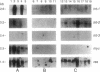
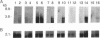Abstract
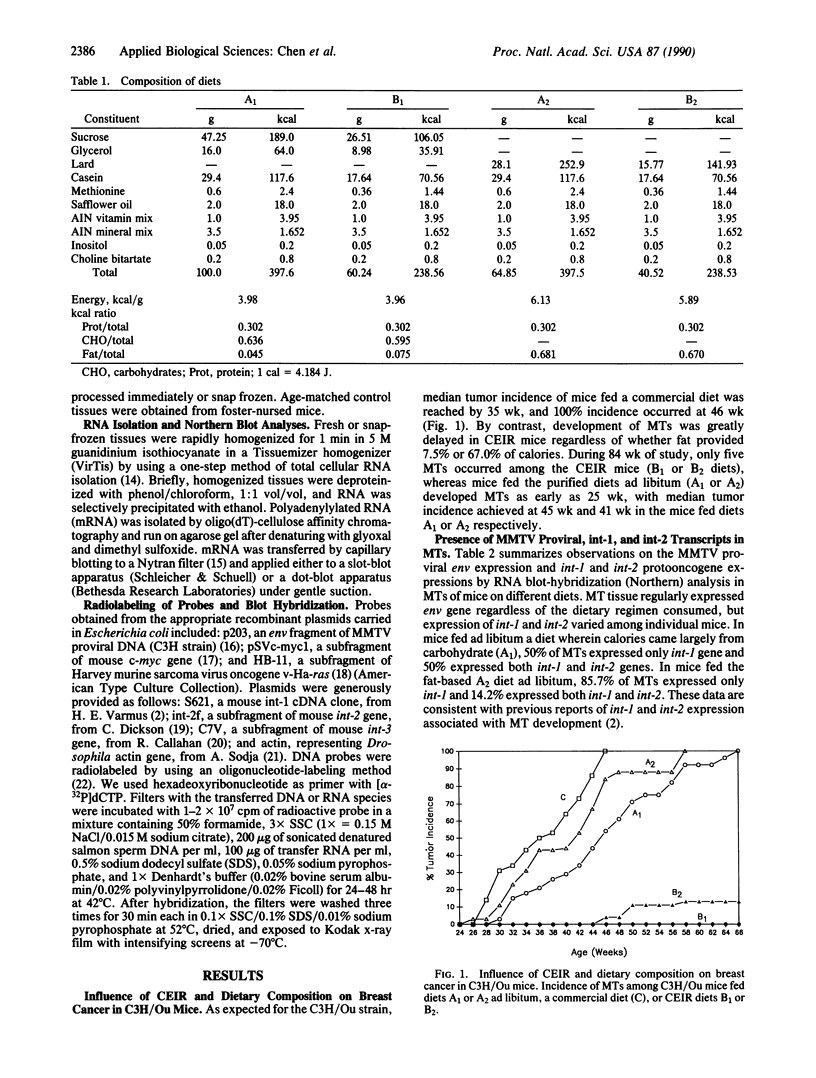
Chronic energy intake restriction (CEIR) reduces mouse mammary tumor virus (MMTV)-induced mammary tumors in C3H/Ou mice. Fewer than 10% of C3H/Ou mice developed mammary tumors during 88 wk of study when subjected to CEIR regardless of calorie source (fat vs. carbohydrate). By contrast, 100% of mice fed ad libitum diets relatively high in fat or carbohydrate or a commercial diet developed tumors by 35-40 wk. MMTV proviral DNA transcription was shown to be activated in spleen, liver, lung, kidney, small intestine, and mammary gland of mice consuming these diets ad libitum. By contrast, these messages were suppressed by CEIR in all tissues analyzed except spleen. MMTV proviral messages in liver and mammary gland increased with age in full-fed mice and were suppressed by CEIR. These findings suggest that the nutritional regulation of MMTV proviral DNA expression is tissue-specific. In CEIR mice the suppressed MMTV proviral DNA transcripts in mammary gland and liver increased with time in association with the delayed onset of mammary tumors. Mammary tumorigenesis in C3H mice is associated with integration of MMTV proviral DNA, which appears to activate a putative mammary tumor protooncogene, int-1. CEIR apparently decreases the frequency of viral reintegration adjacent to the int-1 gene and thus inhibits expression of int-1 and probably an initiation step in mammary tumorigenesis. Expression of other putative protooncogenes, int-2 and ras, in liver tissue was also reduced by CEIR. These findings indicate that both initiation and promotion of mammary tumorigenesis are influenced by CEIR in C3H/Ou mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albanes D. Total calories, body weight, and tumor incidence in mice. Cancer Res. 1987 Apr 15;47(8):1987–1992. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Engelman R. W., Day N. K., Chen R. F., Tomita Y., Bauer-Sardiña I., Dao M. L., Good R. A. Calorie consumption level influences development of C3H/Ou breast adenocarcinoma with indifference to calorie source. Proc Soc Exp Biol Med. 1990 Jan;193(1):23–30. doi: 10.3181/00379727-193-42984. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Suppression of adenocarcinoma by the immunological consequences of calorie restriction. Nature. 1976 Oct 7;263(5577):504–507. doi: 10.1038/263504b0. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Gallahan D., Kozak C., Callahan R. A new common integration region (int-3) for mouse mammary tumor virus on mouse chromosome 17. J Virol. 1987 Jan;61(1):218–220. doi: 10.1128/jvi.61.1.218-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrard D., Ross S. R. Endogenous mouse mammary tumor virus is expressed in several organs in addition to the lactating mammary gland. J Virol. 1988 Aug;62(8):3046–3049. doi: 10.1128/jvi.62.8.3046-3049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers J., Bentvelzen P. Interaction between viral and genetic factors in murine mammary cancer. Adv Cancer Res. 1978;26:143–195. doi: 10.1016/s0065-230x(08)60087-1. [DOI] [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Johnson B. C., Gajjar A., Kubo C., Good R. A. Calories versus protein in onset of renal disease in NZB x NZW mice. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5659–5662. doi: 10.1073/pnas.83.15.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C., Peters G., Pauley R., Morris V., Michalides R., Dudley J., Green M., Davisson M., Prakash O., Vaidya A. A standardized nomenclature for endogenous mouse mammary tumor viruses. J Virol. 1987 May;61(5):1651–1654. doi: 10.1128/jvi.61.5.1651-1654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky D., Weber M. M., Klurfeld D. M. Dietary fat versus caloric content in initiation and promotion of 7,12-dimethylbenz(a)anthracene-induced mammary tumorigenesis in rats. Cancer Res. 1984 Aug;44(8):3174–3177. [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Moore D. H., Long C. A., Vaidya A. B., Sheffield J. B., Dion A. S., Lasfargues E. Y. Mammary tumor viruses. Adv Cancer Res. 1979;29:347–418. doi: 10.1016/s0065-230x(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Outzen H. C., Corrow D., Shultz L. D. Attenuation of exogenous murine mammary tumor virus virulence in the C3H/HeJ mouse substrain bearing the Lps mutation. J Natl Cancer Inst. 1985 Nov;75(5):917–923. doi: 10.1093/jnci/75.5.917. [DOI] [PubMed] [Google Scholar]
- Peters G., Lee A. E., Dickson C. Concerted activation of two potential proto-oncogenes in carcinomas induced by mouse mammary tumour virus. Nature. 1986 Apr 17;320(6063):628–631. doi: 10.1038/320628a0. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Fernandes G., Telang N. T., Kourides I. A., Good R. A. Low-calorie diet prevents the development of mammary tumors in C3H mice and reduces circulating prolactin level, murine mammary tumor virus expression, and proliferation of mammary alveolar cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7758–7762. doi: 10.1073/pnas.79.24.7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn E., Muller W., Pattengale P., Tepler I., Wallace R., Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987 May 22;49(4):465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Pattengale P. K., Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984 Oct;38(3):627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- van Nie R., Verstraeten A. A. Studies of genetic transmission of mammary tumour virus by C3Hf mice. Int J Cancer. 1975 Dec 15;16(6):922–931. doi: 10.1002/ijc.2910160606. [DOI] [PubMed] [Google Scholar]