Abstract
Introduction:
Cryptosporidium species is the most common opportunistic enteric parasite encountered in the immunocompromised patients. Considering the need to diagnose them early relies mostly on rapid tests such as antigen detection by immunochromatographic test (ICT), ELISA, and microscopy. However, the sensitivity and specificity varies with different methods and different kits used. This study was conducted to determine the intestinal parasitic profile in immunocompromised patients and to assess the diagnostic accuracy of the ICT using ImmunoCard STAT kit in detecting Cryptosporidium spp.
Materials and Methods:
The patients in this study were divided into two groups: one group was immunocompromised patients (n = 73) and the other was nonimmunocompromised individuals (n = 73). Stool microscopy, ICT, and polymerase chain reaction (PCR) were carried out for all stool samples.
Results:
Totally, 4 (5.4%) of 73 patients of the study group were positive for Cryptosporidium. The species detected were Cryptosporidium parvum and Cryptosporidium hominis. PCR was taken as gold standard in the current study. PCR detected Cryptosporidium in four samples while ICT in two samples and microscopy in one sample.
Conclusion:
Cryptosporidium was found to be the most common enteric parasite in the immunocompromised patients studied, followed by Cystoisospora, Entamoeba histolytica, and Strongyloides stercoralis. Although the ICT is a rapid test, it was less sensitive and more expensive in comparison to the PCR; hence, its utility appears to be limited in our setting.
Keywords: Cryptosporidium, immunochromatographic test, immunocompromised, polymerase chain reaction
INTRODUCTION
Intestinal parasitic infections are one of the most common causes of morbidity and mortality worldwide, especially among immunocompromised individuals. The various statistical estimations found that there were nearly 340 parasites infecting 3 billion people in developing countries. In developed country, there was an increase in the number of immunosuppressed individuals because of the use of aggressive immunosuppressive drugs and improvement in transplant procedure.[1]
Intestinal parasites such as Giardia intestinalis, Entamoeba histolytica, and Strongyloides stercoralis and coccidian parasites such as Cryptosporidium, Cystoisospora, Cyclospora, and Microsporidia are the common causes of diarrhea in immunocompromised individuals. These enteric parasites were encountered in 30%–60% of HIV positives in developed countries and 90% in developing countries. The individuals with CD4 count <200 cells/µl are at risk of acquiring the opportunistic infection.[2]
Among the opportunistic enteric parasites, Cryptosporidium species is considered an emerging protozoan parasite by the Centers for Disease Control and Prevention (CDC).[3] They usually cause self-limiting illness in the immunocompetent host but may lead to life-threatening disease in immunocompromised individuals. The mode of transmission is through contaminated food and water. Cryptosporidium was listed as a category B pathogen by CDC and the National Institute of Health because of its threat to cause water contamination.[4] A low infective dose of 10 oocysts can initiate the infection. Nearly 22 species of Cryptosporidium have been reported worldwide.[5] Among these, Cryptosporidium parvum and Cryptosporidium hominis are the most common species infecting humans. The infections with other species such as Cryptosporidium felis and Cryptosporidium meleagridis have also been reported, particularly in immunosuppressed patients.[6] The need for an early diagnosis of these infections is important because the treatment available is not adequate to completely cure the patient. The only method is by following the preventive measures such as personal hygiene.
In India, cryptosporidiosis is an important cause of morbidity in HIV-infected individuals, resulting in chronic diarrhea.[7] There are many diagnostic modalities such as microscopy, antigen detection by immunochromatographic test (ICT), ELISA, and polymerase chain reaction (PCR). However, the sensitivity and specificity varies with different methods and different kits used. There are some studies in India on opportunistic intestinal parasites occurring in immunocompromised individuals[2,8,9] and also studies comparing the various diagnostic modalities.[10,11,12] This study was conducted to study the intestinal parasitic profile in immunocompromised patients and to assess the diagnostic accuracy of the ICT using ImmunoCard STAT kit in detecting Cryptosporidium spp.
MATERIALS AND METHODS
This was an analytical study conducted in the Department of Microbiology from October 2013 to June 2015 in collaboration with the Departments of Pediatrics and Medicine in a tertiary care hospital after getting approval from the Institute Research and Ethics Committee. This study compared the stool microscopy, immunochromatography, and PCR. The study population was divided into two groups. Group I was the study group which comprised immunocompromised patients (n = 73) which included HIV seropositive (n = 21), transplant recipients (n = 16), long-term steroids (n = 14), malignant patients on chemotherapy and radiotherapy (n = 18), and primary immunodeficiency (n = 4). Group II was the control group which comprised nonimmunocompromised individuals (n = 73) who attended the outpatient department (OPD) for reasons other than gastrointestinal illnesses but were not admitted. Both study and control groups were age and gender matched. All individuals on antimicrobials within a week were excluded from the study.
The stool microscopy was done by saline and iodine wet mount examination after concentration by Sheather's sucrose floatation technique,[13] and modified acid-fast staining was done using 1% concentrated sulfuric acid. ImmunoCard STAT Crypto/Giardia rapid assay (M/S Meridian Bioscience, Europe) was used for the qualitative detection of Cryptosporidium- and G. intestinalis-specific antigens in the aqueous extract of fecal specimens. The test was performed according to the manufacturer's instructions. Stool DNA was extracted using the QIAamp DNA Stool Mini Kit according to the manufacturer's instructions. A conventional PCR was performed on the palm cycler (Eppendorf Mastercycler gradient thermocycler, Germany). The genus Cryptosporidium was detected by targeting the 18S rRNA gene using Forward primer - 5’AGTGACAAGAAATAACAATACAGG3’ and Reverse primer - 5’CCTGCTTTAAGCACTCTAATTTTC3’ and amplification of the extracted DNA was done using 25 µl reaction volume with little modifications.[10] The primers and DNA used were 12.6 pmol (each of the forward and reverse) and 4 µl, respectively. Samples were heated to 96°C for 2 min followed by 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s and 1 cycle of 72°C for 8 min. After the amplification, the final product was analyzed by electrophoresis using 1.5% gel in 25X TBE buffer and stained with ethidium bromide solution to look for 298 bp product. The results were recorded, and the data were analyzed in the form of frequency and distribution. The sequencing of the positive amplicons of the PCR was done by the ABI BigDye terminator method (BioServe Biotechnologies Inc. India Pvt. Ltd, Hyderabad, India). The database search was performed using the BLASTN program (https://blast.ncbi.nlm.nih.gov/blast).
RESULTS
A total of 73 nonduplicate stool samples were collected from immunocompromised patients attending the OPD and admitted to the tertiary care hospital during the study period from October 2013 to June 2015. Additional 73 nonduplicate stool samples were collected from nonimmunocompromised individuals who attended the OPD for reasons other than gastrointestinal illnesses but were not admitted to the hospital during the same study period. All the samples were collected after obtaining written informed consent. Only one stool sample was collected from individuals attending the OPD, but more than one sample was collected from those admitted to the wards in the hospital. The number of patients included in the study on OPD basis was 100 (68.4%) while the number of patients admitted was 46 (31.5%).
Of the 146 patients in the study and control groups, the majority were aged between 21 and 30 years (30%). The lowest age documented in this study was 11 months. The mean age of the patients was 36.6 years (ranging from 11 months to 83 years).
Among the six children included, two were seropositive for HIV, two had primary immunodeficiency, one had celiac disease, and one had atopic dermatitis with Grade I protein energy malnutrition (PEM). Of these, one child with primary immunodeficiency was positive for Cryptosporidium by all the three tests, that is, microscopy, ICT, and PCR while children with HIV infection and PEM had cysts of G. intestinalis in their stool.
The majority (28.7%) of the patients in this study were the people living with HIV and AIDS (PLHA) [Figure 1].
Figure 1.
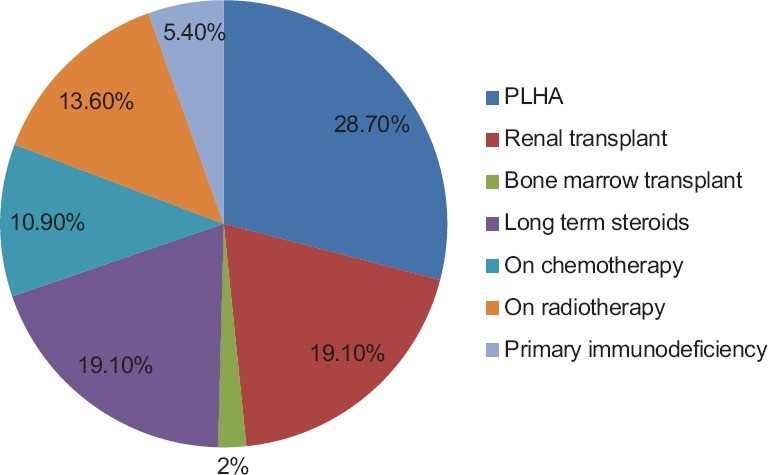
Clinical profile of immunocompromised patients
In this study, the most common intestinal parasites seen using unstained wet mount preparation by microscopy were cysts of Entamoeba spp. and larvae of S. stercoralis (4.1% each) [Figure 2].
Figure 2.
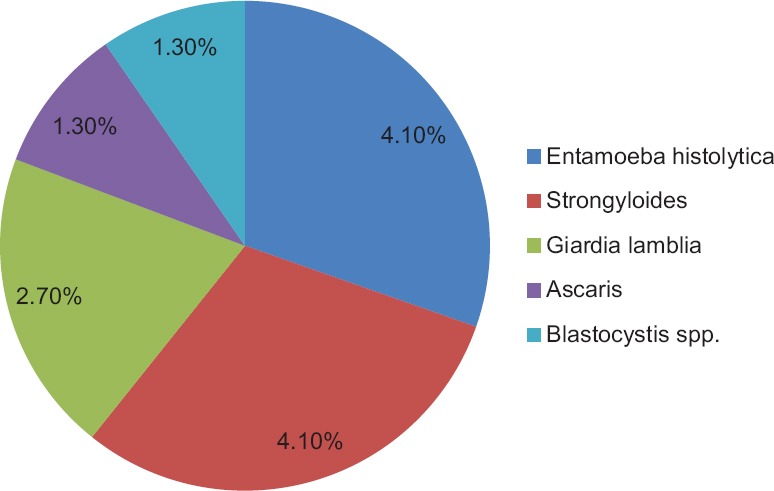
Profile of parasites detected by wet mount microscopic examination
All the individuals in the control group were found to be negative for any of the stool pathogens except for three who had cysts of Blastocystis. The load of the cysts in the stool sample was very less (one cyst in ten fields) and none of these individuals were symptomatic.
The most common immunocompromised condition associated with the enteric infections in this study was PLHA (47%) followed by postrenal transplant and primary immunodeficiency (17.6%). The parasite commonly associated with PLHA was oocyst of Cystoisospora while in postrenal transplant individuals it was Cryptosporidium [Figure 3 and Table 1]. Of the 21 PLHA patients, 5 had CD4 count <200 cells/µl, 3 of whom had detectable parasites in their stool samples; in 7 patients, the CD4 count was 200–500 cells/µl, 3 of whom had detectable parasites in their stool samples. In the remaining nine patients, the CD4 count was found to be >500 cells/µl, of which two patients had detectable parasites in their stool samples [Figure 4]. Overall, 8 PLHA patients had detectable parasites in their stool samples.
Figure 3.
Modified acid-fast stain showing oocyst of Cryptosporidium
Table 1.
Detection of acid-fast structures by modified acid-fast staining method

Figure 4.
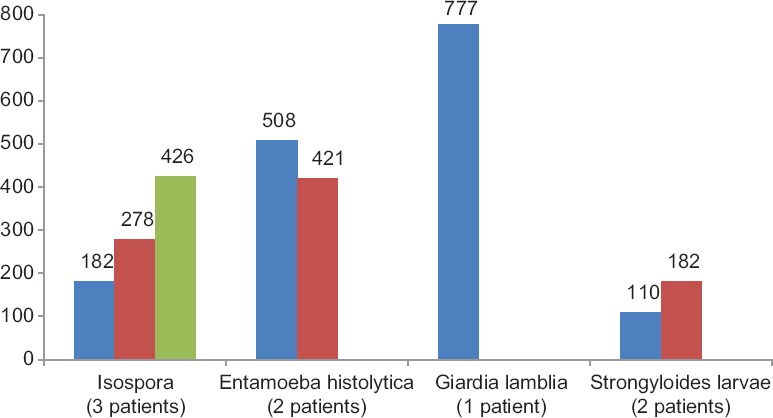
Profile of intestinal parasites in people living with HIV and AIDS according to their CD4 count
Comparison of microscopy and immunochromatographic test with the gold standard polymerase chain reaction for the detection of Cryptosporidium in stool samples
The genus-specific PCR could detect Cryptosporidium in 4 samples [Figure 5] when compared to other methods used in this study. Of the four positives of Cryptosporidium, PCR detected three samples more than microscopy. ICT detected only two samples [Figure 6] while additional two more samples were detected by PCR. The prevalence of Cryptosporidium in the study group was found to be 5.4% (4/73).
Figure 5.
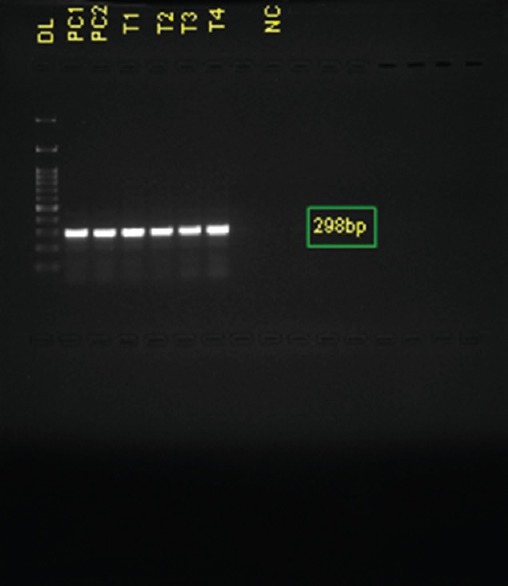
Detection of Cryptosporidium by polymerase chain reaction. Lane 1-100 bp DNA ladder; Lane 2 - Positive control of Cryptosporidium parvum; Lane 3 - Positive control of Cryptosporidium hominis; Lane 4–7 - Samples showing positive band by polymerase chain reaction; Lane 9 - Negative control
Figure 6.
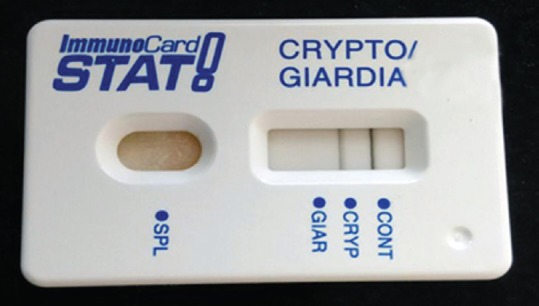
Immunochromatography test showing positive for Cryptosporidium
Results of sequence analysis of polymerase chain reaction amplicons
The species detected in this study was C. parvum and C. hominis. The sequences had identical match and coverage of 100% with GenBank accession numbers KP334118.1, KP098564.1, and KP213125.1.
DISCUSSION
Cryptosporidiosis is one of the most common causes of diarrhea in immunocompromised patients leading to significant morbidity and mortality worldwide.[14] Most of the laboratories routinely use microscopic methods for the diagnosis of cryptosporidiosis. Unfortunately, this method has many limitations as it requires a minimum threshold of 50,000 oocysts/ml of the stool sample for its detection. Hence it is difficult to detect the parasite by routine microscopy. Moreover, this technique requires expertise for reporting. To overcome all these drawbacks, antigen/antibody-based detection methods such as ICT and ELISA or molecular techniques such as PCR can be used. PCR has shown to be more sensitive compared to these methods. Batch testing is possible using PCR, and it permits differentiation between the species. In this study, we compared the performance of ICT with PCR (targeting 18S rRNA gene).
Of the 146 individuals (73 patients and 73 healthy controls) studied, the most common age group affected by the parasitic infestation in the study population was 21–30 years, followed by 31–40 years. This is in comparison with the study by Kashyap et al.[2] where the most common age group was 31–40 years. There was no significant association between gender and the immunocompromised state.
The majority (28.7%) of the immunocompromised patients in our study comprised PLHA as was observed in many previous studies.[8,11,15] The other patients were postrenal transplant recipients, patients with underlying malignancy on chemotherapy and/or radiotherapy, patients with primary immunodeficiency, and patients on long-term steroids and immunosuppressants such as psoriasis vulgaris, pemphigus vulgaris, Sjogren's syndrome, ulcerative colitis, and rheumatoid arthritis with eosinophilia [Figure 1].
In this study, we found enteric parasites in 14 patients. Of the various enteric parasites detected by microscopy, the predominant ones were Entamoeba spp. (4.1%), S. stercoralis larvae (4.1%), the coccidian parasite Cystoisospora (4.1%), G. intestinalis (2.7%), Cryptosporidium spp. (1.3%), Ascaris lumbricoides egg (1.3%), and Blastocystis spp. (1.3%).
A study done from Northern India showed an equal proportion of Cryptosporidium spp. and Cystoisospora (7%) as common parasites in PLHA, postrenal transplant recipients, and patients with hematological malignancies. Among the PLHA, a study from the western part of the country documented the prevalence of Cystoisospora, Entamoeba spp, G. intestinalis, and Cryptosporidium to be 2.5%–31%, 1.7%–7.7%, 2.2%–8.3%, and 10.8%–82%, respectively.[8] Contrarily, in the PLHA patients we studied, the positivity rate was (3/21) 14.2% for Cystoisospora, (2/21) 9.5% for Entamoeba spp., (1/21) 4.7% for G. intestinalis while no Cryptosporidium could be detected. Our observations are similar to another study conducted in the neighboring city of Chennai where Cystoisospora was found to be the most predominant (18%) parasite in PLHA patients.[16] However, our findings are in contrast to a study from the southern part of the country by Jayalakshmi et al., where Cryptosporidium was shown to be the most predominant enteric parasite in the PLHA patients.[15] Another study showed the predominance of Cryptosporidium (15.87%) over the others in the PLHA patients.[17] All these studies had the majority of the PLHA patients with CD4 count <200, which can be the reason for the difference in the number of Cryptosporidium cases which is much less in our study group [Figure 4].
Among the renal transplant patients, 3 (75%) had Cryptosporidium oocysts. A study was done by Raja et al.[18] found 53% of Cryptosporidium infection among renal transplant recipients, while another study showed a positivity rate of 16.6% for Cryptosporidium in posttransplant patients.[19] These patients are particularly vulnerable to acquiring such infections as a result of the usage of immunosuppressive therapy such as cyclosporine, tacrolimus, and steroids. In all these previous studies mentioned, the sample size was high compared to ours; this could reflect the differences in the positivity rates for Cryptosporidium.
Among the two bone marrow transplant recipients included in the present study, we found cysts of Entamoeba in one patient. Previous studies have shown 10.7%, 2.9%, and 1.7% positivity rate for Cryptosporidium.[7,20,21] We could not detect any Cryptosporidium in any of these two patients by either ICT or PCR.
We detected Cryptosporidium in a 2-year-old child with primary immunodeficiency who presented with diarrhea and weight loss for 3 months by all the three tests, that is, microscopy, ICT, and PCR. There are only sporadic reports referring to the same.[22,23]
The ICT is a rapid and sensitive tool for the detection of Cryptosporidium.[12] A study comparing the commercially available ICT kits showed that the sensitivity varied from 58% to 100% while the specificity ranged from 50% to 100%. The ImmunoCard STAT showed a sensitivity of 100%, specificity of 50%, and positivity rate of 10.2% in the same study,[12] while in the present study using the same ImmunoCard STAT kit, a sensitivity of 50%, specificity of 100%, and positivity rate 2.7% obtained. This difference could probably be due to the fact that in the previous studies the results of the ICT were not compared to the gold standard, that is, PCR. Furthermore, the differences in the positivity rates could be due to the relative differences in the number of patients included in this study when compared to the others. Moreover, only 3/73 (4.1%) samples were positive by PCR alone, while in one (1.3%) patient, Cryptosporidium could be detected by all the three tests performed. Probably, the burden of this parasite was low in the three patients, so that only PCR could detect the parasites.
In the Indian scenario, the species of Cryptosporidium most commonly detected are C. hominis and C. parvum.[24] The most common species identified by sequencing of the PCR amplicons in our study were C. parvum and C. hominis.
The major limitations of the study were that we did not have equal representation of categories of immunocompromised states in the study group; this could have led to a difference in the detection of the various parasites including Cryptosporidium. The socioeconomic status of the patients could not be studied since it was a hospital-based study and not community based. In this study, we used the genus (18SrRNA) specific primers for Cryptosporidium and hence were unable to differentiate between the species without employing further sequencing.
Our future perspective is to study a large number of patients into equal representative members with the above-mentioned immunocompromised states to understand the actual burden of the parasite.
CONCLUSION
PCR is better than microscopy and ICT for the detection of Cryptosporidium in terms of cost, sensitivity, and specificity, and long-term community-based studies into larger number of patients with a more homogeneous representation of all categories of immunocompromised patients are required to understand the burden of cryptosporidiosis better in our patient population and region.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Stark D, Barratt JL, van Hal S, Marriott D, Harkness J, Ellis JT. Clinical significance of enteric protozoa in the immunosuppressed human population. Clin Microbiol Rev. 2009;22:634–50. doi: 10.1128/CMR.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashyap A, Singh MP, Madhu, Ghoshal U. Occurrence of gastrointestinal opportunistic parasites in immunocompromised patients in Northern India. J Biol. 2013;1:77–80. [Google Scholar]
- 3.Iqbal A, Lim YA, Mahdy MA, Dixon BR, Surin J. Epidemiology of cryptosporidiosis in HIV-infected individuals: A global perspective. Open Access Sci Rep. 2012;1:431. [Google Scholar]
- 4.Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. Public health assessment of potential biological terrorism agents. Emerg Infect Dis. 2002;8:225–30. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fayer R. Cryptosporidium: A water-borne zoonotic parasite. Vet Parasitol. 2004;126:37–56. doi: 10.1016/j.vetpar.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Joachim A. Human cryptosporidiosis: An update with special emphasis on the situation in Europe. J Vet Med B Infect Dis Vet Public Health. 2004;51:251–9. doi: 10.1111/j.1439-0450.2004.00765.x. [DOI] [PubMed] [Google Scholar]
- 7.Ajjampur SS, Sankaran P, Kang G. Cryptosporidium species in HIV-infected individuals in India: An overview. Natl Med J India. 2008;21:178–84. [PubMed] [Google Scholar]
- 8.De A. Current laboratory diagnosis of opportunistic enteric parasites in human immunodeficiency virus-infected patients. Trop Parasitol. 2013;3:7–16. doi: 10.4103/2229-5070.113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jha AK, Uppal B, Chadha S, Bhalla P, Ghosh R, Aggarwal P, et al. Clinical and microbiological profile of HIV/AIDS cases with diarrhea in North India. J Pathog 2012. 2012:971958. doi: 10.1155/2012/971958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan UM, Pallant L, Dwyer BW, Forbes DA, Rich G, Thompson RC. Comparison of PCR and microscopy for detection of Cryptosporidium parvum in human fecal specimens: Clinical trial. J Clin Microbiol. 1998;36:995–8. doi: 10.1128/jcm.36.4.995-998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khurana S, Sharma P, Sharma A, Malla N. Evaluation of Ziehl-Neelsen staining, auramine phenol staining, antigen detection enzyme linked immunosorbent assay and polymerase chain reaction, for the diagnosis of intestinal cryptosporidiosis. Trop Parasitol. 2012;2:20–3. doi: 10.4103/2229-5070.97234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minak J, Kabir M, Mahmud I, Liu Y, Liu L, Haque R, et al. Evaluation of rapid antigen point-of-care tests for detection of Giardia and Cryptosporidium species in human fecal specimens. J Clin Microbiol. 2012;50:154–6. doi: 10.1128/JCM.01194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dryden MW, Payne PA, Ridley R, Smith V. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet Ther. 2005;6:15–28. [PubMed] [Google Scholar]
- 14.Dehkordy AB, Rafiei A, Alavi S, Latifi S. Prevalence of cryptosporidium infection in immunocompromised patients, in South-west of Iran, 2009-10. Iran J Parasitol. 2010;5:42–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Jayalakshmi J, Appalaraju B, Mahadevan K. Evaluation of an enzyme-linked immunoassay for the detection of Cryptosporidium antigen in fecal specimens of HIV/AIDS patients. Indian J Pathol Microbiol. 2008;51:137–8. doi: 10.4103/0377-4929.40427. [DOI] [PubMed] [Google Scholar]
- 16.Kumar SS, Ananthan S, Lakshmi P. Intestinal parasitic infection in HIV infected patients with diarrhoea in Chennai. Indian J Med Microbiol. 2002;20:88–91. [PubMed] [Google Scholar]
- 17.Naik VR, Ravichandraprakash H, Ukey PM, Vijayanath V, Shreeharsha G, Chandak VK, et al. Opportunistic intestinal parasitic infections in HIV/AIDS patients presenting with diarrhea and their correlation with CD4 + T-lymphocyte counts. Biol Sci. 2012;2:293–9. [Google Scholar]
- 18.Raja K, Abbas Z, Hassan SM, Luck NH, Aziz T, Mubarak M. Prevalence of cryptosporidiosis in renal transplant recipients presenting with acute diarrhea at a single center in Pakistan. J Nephropathol. 2014;3:127–31. doi: 10.12860/jnp.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udgiri N, Minz M, Kashyap R, Heer M, Gupta CS, Mohandas K, et al. Intestinal cryptosporidiasis in living related renal transplant recipients. Transplant Proc. 2004;36:2128–9. doi: 10.1016/j.transproceed.2004.08.107. [DOI] [PubMed] [Google Scholar]
- 20.Kang G, Srivastava A, Pulimood AB, Dennison D, Chandy M. Etiology of diarrhea in patients undergoing allogeneic bone marrow transplantation in South India. Transplantation. 2002;73:1247–51. doi: 10.1097/00007890-200204270-00010. [DOI] [PubMed] [Google Scholar]
- 21.George B, Mathews V, Viswabandya A, Srivastava A, Chandy M. Infections in children undergoing allogeneic bone marrow transplantation in India. Pediatr Transplant. 2006;10:48–54. doi: 10.1111/j.1399-3046.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 22.Wolska-Kusnierz B, Bajer A, Caccio S, Heropolitanska-Pliszka E, Bernatowska E, Socha P, et al. Cryptosporidium infection in patients with primary immunodeficiencies. J Pediatr Gastroenterol Nutr. 2007;45:458–64. doi: 10.1097/MPG.0b013e318054b09b. [DOI] [PubMed] [Google Scholar]
- 23.Jo EK, Kim HS, Lee MY, Iseki M, Lee JH, Song CH, et al. X-linked hyper-IgM syndrome associated with Cryptosporidium parvum and Cryptococcus neoformans infections: The first case with molecular diagnosis in Korea. J Korean Med Sci. 2002;17:116–20. doi: 10.3346/jkms.2002.17.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai NT, Sarkar R, Kang G. Cryptosporidiosis: An under-recognized public health problem. Trop Parasitol. 2012;2:91–8. doi: 10.4103/2229-5070.105173. [DOI] [PMC free article] [PubMed] [Google Scholar]


