbkbfl/fl mice were obtained from the laboratory of Dr. Michael Karin [30] and mated with GfapCreER/wt mice. Breeding in our facility generated the littermates used in the experiments that were GfapCreER/wtIkbkbfl/fl (IKKβ-AKO) and Gfapwt/wtIkbkbfl/fl (Ctl). Genotyping was performed by PCR using ear genomic DNA and the following primers: Ikbkb floxed allele (forward-CCT TGT CCT ATA GAA GCA CAA C; reverse-GTC ATT TCC ACA GCC CTG TGA); GFAP-CreERT2 allele (forward-GCC AGT CTA GCC CAC TCC TT; reverse-TCC CTG AAC ATG TCC ATC AG). All mice, including controls, were administered 2 subcutaneous injections (48 h apart, 4 mg dissolved in 200 μl warm purified corn oil) of tamoxifen (TMX; Sigma, T5648) to induce CreER-mediated recombination. Mice were housed with ad libitum access to water and diet in a temperature-controlled room with a 12 h:12 h light:dark cycle under specific-pathogen free (SPF) conditions. After weaning, all mice were fed standard rodent chow (5001; 13% (kcal) fat, LabDiet, St. Louis MO) or were switched to 60% HFD (D12492; Research Diets, Inc.; USA). One cohort (n = 6–9 per group) received TMX at 6 wks of age (TMX-HFD) while the other (n = 5–7 per group) received 6 wks of HFD prior to TMX treatment (HFD-TMX). All procedures were performed in accordance with NIH Guidelines for Care and Use of Animals and were approved by the Institutional Animal Care and Use Committee at the University of Washington.
2.2. Body composition and indirect calorimetry
In vivo body composition analysis of lean mass and fat mass from conscious, immobilized mice was performed by the NIDDK-funded Nutrition Obesity Research Center (NORC) Energy Balance and Glucose Metabolism (EBGM) Core, using quantitative magnetic resonance spectroscopy (QMR) (EchoMRI 3-in-1; Echo Medical Systems). For calorimetric analyses, mice were acclimated to metabolic cages 3 weeks after TMX treatment (wk 9 of HFD in the HFD-TMX model) after which energy expenditure was measured using a computer-controlled indirect calorimetry system (Promethion, Sable Systems, Las Vegas, NV) run by the EBGM Core. For each animal, O2 consumption and CO2 production were measured for 1 min at 10-min intervals. Respiratory exchange ratio (RER) was calculated as the ratio of CO2 production to O2 consumption. Energy expenditure was calculated using the Weir equation without normalization, since body weight and composition did not differ between groups at this time point. Light and dark cycle energy expenditure were determined using the average of all 72 data points per 12-h light cycle of 3 consecutive days, and these, in turn, were averaged to obtain total 24-h energy expenditure. Ambulatory activity was measured continuously with consecutive adjacent infrared beam breaks in the x-, y- and z-axes scored as an activity count that was recorded every 10 min as previously described. Data acquisition and instrument control were coordinated by MetaScreen v.2.0.0.9, and raw data was processed using ExpeData v.1.6.4 (Sable Systems) with an analysis script documenting all aspects of data transformation.
2.3. Glucose and insulin tests
For the glucose tolerance test (GTT), mice were fasted 4 h and then administered 2 g/kg body weight d-glucose i.p., and blood glucose from tail was measured by glucometer (Freestyle Lite, Abbot Diabetes Care Inc.). In a separate experiment that analyzed glucose-stimulated insulin secretion (GSIS), blood samples were taken at t = 0 and 15 min for measurement of serum insulin by ELISA (Crystal Chem Inc). For the insulin tolerance test (ITT), food was removed from mice 4 h prior to experiment and recombinant insulin (Humulin R, Eli Lilly & Co.) was administered i.p. at 1.25 U/kg. Area-under-curve (AUC) and area-over-curve (AOC) were calculated by the trapezoid rule.
2.4. Primary astrocyte culture
Control and IKKβ-AKO mice aged P1 to P4 were sacrificed by decapitation, meninges removed, and cortices isolated under a dissecting microscope. Four cortices per group were pooled and minced, and incubated in Hanks Buffered Salt Solution (HBSS) with 2.5% Trypsin/EDTA at 37 °C for 30 min with shaking. After centrifugation, the tissue was resuspended in media and further dissociated by pipetting into a single cell suspension. Mixed cortical cells were seeded onto a poly-d-lysine coated T75 flask with culture media (Dulbecco's Modified Eagle's Medium (DMEM), 4.5 g/L glucose, 10% fetal bovine serum, l-glutamine, 25 mM HEPES, and 1% penicillin/streptomycin) and incubated at 37 °C and 5% CO2/air. After cells reached confluency, microglia were removed by shaking at 180 rpm for 30 min and discarding the supernatant, followed by further shaking at 240 rpm for 6 h to remove oligodendrocyte precursors. To induce recombination, the resulting astrocyte-enriched culture was incubated in culture media containing 5 μM 4-hydroxy tamoxifen (4-OHT, Sigma) 3 days prior to experiments.
2.5. Quantitative real time PCR (qRT-PCR)
Total RNA was extracted using RNeasy micro kit according to manufacturer's instructions (Qiagen) and reverse-transcribed with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Levels of transcripts were measured by quantitative real-time PCR on an ABI Prism 7900 HT (Applied Biosystems) using the standard curve method and specific primer sequences (Supplementary Table 1).
2.6. Histological analyses
Mice were perfused with PBS and then 4% paraformaldehyde (PFA). Brains were removed from the skull, post-fixed in PFA, and cryoprotected in 25% sucrose/phosphate buffered saline (PBS). Brain sections containing the hypothalamus were mounted in OCT, and 30-40 μm-thick coronal sections were cut using a cryostat (Leica CM1850). Immunohistochemistry was performed in free-floating sections with the following primary and secondary antibodies: mouse monoclonal anti-GFAP-Cy3 (Sigma, catalog #C9205), rabbit anti-Iba1 (Wako, catalog # 019-19741), and goat anti-rabbit Alexa Fluor 488 (Life Technologies, A11008). Sections were mounted onto slides and imaged by epifluorescence microscopy. Immunostaining in the ARC and MBH was performed blinded and fluorescence was analyzed using Image J software with threshold values to standardize intensity, followed by quantification of mean pixel area per region of interest (ARC, MBH, and DMH) or per cell. Total cell counts were measured manually using the Image J cell counter plugin.
2.7. Statistical analyses
All results are presented as mean ± SEM. For between-subject comparisons, statistical significance was determined by unpaired two-tail Student's t test or two-way and repeated measures ANOVA with Bonferroni post-hoc tests using statistical software (Prism 6.0, GraphPad). Probability P values of less than 0.05 were considered significant.
3. Results
3.1. Tamoxifen treatment prior to HFD exposure does not affect diet-induced obesity in GfapCreERIkbkbfl/fl mice
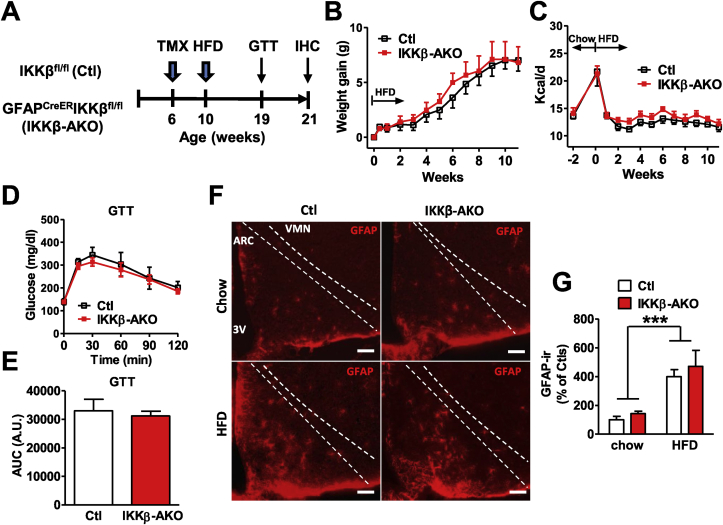
We have previously shown that HFD consumption in rodents is associated with hypothalamic inflammation and activation of astrocytes prior to the onset of weight gain [10]. To determine whether astrocyte activation promotes the development of obesity through the generation of inflammatory mediators, we studied mice in which astrocyte-specific deletion of IKKβ is accomplished by administration of tamoxifen (GfapCreERIkbkbfl/fl, IKKβ-AKO). First, we verified the deletion of IKKβ transcript and absence of a proinflammatory response (Tnfa, Il1b, and Il6) to lipopolysaccharide in primary astrocytes derived from IKKβ-AKO mice compared with cells from littermate controls (Ikbkbfl/fl, Ctl) (Supplementary Figure S1A–D). Next, we investigated the metabolic consequences of astrocyte NF-κB inactivation, accomplished by treating IKKβ-AKO and Ctl mice with tamoxifen 4 wks prior to the introduction of HFD (Figure 1A). Weight gain on chow (ages 6–10 wks; data not shown) and after 11 wks of HFD exposure were equivalent between IKKβ-AKO and Ctl mice (Figure 1B), as were the degree of initial HFD-induced hyperphagia (week 0–1; Figure 1C) and average daily food intake throughout the study (Figure 1C). To determine whether reduction of astrocyte NF-κB signaling has an independent effect on glucose homeostasis, we performed a glucose tolerance test (GTT; 2 mg/kg i.p.). At week 9 of HFD, glucose excursions were not different between IKKβ-AKOs and Ctls (Figure 1D–E). Given the lack of metabolic alterations despite reduced astrocyte inflammatory capacity in vitro (Supplemental Figure S1A–C), we then sought to determine the activation status of MBH astrocytes in vivo by monitoring hypothalamic expression of GFAP, an NF-κB response gene that indicates reactive astrocytosis [31]. Immunohistochemical (IHC) analysis of MBH nuclei (arcuate (ARC) and ventromedial (VMN)) confirmed the marked induction of GFAP immunoreactivity by HFD feeding (Figure 1F–G), but showed no differences between IKKβ-AKO and Ctl mice. Thus, astrocyte NF-κB inactivation prior to HFD exposure neither prevented diet-induced MBH astrogliosis nor altered diet-induced weight gain and glucose intolerance.
Figure 1.
Deletion of IKKβ in astrocytes prior to HFD introduction does not alter DIO sensitivity or hypothalamic astrogliosis. (A) Experimental design. (B) Weight gain after HFD exposure (n = 6 per group). (C) Food intake prior to and after introduction of HFD (n = 6 per group). (D) Glucose tolerance test (GTT, 2 mg/kg dextrose i.p.) (n = 6–9 per group). (E) Area-under-curve of GTT. (F) Representative images of GFAP immunostaining in the MBH (ARC and VMN regions) of chow and HFD-fed mice at 21 weeks old. Scale bar is 100 μm. (G) Relative quantification of GFAP immunoreactivity (ir) in the MBH (4 sections per mouse analyzed, n = 4 per group). Values are mean ± SEM, 2-way ANOVA with Bonferroni post-hoc test, ***p < 0.001.
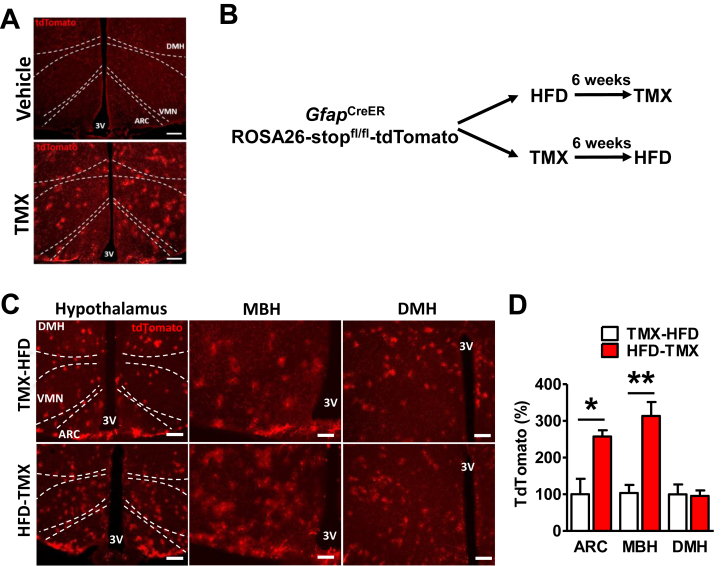
3.2. HFD consumption increases CreER-mediated recombination in the MBH of GfapCreER reporter mice
The GfapCreER mouse is a commonly used model to modify astrocyte gene expression in a temporally-controlled manner using tamoxifen administration [25], [28]. However, astrocytes are heterogeneous, and only a subpopulation express GFAP at significant levels in the uninflamed CNS [20], [32]. Indeed, we found using GfapCreERROSA26-stopfl/fl-tdTomato reporter mice (Figure 2A–B) that TMX induced minimal recombination in the ARC and MBH of chow-fed mice subsequently fed HFD (TMX-HFD, Figure 2C–D). However, when age-matched reporter mice received TMX after 6 weeks of HFD feeding (HFD-TMX), there was a large increase in CreER-mediated recombination in the ARC and MBH, but not the DMH (Figure 2C–D), consistent with the specific upregulation of MBH GFAP by HFD feeding (Figure 1F–G) [10], [16]. Thus, the minimal IKKβ deletion in the MBH may account for the lack of metabolic alterations in IKKβ-AKO mice that receive tamoxifen before HFD exposure.
Figure 2.
Efficiency of Cre-mediated recombination in MBH astrocytes is increased by HFD feeding. (A) Representative images of tdTomato fluorescence in vehicle (corn oil) and TMX-treated GfapCreERROSA26-stopfl/fl-tdTomato reporter mice. Scale bars are 200 μm. (B) Experimental design to investigate the effect of HFD on CreER-mediated recombination. (C) Representative images of tdTomato fluorescence in hypothalami from age-matched mice administered TMX before (top row, TMX-HFD) or after 6 weeks HFD (bottom row, HFD-TMX). Scale bars: 200 μm for hypothalamus, 100 μm for MBH and DMH. (D) Relative quantification of tdTomato fluorescence in hypothalamic regions (2–4 sections per mouse analyzed, n = 3–4 per group). Values are mean ± SEM, Student's t-test, **p < 0.01.
3.3. Conditional deletion of astrocyte IKKβ in mice following 6 weeks of HFD feeding results in protection from DIO
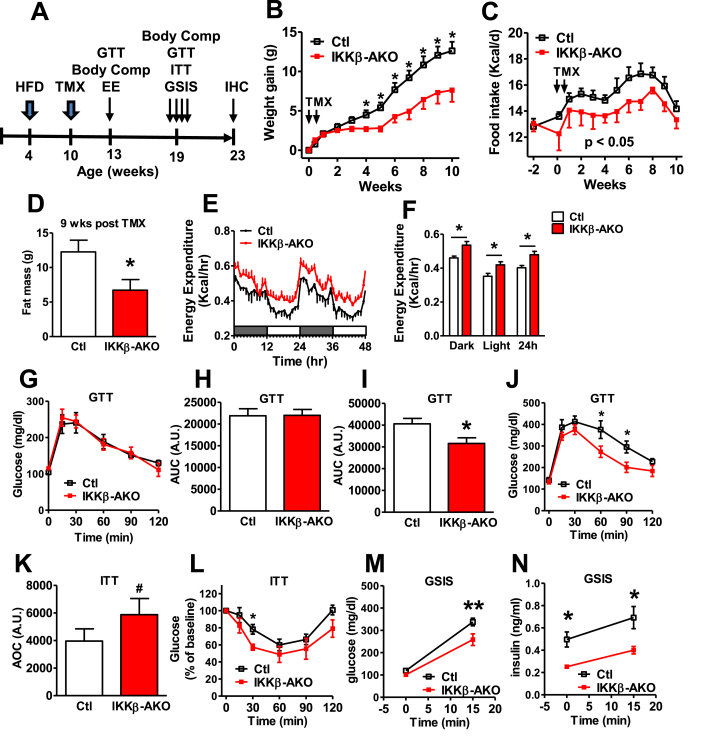
Based on the results from the reporter experiment (Figure 2), we reassessed the IKKβ-AKO model using the HFD-TMX paradigm to enhance silencing of astrocyte inflammation in the MBH (Figure 3A). Remarkably, TMX treatment after 6 weeks of HFD feeding immediately reduced HFD-associated weight gain and food intake in IKKβ-AKO mice relative to Ctls (Figure 3B–C). The reduced body weight specifically resulted from decreased fat mass (Figure 3D), as lean mass was unaffected (data not shown). To assess energy expenditure in the HFD-TMX IKKβ-AKO mice, we performed indirect calorimetry in a separate cohort studied 3 weeks after TMX administration prior to the divergence of body weight and body composition between genotypes (Supplemental Figure S2A–C). While RER and ambulatory activity were unchanged in IKKβ-AKO mice compared to Ctls (Supplemental Figure S2D–G), metabolic rate was significantly increased over all photoperiods (Figure 3E–F). Thus, reduced MBH astrocyte NF-κB signaling limits DIO through both decreased food intake and increased energy expenditure.
Figure 3.
Astrocyte-specific IKKβ deletion following 6 weeks of HFD feeding results in DIO resistance and metabolic improvements. (A) Experimental design. (B) Weight gain after TMX treatment. (C) HFD food intake prior to and after TMX. (G–J) GTT assessed at 3 weeks (G and H) and 9 weeks (I and J) after TMX. (K–L) Insulin tolerance test (ITT, 1.5 U/kg i.p.) at 20 weeks after TMX. (M–N) Glucose-stimulated insulin secretion (GSIS, 2 mg/kg dextrose i.p.) at 20 weeks after TMX. All analyses n = 5–7 per group. Values are mean ± SEM, repeated measures ANOVA and Student's t-test, #p = 0.06, ∗p < 0.05, **p < 0.01.
3.4. Glucose homeostasis is improved in IKKβ-AKO mice fed chronic HFD
Using cohorts of HFD-TMX IKKβ-AKO mice (Figure 3A), we assessed glucose homeostasis both early (3 wks post-TMX) and late (9 wks post-TMX) during HFD feeding. While glucose tolerance was unaffected by astrocyte IKKβ deletion at the early time point (Figure 3G–H), IKKβ-AKO mice had improved glucose tolerance and insulin sensitivity after body weight divergence (Figure 3I–L). Twenty weeks after TMX administration, IKKβ-AKO mice also had lower fasted and glucose-stimulated insulin levels than Ctls (Figure 3M–N), suggesting glucose tolerance is a result of preserved insulin sensitivity rather than enhanced insulin secretion. Overall, these results indicate that reducing MBH astrocyte inflammatory capacity improves glucose homeostasis indirectly as a consequence of lower adiposity.
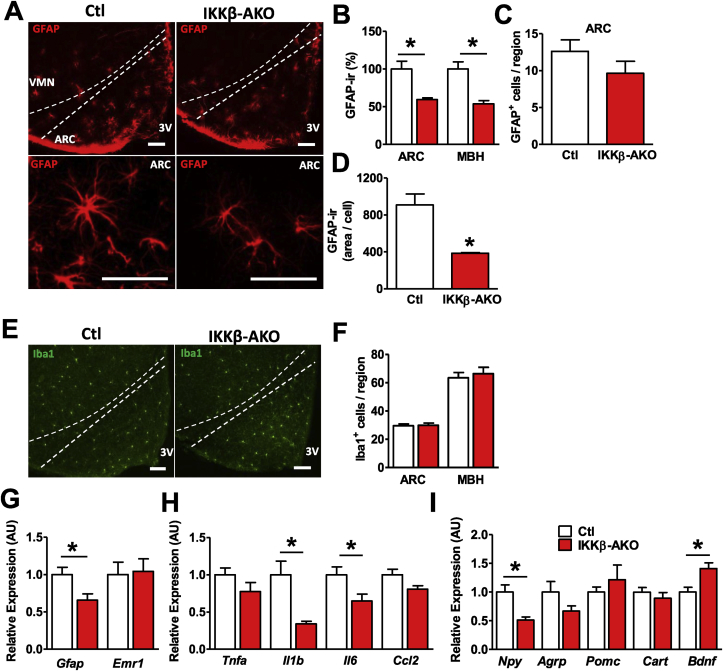
3.5. HFD-TMX IKKβ-AKO mice have reduced hypothalamic astrogliosis and inflammation
HFD consumption and obesity are associated with increased hypothalamic levels of inflammatory mediators and activation and proliferation of MBH glial cells [3]. Given that IKKβ-AKO mice administered TMX prior to HFD feeding show neither decreased hypothalamic gliosis nor altered DIO susceptibility, we investigated whether HFD-TMX IKKβ-AKO mice show reduced astrocyte activation along with their DIO resistance. IHC analysis revealed a near 50% reduction in GFAP immunoreactivity in the ARC and MBH of IKKβ-AKO mice (Figure 4A–B). While the number of GFAP-positive astrocytes in the ARC did not differ between IKKβ-AKO and Ctl mice (Figure 4C), cell size was significantly decreased in IKKβ-AKO mice (Figure 4A,D), suggesting that activation of hypothalamic astrocytes in response to HFD requires intact inflammatory signaling. In contrast, ARC and MBH microglial number assessed by Iba1 staining was not significantly affected by astrocyte IKKβ deletion (Figure 4E–F). Similarly, hypothalamic gene expression analysis revealed significantly less Gfap but unchanged Emr1 (microglia-specific gene) mRNA levels in IKKβ-AKO mice compared with Ctls (Figure 4G). In addition to reduced markers of astrogliosis, IKKβ-AKO mice had significantly lower hypothalamic expression of the cytokines Il1b and Il6, although there were no changes in Tnfa or the chemokine Ccl2 (Figure 4H). Expression of the orexigenic neuropeptide Npy was significantly lower in IKKβ-AKO mice while levels of the potent anorexigenic neuropeptide Bdnf were increased (Figure 4I) [33], [34]. Taken together, these data indicate that deletion of IKKβ in astrocytes restrains HFD-induced hypothalamic inflammation and astrogliosis and promotes an anorexigenic neuropeptide profile.
Figure 4.
HFD-TMX astrocyte IKKβ KO mice have decreased hypothalamic astrogliosis and inflammation. (A) Representative images of GFAP-ir in the MBH (top) and ARC (bottom). (B) Relative quantification of GFAP-ir (6 sections per mouse analyzed, n = 3 per group). (C) Total GFAP+ cell counts per unilateral ARC. (D) Quantification of individual cell area based on GFAP-ir. (E) Representative images of Iba1-ir. (F) Relative quantification of Iba1-ir. Hypothalamic expression by qRT-PCR (n = 4–6 per group) of (G) glial markers, (H) cytokines, and (I) neuropeptides. All scale bars are 100 μm. Values are mean ± SEM, Student's t-test, *p < 0.05.
4. Discussion
Reactive astrocytosis is a prominent early hypothalamic response to obesity and HFD feeding in rodents and humans, but its functional significance to DIO susceptibility remains uncertain. Here, we demonstrate for the first time that astrocytes are critical contributors to obesity pathogenesis induced by HFD feeding. Specifically, we show that IKKβ-AKO mice receiving TMX after 6 wks of HFD feeding show reduced MBH astrocyte activation and hypothalamic inflammation, though no change in microgliosis. Remarkably, these mice become DIO resistant with lower food intake and increased energy expenditure and show weight-dependent improvements in glucose homeostasis. In contrast, mice without prior HFD exposure have minimal gene deletion in MBH astrocytes after TMX administration and no distinguishable metabolic characteristics during HFD feeding. Together, these data demonstrate the fundamental contribution of astrocyte signaling to obesity pathogenesis and highlight the potential pitfalls associated with commonly used astrocyte gene deletion models.
In the two experimental approaches used in this study, we found that only TMX treatment after prolonged HFD consumption had significant effects on inactivating hypothalamic astrocytes and altering the metabolic phenotype of IKKβ-AKO mice. While these results highlight the importance of hypothalamic astrocyte inflammation in promoting obesity, the data from both approaches also suggest that IKKβ deletion from the population of GfapCreER-expressing cells in unchallenged (chow-fed) mice is not sufficient to limit hypothalamic inflammatory potential or alter metabolic parameters during HFD feeding. Thus, we hypothesize that HFD exposure triggers increased GFAP expression and activation in subpopulations of MBH astrocytes (primarily in the ARC) that promote susceptibility to DIO. Future studies are required to characterize the heterogeneous astrocyte response to HFD feeding and identify specific populations in the MBH and other brain regions that contribute to the regulation of energy balance.
Gfap promoter-based transgenic mice are the most well-established models used to study astrocyte function [28], [35], [36], [37]. Their application to the study of energy homeostasis is problematic, however, due to inefficient transgene expression in the MBH [32]. Interestingly, prior HFD exposure circumvented this limitation by increasing the degree of MBH astrocytic recombination induced by GfapCreER mice. Though the exact mechanism by which this occurs is not yet known, a likely possibility is the enhanced CreER transcription from diet-induced upregulation of the transgenic Gfap promoter. This hypothesis is consistent with the finding that HFD feeding triggers MBH astrocyte activation and increased GFAP expression [10], [13], [15], [16] through an NF-κB-dependent mechanism.
A previous study reported on a 7 d HFD feeding paradigm in a doxycycline-inducible model of astrocyte NF-κB inactivation with overexpression of dominant-negative IκB (GFAP-tTA × IκB-DN). The authors found a modest increase in HFD intake on the first day of exposure, but no other effect on energy balance was reported [19]. In the present study, we observed no differences in the initial hyperphagic response to HFD in the TMX-HFD IKKβ-AKO mice and could not assess this parameter in the HFD-TMX cohort. However, we did observe increased energy expenditure, reduced chronic HFD intake, and prevention of fat mass accumulation in the HFD-TMX IKKβ-AKO mice. These discrepancies with Buckman et al. likely relate to the long-term HFD study we conducted, the greater MBH astrocyte NF-κB inactivation achieved with 6 weeks of pre-exposure to HFD, the use of doxycycline and Splenda®-infused water for transgene activation in Buckman et al., and differences in background strain of mice (C57BL6/J in this study vs. FVB; BL6 F1 hybrid) [19]. Additionally, the differences between the “tamoxifen first” and “HFD first” cohorts suggest that deletion of astrocyte inflammatory capacity specifically in the MBH may be required to reduce DIO susceptibility.
5. Conclusion
In summary, we have developed an approach to increase the recombination efficiency of the GfapCreER mouse in the MBH to demonstrate that astrocyte inflammatory signaling sustains hypothalamic inflammation and DIO sensitivity during chronic HFD feeding. The reduced adiposity of HFD-TMX IKKβ-AKO mice results from both decreased food intake and increased energy expenditure, suggesting links between astrocyte signaling and hypothalamic neuronal circuits that regulate multiple aspects of energy balance. Overall, these results highlight the importance of non-neuronal cells in metabolic disease and demonstrate the potential of astrocyte-directed interventions to provide novel obesity therapeutics.
Acknowledgments
We thank members of the Thaler lab for fruitful discussions about the manuscript. This work was supported by the American Diabetes Association (Pathway Award 1-14-ACE-51 to JPT), and the NIDDK (K08 DK088872 to JPT, T32 DK7247-37 and F32 DK108473-01 to JDD). Metabolic phenotyping assistance was provided by the NIH-funded Nutrition Obesity Research Center (NORC, DK035816) and the Diabetes Research Center (DRC, DK017047) at the University of Washington.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.01.010.
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Inflammatory response is reduced in IKKβ-AKO primary astrocytes. Primary cortical astrocytes isolated from control and IKKβ-AKO mice were treated for 24 h with media or media containing 100 ng/ml LPS. Gene expression was measured by qRT-PCR for (A) Ikbkb (B) Tnfa (C) Il1b (D) Il6 cytokines. n = 3–4 replicates per treatment group. Values are mean ± SEM, 2-way ANOVA with Bonferroni post hoc test, p < 0.05 shown by different lower-case letters.
IKKβ-AKO mice at 3 weeks after tamoxifen do not differ in body composition, RER, or ambulatory activity. Calorimetry was performed 3 weeks after TMX administration in HFD-TMX IKKβ-AKO mice. Body composition was assessed at time of analyses. There were no differences in (A) body weight, (B) fat mass, or (C) lean mass. (D) 48-h timeline of respiratory exchange ratio (RER) and (D) photoperiod averages of RER. (F) 48-h timeline of ambulatory activity (measured by consecutive beam breaks) and (G) average photoperiod total activity. All analyses n = 5–8 per group. Values are mean ± SEM, Student's t-test, *p < 0.05.
References
- 1.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. Journal of Clinical Investigation. 2011:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglass J.D., Dorfman M.D., Thaler J.P. Glia: silent partners in energy homeostasis and obesity pathogenesis. Diabetologia. 2016;60(2) doi: 10.1007/s00125-016-4181-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Souza C.T., Araujo E.P., Bordin S., Ashimine R., Zollner R.L., Boschero A.C. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135(1):61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinridders A., Schenten D., Konner A.C., Belgardt B.F., Mauer J., Okamura T. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metabolism. 2009;10(4):249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Posey K.A., Clegg D.J., Printz R.L., Byun J., Morton G.J., Vivekanandan-Giri A. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. American Journal of Physiology, Endocrinology and Metabolism. 2009;296(5):E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry C.J., Huang Y., Wynne A., Hanke M., Himler J., Bailey M.T. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. Journal of Neuroinflammation. 2008;5(1):15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milanski M., Degasperi G., Coope A., Morari J., Denis R., Cintra D.E. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. Journal of Neuroscience. 2009;29(2):359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thaler J.P., Yi C.-X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O. Obesity is associated with hypothalamic injury in rodents and humans. Journal of Clinical Investigation. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benzler J., Ganjam G.K., Pretz D., Oelkrug R., Koch C.E., Legler K. Central inhibition of IKKβ/NF-κB signaling attenuates high-fat diet-induced obesity and glucose intolerance. Diabetes. 2015;64(6):2015–2027. doi: 10.2337/db14-0093. [DOI] [PubMed] [Google Scholar]
- 12.Guyenet S.J., Nguyen H.T., Hwang B.H., Schwartz M.W., Baskin D.G., Thaler J.P. High-fat diet feeding causes rapid, non-apoptotic cleavage of caspase-3 in astrocytes. Brain Research. 2013;1512:97–105. doi: 10.1016/j.brainres.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckman L.B., Thompson M.M., Moreno H.N., Ellacott K.L.J. Regional astrogliosis in the mouse hypothalamus in response to obesity. Journal of Comparative Neurology. 2013;521(6):1322–1333. doi: 10.1002/cne.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckman L.B., Hasty A.H., Flaherty D.K., Buckman C.T., Thompson M.M., Matlock B.K. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain, Behavior, and Immunity. 2014;35:33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkseth K.E., Guyenet S.J., Melhorn S.J., Lee D., Thaler J.P., Schur E.A. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: a combined immunohistochemical and magnetic resonance imaging study. Endocrinology. 2014;155(8):2858–2867. doi: 10.1210/en.2014-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee D., Thaler J.P., Berkseth K.E., Melhorn S.J., Schwartz M.W., Schur E.A. Longer T(2) relaxation time is a marker of hypothalamic gliosis in mice with diet-induced obesity. American Journal of Physiology, Endocrinology and Metabolism. 2013;304:E1245–E1250. doi: 10.1152/ajpendo.00020.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdearcos M., Robblee M.M., Benjamin D.I., Nomura D.K., Xu A.W., Koliwad S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Reports. 2014;9(6):2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.André C., Guzman-Quevedo O., Rey C., Rémus-Borel J., Clark S., Castellanos-Jankiewicz A. Inhibiting microglia expansion prevents diet-induced hypothalamic and peripheral inflammation. Diabetes. 2016 doi: 10.2337/db16-0586. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Buckman L.B., Thompson M.M., Lippert R.N., Blackwell T.S., Yull F.E., Ellacott K.L.J. Evidence for a novel functional role of astrocytes in the acute homeostatic response to high-fat diet intake in mice. Molecular Metabolism. 2015;4(1):58–63. doi: 10.1016/j.molmet.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathologica. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamanaka K., Chun S.J., Boillee S., Fujimori-Tonou N., Yamashita H., Gutmann D.H. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nature Neuroscience. 2008;11(3):251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborn L.M., Kamphuis W., Wadman W.J., Hol E.M. Astrogliosis: an integral player in the pathogenesis of Alzheimer's disease. Progress in Neurobiology. 2016;144:121–141. doi: 10.1016/j.pneurobio.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Yang L., Qi Y., Yang Y. Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Reports. 2015;11(5):798–807. doi: 10.1016/j.celrep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Chen N., Sugihara H., Kim J.J.J., Fu Z., Barak B., Sur M. Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. eLife. 2016;5:5599–5609. doi: 10.7554/eLife.18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J.G., Suyama S., Koch M., Jin S., Argente-Arizon P., Argente J. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nature Neuroscience. 2014;17(7):908–910. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuente-Martín E., García-Cáceres C., Argente-Arizón P., Díaz F., Granado M., Freire-Regatillo A. Ghrelin regulates glucose and glutamate transporters in hypothalamic astrocytes. Science Reports. 2016;6:23673. doi: 10.1038/srep23673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiner D.J., Mietlicki-Baase E.G., McGrath L.E., Zimmer D.J., Bence K.K., Sousa G.L. Astrocytes regulate GLP-1 receptor-mediated effects on energy balance. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2016;36(12):3531–3540. doi: 10.1523/JNEUROSCI.3579-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Caceres C., Quarta C., Varela L., Gao Y., Gruber T., Legutko B. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell. 2016;166(4):867–880. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schur E.A., Melhorn S.J., Oh S.-K., Lacy J.M., Berkseth K.E., Guyenet S.J. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity (Silver Spring, Md.) 2015;23(11):2142–2148. doi: 10.1002/oby.21248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z.-W., Omori S.A., Labuda T., Karin M., Rickert R.C. IKK beta is required for peripheral B cell survival and proliferation. Journal of Immunology (Baltimore, Md.: 1950) 2003;170(9):4630–4637. doi: 10.4049/jimmunol.170.9.4630. [DOI] [PubMed] [Google Scholar]
- 31.Kaltschmidt B., Kaltschmidt C. NF-kappaB in the nervous system. Cold Spring Harbor Perspectives in Biology. 2009;1(3):a001271. doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kälin S., Heppner F.L., Bechmann I., Prinz M., Tschöp M.H., Yi C.-X. Hypothalamic innate immune reaction in obesity. Nature Reviews, Endocrinology. 2015;11(6):339–351. doi: 10.1038/nrendo.2015.48. [DOI] [PubMed] [Google Scholar]
- 33.Lapchak P.A., Hefti F. BDNF and NGF treatment in lesioned rats: effects on cholinergic function and weight gain. Neuroreport. 1992;3(5):405–408. doi: 10.1097/00001756-199205000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Unger T.J., Calderon G.A., Bradley L.C., Sena-Esteves M., Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27(52):14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganat Y.M., Silbereis J., Cave C., Ngu H., Anderson G.M., Ohkubo Y. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(33):8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beckervordersandforth R., Tripathi P., Ninkovic J., Bayam E., Lepier A., Stempfhuber B. In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell. 2010;7(6):744–758. doi: 10.1016/j.stem.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 37.Moon Y., Kim H.J., Kim J.Y., Kim H., Kim W.R., Sun W. Different expression of human GFAP promoter-derived GFP in different subsets of astrocytes in the mouse brain. Animal Cells and Systems. 2011;15(4):268–273. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inflammatory response is reduced in IKKβ-AKO primary astrocytes. Primary cortical astrocytes isolated from control and IKKβ-AKO mice were treated for 24 h with media or media containing 100 ng/ml LPS. Gene expression was measured by qRT-PCR for (A) Ikbkb (B) Tnfa (C) Il1b (D) Il6 cytokines. n = 3–4 replicates per treatment group. Values are mean ± SEM, 2-way ANOVA with Bonferroni post hoc test, p < 0.05 shown by different lower-case letters.
IKKβ-AKO mice at 3 weeks after tamoxifen do not differ in body composition, RER, or ambulatory activity. Calorimetry was performed 3 weeks after TMX administration in HFD-TMX IKKβ-AKO mice. Body composition was assessed at time of analyses. There were no differences in (A) body weight, (B) fat mass, or (C) lean mass. (D) 48-h timeline of respiratory exchange ratio (RER) and (D) photoperiod averages of RER. (F) 48-h timeline of ambulatory activity (measured by consecutive beam breaks) and (G) average photoperiod total activity. All analyses n = 5–8 per group. Values are mean ± SEM, Student's t-test, *p < 0.05.