Abstract
Despite a good initial response to chemotherapy, the majority of patients with epithelial ovarian cancer will eventually recur and die of their disease. The introduction of targeted therapies to traditional chemotherapy regimens has done little to improve overall survival in women with ovarian cancer. It has become increasingly apparent that the cancer epigenome contributes significantly to the pathogenesis of ovarian cancer and may play an important role in cell proliferation, metastasis, chemoresistance, and immune tolerance. Epigenetic therapies such as DNA methyltransferase inhibitors and histone deacetylase inhibitors have the potential to reverse these epigenetic changes; however, more research is needed to determine how to incorporate these agents into clinical practice. In this review, we discuss the common epigenetic changes that occur in epithelial ovarian cancer, the current epigenetic therapies that may target these changes, and the clinical experience with epigenetic therapy for the treatment of epithelial ovarian cancer.
Highlights
-
•
Epigenetic changes are important in the pathogenesis of ovarian cancer.
-
•
Histone modification and DNA methylation are the most common epigenetic changes.
-
•
Targeting the epigenome in ovarian cancer may improve response to other therapies.
1. Introduction
With an estimated 22,280 new cases of ovarian cancer and 14,240 deaths projected in 2016, ovarian cancer remains the fifth-leading cause of cancer death in women (Siegel et al., 2016). While the majority of patients respond to primary platinum and taxane-based chemotherapy, recurrence rates are high with over 75% of patients ultimately relapsing (Ozols et al., 2003). Advances in cytotoxic chemotherapy and development of novel targeted therapies such as the poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitors have improved progression-free survival (PFS) but have failed to significantly impact overall survival (OS) (Armstrong et al., 2006, Ledermann et al., 2012). As long-term prognosis for patients with epithelial ovarian cancer remains poor, there is a need for development of new therapies to augment or replace traditional cytotoxic chemotherapies. One such area of therapeutic potential involves the use of epigenetic therapy.
While germline and somatic mutations in tumor suppressor genes such as BRCA1/2 have long been implicated in the development of ovarian cancer (Welcsh & King, 2001), it has become increasingly apparent that epigenetic changes also play a critical role. Epigenetic changes alter gene expression without affecting the underlying DNA sequence. The two most widely affected epigenetic pathways in cancer are DNA methylation and histone modification (Dawson & Kouzarides, 2012).
2. DNA methylation
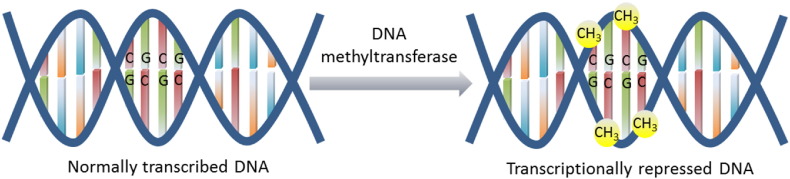
DNA methylation occurs at the carbon-5 position of cytosine residues, usually in cytosine-phosphate-guanine (CpG) dinucleotide sequences, and inhibits gene transcription (Fig. 1). The process of DNA methylation is regulated by a family of enzymes known as the DNA methyltransferases (DNMTs), which consists of DNMT1, DNMT3a, and DNMT3b. DNMT1 maintains appropriate methylation between cell divisions, while DNMT3a and DNMT3b control de novo methylation during embryogenesis (Sarkar et al., 2013). Levels of all three DNMTs have been shown to be upregulated in cancer cells compared to normal cells (Kautiainen and Jones, 1986, Xie et al., 1999).
Fig. 1.
The process of DNA methylation is mediated by a family of enzymes known as the DNA methyltransferases, which add a methyl (CH3) group at the carbon-5 position of cytosine-phosphate-guanine (CpG) dinucleotide sequences. The addition of the methyl groups inhibits DNA transcription and can lead to silencing of various genes.
CpG islands are CpG-rich sequences associated with the promoters of widely expressed genes which are normally protected from methylation. Genome-wide mapping has confirmed that 5–10% of these CpG islands become abnormally methylated in cancer genomes, and this de novo methylation has been implicated in the silencing of multiple tumor suppressor genes, as well as other genes that are critical for regulation of cell growth, angiogenesis, and DNA repair (Dawson & Kouzarides, 2012).
A number of genes have been found to be silenced via hypermethylation in ovarian cancer, and the degree of abnormal methylation has been correlated with disease progression and decreased survival (Watts et al., 2008, Wei et al., 2002). BRCA1 promoter hypermethylation with resultant decreased BRCA1 protein expression has been identified in 15–35% of patients with sporadic ovarian cancer (Bai et al., 2014, Baldwin et al., 2000). The effect of BRCA1 methylation on prognosis is unclear; it has been associated with improved survival in some studies and decreased survival in others (Bai et al., 2014, Chiang et al., 2006). BRCA1 methylation has also been correlated with improved chemosensitivity and response to PARP inhibitors, suggesting that patients with BRCA1 methylation have a similar phenotype to patients with germline BRCA1 mutations (Chaudhry et al., 2009, Veeck et al., 2010). Hypermethylation has been found to contribute to silencing of multiple other tumor suppressor genes in ovarian cancer, including p53, MLH1, HIC1, p16, E-cadherin, and APC (Strathdee et al., 2001, Makarla et al., 2005, Chmelarova et al., 2013), and both hypermethylation of multiple genes and increased expression of DMNTs have been associated with the development of platinum resistance (Li et al., 2009, Matei and Nephew, 2010).
While ovarian cancer is characterized by hypermethylation of numerous promoter CpG islands, the ovarian cancer genome is hypomethylated as a whole (Watts et al., 2008). Hypomethylation of unstable satellite DNA sequences has been shown to play an important role in carcinogenesis and is thought to contribute to genomic instability (Feinberg and Vogelstein, 1983, Widschwendter et al., 2004). Patients with ovarian cancer have significantly increased hypomethylation of satellite DNA compared to patients with benign or borderline ovarian tumors, and this extensive hypomethylation is strongly correlated with advanced stage and poor prognosis (Watts et al., 2008, Widschwendter et al., 2004).
3. Histone acetylation
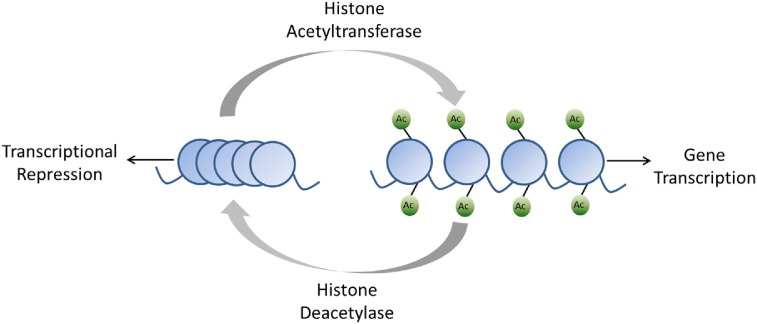
Histones are proteins that package DNA into nucleosomes which are the functional unit of chromatin. Post-translational histone modification can occur through several mechanisms; acetylation at the ε-amino group of lysine residues on the amino-terminal tails of the histone proteins is the best understood (Dawson & Kouzarides, 2012). Histone acetylation converts chromatin to an open or transcriptionally permissive state and is regulated by a class of enzymes known as histone acetyltransferases (HACs). Conversely, deacetylation is regulated by the histone deacetylases (HDACs) and converts chromatin to a more condensed or transcriptionally repressive state due to increase in electrostatic interactions between the histones and DNA (Fig. 2) (Dawson & Kouzarides, 2012). HDACs are also involved in acetylation of lysine residues of several non-histone proteins, including the estrogen and androgen receptors, p53, c-Myc, and STAT3 (Kim & Bae, 2011).
Fig. 2.
Histone acetylation converts chromatin to an open or transcriptionally permissive state and is regulated by the histone acetyltransferases. Deacetylation is regulated by the histone deacetylases and converts chromatin to a condensed or transcriptionally repressive state.
Eighteen distinct HDACs have been identified and separated into four classes based on sequence homology with yeast (Dawson & Kouzarides, 2012). Classes I, II, and IV are zinc-dependent, while class III is characterized by NAD + dependence. Class I HDACs are found only in the nucleus and are the most prevalent, while class II, III, and IV HDACs are found both in the nucleus and cytoplasm (Kim & Bae, 2011).
High levels of HDACs with resultant histone hypoacetylation have been identified in multiple cancers (Nakagawa et al., 2007). HDAC1, 2, and 3 are all class I HDACs that are expressed at high levels in ovarian cancer and are associated with poor prognosis (Khabele et al., 2007, Weichert et al., 2008). Expression of the class I HDACs has been shown to increase in a stepwise fashion when moving from benign to borderline to malignant ovarian tumors, indicating that these HDACs may play an important role in carcinogenesis. Specifically, HDAC1 and 2 expression correlate with increased cell proliferation in ovarian cancer cells, while HDAC3 expression inversely correlates with E-cadherin expression, suggesting a role in cell migration and metastasis (Hayashi et al., 2010). Additionally, HDAC overexpression has been correlated with development of platinum resistance in ovarian cancer (Kim et al., 2012).
4. Epigenetic therapy
4.1. DNA methyltransferase inhibitors
The DNMT inhibitors are cytosine analogues that are incorporated into DNA during replication and covalently bind to the DNMT enzymes inhibiting their function. At higher doses, these agents can also trap the DNMT enzyme leading to enzyme degradation and cytotoxicity (Heninger et al., 2015). 5-azacytidine (5-AZA) and decitabine (5-aza-2′-deoxycytidine) are the two most commonly used DNMT inhibitors and were initially developed in the 1960s as cytotoxic drugs for use in the treatment of hematologic malignancies. Their ability to inhibit DNA methylation was discovered later (Heninger et al., 2015). Both of these drugs are currently FDA-approved for the treatment of myelodysplastic syndromes, but they have been investigated in numerous solid tumors (FDA approval for decitabine for injection (Dacogen) to treat myelodysplastic syndromes. 2006 December 13, 2016; Kaminskas et al., 2005).
The major toxicity for 5-AZA and decitabine is myelosuppression which can be severe and dose-limiting (FDA approval for decitabine for injection (Dacogen) to treat myelodysplastic syndromes. 2006 December 13, 2016; Kaminskas et al., 2005). Given this substantial toxicity, several other DNMT inhibitors are currently being investigated, including less-toxic nucleoside inhibitors such as zebularine, non-nucleoside inhibitors such as the local anesthetic procaine, the main polyphenol compound from green tea epigallocatechin-3-gallate (EGCG), and the small-molecule inhibitor RG108 (Heninger et al., 2015, Stresemann et al., 2006).
4.2. Histone deacetylase inhibitors
In the 1970′s, Riggs et al. discovered that the drug sodium butyrate was an effective and specific inhibitor of HDAC activity. Subsequently, sodium butyrate was found to induce cell differentiation and inhibit tumor cell growth, prompting the development of several HDAC inhibitors designed for clinical use (Lane & Chabner, 2009). All of the current HDAC inhibitors act by targeting the zinc ion required for catalytic function of the class I, II, and IV HDACs. The class III HDACs, which are not zinc dependent, are not inhibited by any of the available HDAC inhibitors (Lane and Chabner, 2009, Bolden et al., 2006).
HDAC inhibitors can be classified by their specificity (pan-HDAC inhibitors versus class-specific inhibitors) or by their molecular structure. Structurally, HDAC inhibitors can be divided into four classes—hydroxamic acids, cyclic tetrapeptides, benzamides, and short-chain aliphatic acids (Kim and Bae, 2011, Lane and Chabner, 2009). The hydroxamic acids are the largest class of HDAC inhibitors and include vorinostat, belinostat, and panobinostat; all are pan-HDAC inhibitors that have been FDA-approved for the treatment of hematologic malignancies (Mann et al., 2007, Lee et al., 2015, Laubach et al., 2015). Romidepsin, a class I HDAC-specific cyclic tetrapeptide, is FDA-approved for the treatment of cutaneous T-cell lymphoma (Barbarotta & Hurley, 2015). While the class I specific benzamide entinostat (MS-275) is not currently FDA-approved, it has been granted a breakthrough designation when used in combination with exemestane for recurrent or metastatic estrogen-receptor positive breast cancer in postmenopausal women who have progressed after aromatase inhibitor therapy (FDA grants breakthrough therapy status to entinostat for advanced breast cancer, 2013). It is also being investigated in multiple other disease sites (Ngamphaiboon et al., 2015). The short-chain aliphatic acids, such as valproic acid, are relatively weak HDAC inhibitors making them less clinically attractive (Kim and Bae, 2011, Lane and Chabner, 2009).
The clinical activity of HDAC inhibitors, which includes arrest of cell growth (Bolden et al., 2006), promotion of cell differentiation and apoptosis (Rosato et al., 2003), and inhibition of angiogenesis (Kim et al., 2001), is achieved by selective alteration of gene transcription. This occurs through chromatin remodeling, changes in structure of transcription factor complexes, and regulation of multiple non-histone proteins (Bolden et al., 2006). HDAC inhibition alone does not result in transcriptional changes of all genes. It is estimated that 20% of known genes are affected by HDAC inhibitors, with approximately half of those being upregulated and the remainder being downregulated (Peart et al., 2005). Importantly, when compared to tumor cells, normal cells are relatively resistant to the effects of HDAC inhibitors (Johnstone, 2002).
4.3. Other epigenetic therapies
While histone acetylation/deacetylation is the best understood pathway of histone modification, there are several other pathways that are important in regulating chromatin structure and gene transcription, including methylation and phosphorylation, which may represent additional therapeutic targets (Dawson & Kouzarides, 2012). One such example is the histone lysine methyltransferases EZH2, which mediates methylation of a lysine residue on histone H3. Its overexpression has been correlated with aggressive behavior, metastasis, and poor prognosis in multiple cancers, prompting development of small-molecule inhibitors that are currently being investigated in clinical trials (McCabe & Creasy, 2014).
The primary readers of acetylated lysine residues are the bromodomain proteins, which include the BET family (BRD2, BRD3, BRD4, and BRDt). These proteins play an important role in transcription elongation and cell-cycle progression by RNA polymerase II. Inhibition of the BET bromodomain family has been shown to inhibit MYC transcription, resulting in decreased cell proliferation and increased apoptosis (Fu et al., 2015). BET inhibitors have efficacy in several hematologic malignancies and are being studied in solid tumors as well (Chaidos et al., 2015).
5. Use of epigenetic therapy in ovarian cancer
Both HDAC inhibitors and DNMT inhibitors have been investigated in ovarian cancer as single agents and in combination with other therapies. The current clinical experience is summarized in Table 1.
Table 1.
Clinical Experience with Epigenetic Therapy in Epithelial Ovarian Cancer.
| Citation | 1st Author | Year | Study Type | Regimen | # Pts | Population | Findings |
|---|---|---|---|---|---|---|---|
| HDAC Inhibitors | |||||||
| (Modesitt et al., 2008) | Modesitt | 2008 | Phase 2 | Vorinostat | 27 | Platinum-resistant | 1 PR, 9 SD, only 2 patients had PFS > 6 months |
| (Mendivil et al., 2013) | Mendivil | 2013 | Phase 2 | Vorinostat + paclitaxel/carbo | 18 | Primary therapy | 7 CR, 2 PR, 2 SD, ORR 50%. Terminated early due to GI perforation in 3 patients |
| (Matulonis et al., 2015) | Matulonis | 2015 | Phase 1 | Vorinostat + gemcitabine/carbo | 15 | 1st recurrence, platinum-sensitive | 6 PR, 1 SD. Terminated early due to hematologic toxicity |
| (Mackay et al., 2010) | Mackay | 2010 | Phase 2 | Belinostat | 32 | Platinum-resistant EOC or LMP | LMP: 1 PR, 10 SD EOC: 9 SD |
| (Dizon et al., 2012a) | Dizon | 2012 | Phase 2 | Belinostat + carbo | 27 | Platinum-resistant | ORR 7.4%. Terminated early due to lack of activity |
| (Dizon et al., 2012b) | Dizon | 2012 | Phase 1b/2 | Belinostat + paclitaxel/carbo | 35 | Recurrent EOC | 3 CR, 12 PR, ORR 43% |
| DNMT Inhibitors | |||||||
| (Fu et al., 2011) | Fu | 2011 | Phase 1 | 5AZA + carbo | 17 | Platinum-resistant | 1 CR, 3 PR, 10 SD. ORR 22% |
| (Falchook et al., 2013) | Falchook | 2013 | Phase 1 | 5AZA + VPA + carbo | 32 (10 EOC) | Platinum-resistant | 3/10 EOC patients had minor response or SD > 4 months |
| (Fang et al., 2010) | Fang | 2010 | Phase 1 | Decitabine + carbo | 9 | Platinum-resistant | 1 CR, 3 SD > 6 months |
| (Matei et al., 2012) | Matei | 2012 | Phase 2 | Decitabine + carbo | 17 | Platinum-resistant | 1 CR, 5 PR, 6 SD. 35% ORR |
| (Odunsi et al., 2014) | Odunsi | 2014 | Phase 1 | NY-ESO-1 vaccine + decitabine + PLD | 10 | Recurrent EOC | 5 SD, 1 PR |
Carbo = carboplatin; 5AZA = 5-azacytidine; VPA = valproic acid; PLD = pegylated liposomal doxorubicin; EOC = epithelial ovarian cancer; LMP = low malignant potential; CR = complete response; PR = partial response; SD = stable disease; ORR = objective response rate; PFS = progression-free survival.
5.1. Single agents
Similar to clinical findings in other disease sites, HDAC inhibitors have limited utility as single agents in ovarian cancer. Vorinostat and belinostat have been studied as single agents in patients with platinum-resistant ovarian cancer and while well-tolerated have minimal antitumor activity (Mackay et al., 2010, Modesitt et al., 2008). In a phase 2 study that enrolled 27 patients with recurrent, platinum-sensitive ovarian cancer, only two patients had a PFS longer than 6 months with single-agent vorinostat (Modesitt et al., 2008). Single agent belinostat was evaluated in a cohort of 32 patients with platinum-resistant recurrent disease, 18 with epithelial ovarian cancer and 14 with ovarian tumors of low malignant potential. For the patients with epithelial ovarian cancer, the median PFS was 2.3 months and the best response was stable disease in nine patients (Mackay et al., 2010). Given this lack of activity, attention has shifted to using these drugs in combination with other agents.
5.2. Restoration of platinum-sensitivity
Due to preclinical data indicating that both hypermethylation and histone modification may play an important role in the development of chemotherapy resistance (Li et al., 2009, Matei and Nephew, 2010), both DNMT inhibitors and HDAC inhibitors have been investigated as a means to mitigate platinum resistance in patients with recurrent disease.
While previously published data suggests that < 10% of patients with platinum-resistant ovarian cancer will have an objective response to re-treatment with platinum, pretreatment with azacytidine or decitabine produced objective response rates (ORRs) of 22% and 35%, respectively (Fu et al., 2011, Matei et al., 2012). Low-dose decitabine led to demethylation of multiple genes in pathways involved in Wnt signaling and apoptosis, as well as several individual genes including MLH1, RASSF1A, HOXA10, HOXA11, and BRCA1 (Matei et al., 2012, Fang et al., 2010). Although toxicity is a significant concern with these agents, both were well-tolerated in combination with carboplatin dosed for an area under the curve (AUC) of 5 [51–53]. While these studies were small, the results indicate that treatment with DNMT inhibitors may improve response to platinum in patients with platinum-resistant disease.
Experience with the HDAC inhibitors has been less encouraging in patients with platinum resistance. A phase 2 study of belinostat and carboplatin included 27 patients with platinum-resistant disease. The results were disappointing with an ORR of only 7.4%, and the study was terminated early due to lack of activity (Dizon et al., 2012a).
One phase 1 study has evaluated the combination of an HDAC inhibitor and DNMT inhibitor in patients with advanced malignancy refractory to standard treatment, including 10 patients with platinum-refractory or resistant ovarian cancer. 3 of the 10 ovarian cancer patients in this study had stable disease for > 4 months; however, the combination was poorly tolerated with nearly 80% of patients experiencing grade 3 or higher adverse events (Falchook et al., 2013).
5.3. Combination with cytotoxic chemotherapy
Low-dose decitabine has been studied in combination with dose-reduced paclitaxel and platinum chemotherapy in patients with platinum-resistant/refractory ovarian cancer in a phase 1/2 study. The combination was well-tolerated and produced clinical benefit (either partial response or stable disease) in over 70% of the 17 patients included in the trial (Fu et al., 2011).
Attempts to combine vorinostat with cytotoxic chemotherapy have been unsuccessful due to toxicity. A phase 2 trial combining vorinostat with paclitaxel and carboplatin in the upfront setting was terminated early after gastrointestinal perforation occurred in 3 of 11 patients (Mendivil et al., 2013). Similarly, a phase 1 study of vorinostat in combination with carboplatin and gemcitabine in platinum-sensitive patients with a first recurrence was terminated early due to unacceptable hematologic toxicity (Matulonis et al., 2015).
Belinostat is better tolerated than vorinostat in combination regimens. In a phase 1b/phase 2 trial that included 19 patients with recurrent platinum-sensitive disease and 16 patients with platinum-resistant disease, belinostat in combination with paclitaxel and carboplatin produced an ORR of 43%. The combination was well-tolerated with the only grade 4 toxicity being neutropenia in 14% of patients. The most common toxicities were nausea/vomiting and fatigue (Dizon et al., 2012b).
6. Epigenetic therapy and immunotherapy
While immune checkpoint blockade, which includes monoclonal antibody targeted inhibitors of programmed cell death protein 1 (PD1) and its ligand (PDL1) or anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), has shown promise for patients with metastatic melanoma, the majority of patients with ovarian cancer do not respond to single-agent checkpoint inhibitors (Varga et al., 2015, Gaillard et al., 2016). Several strategies have been developed to enhance response to checkpoint inhibitors, including using these agents in combination with chemotherapy or using dual checkpoint inhibitor therapy (Gaillard et al., 2016). Another exciting possibility involves the use of checkpoint inhibitors in combination with epigenetic therapy.
Interest in combining epigenetic and immunotherapy was sparked after a group of patients with non-small cell lung cancer (NSCLC) progressed through low-dose DNMT inhibitor therapy and crossed over to a checkpoint inhibitor trial. 60% of those patients developed a durable response that persisted at least 2.5 years, significantly better than the patients in the same trial that did not receive prior epigenetic therapy (Chiappinelli et al., 2016, Juergens et al., 2011). Subsequently, NSCLC cell lines treated with 5-AZA were found to have significant upregulation of multiple immune pathways, including increased expression of cancer testes antigen, major histocompatibility complex class I (MHCI), and PDL1 (Wrangle et al., 2013). In diffuse large B cell lymphoma, HDAC inhibitors have been shown to upregulate major histocompatibility complex class II (MHCII) expression on tumor cells via the transcriptional regulator CIITA (Cycon et al., 2013), and increased MHCII expression has been associated with improved immunogenicity and tumor rejection in animal models of breast, prostate, and renal cell carcinoma (Hillman et al., 2004, Mortara et al., 2006). Additionally, MHCII expression has been associated with increased infiltration of CD8 lymphocytes and improved survival in patients with triple negative breast cancer and papillary serous ovarian cancer (Cycon et al., 2013, Forero et al., 2016).
The combination of epigenetic therapy and immunotherapy has shown promising results in preclinical studies. In syngeneic murine models of colorectal (CT26) and breast (4 T1) cancer, the addition of entinostat and 5AZA to anti-PD1 and anti-CTLA4 led to complete tumor regression in 10/11 CT26 tumor-bearing mice and 10/10 4 T1 tumor-bearing mice. This was in comparison to the dual checkpoint inhibitor therapy alone, which resulted in tumor eradication in 36% of the CT26 mice and 30% of the 4T1 mice (Kim et al., 2014). Similarly, in a syngeneic ovarian cancer model, the combination of decitabine and anti-CTLA-4 significantly reduced tumor growth and prolonged survival compared to either agent alone. The enhanced anti-tumor effect appeared to be related to increased recruitment and activation of cytotoxic T lymphocytes (Wang et al., 2015).
The only currently published clinical trial evaluating the combination of epigenetic therapy and immunotherapy in ovarian cancer is a phase 1 study with the cancer testis antigen NY-ESO-1 vaccine plus decitabine and liposomal doxorubicin in patients with recurrent epithelial ovarian cancer. Increased NY-ESO-1 antibodies and associated T cell response were seen in the majority of patients, and 6/10 evaluable patients had either a partial response or stable disease (Odunsi et al., 2014). The combination of entinostat and the PDL1 inhibitor avelumab is currently being evaluated in patients with recurrent epithelial ovarian cancer [NCT02915523], and may provide additional insight into clinical response to combination epigenetic therapy and immunotherapy.
7. Conclusions
Epigenetic alterations such as aberrant DNA methylation and histone modification play an important role in the pathogenesis of epithelial ovarian cancer, and may contribute to multiple cancer phenotypes, including cell proliferation, metastasis, chemoresistance, and immune tolerance. An increasing number of therapeutic agents targeting epigenetic alterations are available, and these therapies, which include DNMT inhibitors and HDAC inhibitors, represent an exciting area of research.
While response rates with single-agent epigenetic therapy have thus far been low, these agents have been able to at least partially mitigate platinum resistance and improve response to immunotherapy in preclinical studies and some early phase clinical trials in epithelial ovarian cancer, indicating that epigenetic agents may be best used in combination with other therapies. Pretreatment with low dose azacytidine or decitabine produced ORRs of 22–35% to carboplatin in patients with documented platinum-resistance ovarian cancer, which is an improvement over the 10% ORR typically seen with carboplatin in this population (Fu et al., 2011, Matei et al., 2012).
In diffuse large B-cell lymphoma, treatment with HDAC inhibitors has resulted in upregulation of the MHCII pathway, which is known to be associated with improved survival in both breast and ovarian carcinoma (Cycon et al., 2013, Forero et al., 2016). In our experience, treatment with the HDAC inhibitor entinostat has resulted in increased MHCII expression in vitro in human and murine ovarian cancer cell lines, as well as in vivo in both a patient-derived xenograft and a syngeneic mouse model (unpublished data). HDAC inhibitors have also been associated with improved response to immune checkpoint inhibitors in patients with NSCLC (Juergens et al., 2011). The results of an ongoing clinical trial (NCT02915523) combining entinostat with a PDL1 inhibitor in ovarian cancer should provide additional information on the best way to combine these two classes of therapy in epithelial ovarian cancer. More studies are needed to determine the best strategy to incorporate these agents into the treatment of ovarian cancer while minimizing toxicity and maximizing clinical benefit.
Conflict of interest statement
None of the authors have any conflicts of interest to disclose.
References
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Ozols R.F. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology group study. J. Clin. Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- Armstrong D.K. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- Ledermann J. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012;366(15):1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- Welcsh P.L., King M.C. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum. Mol. Genet. 2001;10(7):705–713. doi: 10.1093/hmg/10.7.705. [DOI] [PubMed] [Google Scholar]
- Dawson M.A., Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Sarkar S. Cancer development, progression, and therapy: an epigenetic overview. Int. J. Mol. Sci. 2013;14(10):21087–21113. doi: 10.3390/ijms141021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautiainen T.L., Jones P.A. DNA methyltransferase levels in tumorigenic and nontumorigenic cells in culture. J. Biol. Chem. 1986;261(4):1594–1598. [PubMed] [Google Scholar]
- Xie S. Cloning, expression and chromosome locations of the human DNMT3 gene family. Gene. 1999;236(1):87–95. doi: 10.1016/s0378-1119(99)00252-8. [DOI] [PubMed] [Google Scholar]
- Watts G.S. DNA methylation changes in ovarian cancer are cumulative with disease progression and identify tumor stage. BMC Med. Genet. 2008;1:47. doi: 10.1186/1755-8794-1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S.H. Methylation microarray analysis of late-stage ovarian carcinomas distinguishes progression-free survival in patients and identifies candidate epigenetic markers. Clin. Cancer Res. 2002;8(7):2246–2252. [PubMed] [Google Scholar]
- Bai X. BRCA1 promoter hypermethylation in sporadic epithelial ovarian carcinoma: association with low expression of BRCA1, improved survival and co-expression of DNA methyltransferases. Oncol. Lett. 2014;7(4):1088–1096. doi: 10.3892/ol.2014.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R.L. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000;60(19):5329–5333. [PubMed] [Google Scholar]
- Chiang J.W. BRCA1 promoter methylation predicts adverse ovarian cancer prognosis. Gynecol. Oncol. 2006;101(3):403–410. doi: 10.1016/j.ygyno.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Chaudhry P., Srinivasan R., Patel F.D. Utility of gene promoter methylation in prediction of response to platinum-based chemotherapy in epithelial ovarian cancer (EOC) Cancer Investig. 2009;27(8):877–884. doi: 10.1080/07357900902849699. [DOI] [PubMed] [Google Scholar]
- Veeck J. BRCA1 CpG island hypermethylation predicts sensitivity to poly(adenosine diphosphate)-ribose polymerase inhibitors. J. Clin. Oncol. 2010;28(29):e563–e564. doi: 10.1200/JCO.2010.30.1010. (author reply e565–6) [DOI] [PubMed] [Google Scholar]
- Strathdee G. Primary ovarian carcinomas display multiple methylator phenotypes involving known tumor suppressor genes. Am. J. Pathol. 2001;158(3):1121–1127. doi: 10.1016/S0002-9440(10)64059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarla P.B. Promoter hypermethylation profile of ovarian epithelial neoplasms. Clin. Cancer Res. 2005;11(15):5365–5369. doi: 10.1158/1078-0432.CCR-04-2455. [DOI] [PubMed] [Google Scholar]
- Chmelarova M. Methylation in the p53 promoter in epithelial ovarian cancer. Clin. Transl. Oncol. 2013;15(2):160–163. doi: 10.1007/s12094-012-0894-z. [DOI] [PubMed] [Google Scholar]
- Li M. Integrated analysis of DNA methylation and gene expression reveals specific signaling pathways associated with platinum resistance in ovarian cancer. BMC Med. Genet. 2009;2:34. doi: 10.1186/1755-8794-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei D.E., Nephew K.P. Epigenetic therapies for chemoresensitization of epithelial ovarian cancer. Gynecol. Oncol. 2010;116(2):195–201. doi: 10.1016/j.ygyno.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A.P., Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301(5895):89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- Widschwendter M. DNA hypomethylation and ovarian cancer biology. Cancer Res. 2004;64(13):4472–4480. doi: 10.1158/0008-5472.CAN-04-0238. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Bae S.C. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am. J. Transl. Res. 2011;3(2):166–179. [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M. Expression profile of class I histone deacetylases in human cancer tissues. Oncol. Rep. 2007;18(4):769–774. [PubMed] [Google Scholar]
- Khabele D. Drug-induced inactivation or gene silencing of class I histone deacetylases suppresses ovarian cancer cell growth: implications for therapy. Cancer Biol. Ther. 2007;6(5):795–801. doi: 10.4161/cbt.6.5.4007. [DOI] [PubMed] [Google Scholar]
- Weichert W. Expression of class I histone deacetylases indicates poor prognosis in endometrioid subtypes of ovarian and endometrial carcinomas. Neoplasia. 2008;10(9):1021–1027. doi: 10.1593/neo.08474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A. Type-specific roles of histone deacetylase (HDAC) overexpression in ovarian carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates cell migration with downregulation of E-cadherin. Int. J. Cancer. 2010;127(6):1332–1346. doi: 10.1002/ijc.25151. [DOI] [PubMed] [Google Scholar]
- Kim M.G. The relationship between cisplatin resistance and histone deacetylase isoform overexpression in epithelial ovarian cancer cell lines. J. Gynecol. Oncol. 2012;23(3):182–189. doi: 10.3802/jgo.2012.23.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heninger E., Krueger T.E., Lang J.M. Augmenting antitumor immune responses with epigenetic modifying agents. Front. Immunol. 2015;6:29. doi: 10.3389/fimmu.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA approval for decitabine for injection (Dacogen) to treat myelodysplastic syndromes. 2006 December 13. 2016. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm095652.htm Available from:
- Kaminskas E. Approval summary: azacitidine for treatment of myelodysplastic syndrome subtypes. Clin. Cancer Res. 2005;11(10):3604–3608. doi: 10.1158/1078-0432.CCR-04-2135. [DOI] [PubMed] [Google Scholar]
- Stresemann C. Functional diversity of DNA methyltransferase inhibitors in human cancer cell lines. Cancer Res. 2006;66(5):2794–2800. doi: 10.1158/0008-5472.CAN-05-2821. [DOI] [PubMed] [Google Scholar]
- Lane A.A., Chabner B.A. Histone deacetylase inhibitors in cancer therapy. J. Clin. Oncol. 2009;27(32):5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- Bolden J.E., Peart M.J., Johnstone R.W. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 2006;5(9):769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- Mann B.S. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin. Cancer Res. 2007;13(8):2318–2322. doi: 10.1158/1078-0432.CCR-06-2672. [DOI] [PubMed] [Google Scholar]
- Lee H.Z. FDA approval: Belinostat for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. Clin. Cancer Res. 2015;21(12):2666–2670. doi: 10.1158/1078-0432.CCR-14-3119. [DOI] [PubMed] [Google Scholar]
- Laubach J.P. Panobinostat for the treatment of multiple myeloma. Clin. Cancer Res. 2015;21(21):4767–4773. doi: 10.1158/1078-0432.CCR-15-0530. [DOI] [PubMed] [Google Scholar]
- Barbarotta L., Hurley K. Romidepsin for the treatment of peripheral T-cell lymphoma. J. Adv. Pract. Oncol. 2015;6(1):22–36. [PMC free article] [PubMed] [Google Scholar]
- FDA grants breakthrough therapy status to entinostat for advanced breast cancer. 2013 1/3/17]; Available from: http://www.ascopost.com/News/8525.
- Ngamphaiboon N. A phase I study of the histone deacetylase (HDAC) inhibitor entinostat, in combination with sorafenib in patients with advanced solid tumors. Investig. New Drugs. 2015;33(1):225–232. doi: 10.1007/s10637-014-0174-6. [DOI] [PubMed] [Google Scholar]
- Rosato R.R., Almenara J.A., Grant S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 2003;63(13):3637–3645. [PubMed] [Google Scholar]
- Kim M.S. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat. Med. 2001;7(4):437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- Peart M.J. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc. Natl. Acad. Sci. U. S. A. 2005;102(10):3697–3702. doi: 10.1073/pnas.0500369102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone R.W. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 2002;1(4):287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- McCabe M.T., Creasy C.L. EZH2 as a potential target in cancer therapy. Epigenomics. 2014;6(3):341–351. doi: 10.2217/epi.14.23. [DOI] [PubMed] [Google Scholar]
- Fu L.L. Inhibition of BET bromodomains as a therapeutic strategy for cancer drug discovery. Oncotarget. 2015;6(8):5501–5516. doi: 10.18632/oncotarget.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaidos A., Caputo V., Karadimitris A. Inhibition of bromodomain and extra-terminal proteins (BET) as a potential therapeutic approach in haematological malignancies: emerging preclinical and clinical evidence. Ther. Adv. Hematol. 2015;6(3):128–141. doi: 10.1177/2040620715576662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay H.J. Phase II trial of the histone deacetylase inhibitor belinostat in women with platinum resistant epithelial ovarian cancer and micropapillary (LMP) ovarian tumours. Eur. J. Cancer. 2010;46(9):1573–1579. doi: 10.1016/j.ejca.2010.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesitt S.C. A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2008;109(2):182–186. doi: 10.1016/j.ygyno.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Fu S. Phase 1b-2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer. 2011;117(8):1661–1669. doi: 10.1002/cncr.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei D. Epigenetic resensitization to platinum in ovarian cancer. Cancer Res. 2012;72(9):2197–2205. doi: 10.1158/0008-5472.CAN-11-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F. A phase 1 and pharmacodynamic study of decitabine in combination with carboplatin in patients with recurrent, platinum-resistant, epithelial ovarian cancer. Cancer. 2010;116(17):4043–4053. doi: 10.1002/cncr.25204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizon D.S. A phase II evaluation of belinostat and carboplatin in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2012;125(2):367–371. doi: 10.1016/j.ygyno.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchook G.S. Methylation and histone deacetylase inhibition in combination with platinum treatment in patients with advanced malignancies. Investig. New Drugs. 2013;31(5):1192–1200. doi: 10.1007/s10637-013-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendivil A.A. Increased incidence of severe gastrointestinal events with first-line paclitaxel, carboplatin, and vorinostat chemotherapy for advanced-stage epithelial ovarian, primary peritoneal, and fallopian tube cancer. Int. J. Gynecol. Cancer. 2013;23(3):533–539. doi: 10.1097/IGC.0b013e31828566f1. [DOI] [PubMed] [Google Scholar]
- Matulonis U. Phase I study of combination of vorinostat, carboplatin, and gemcitabine in women with recurrent, platinum-sensitive epithelial ovarian, fallopian tube, or peritoneal cancer. Cancer Chemother. Pharmacol. 2015;76(2):417–423. doi: 10.1007/s00280-015-2813-9. [DOI] [PubMed] [Google Scholar]
- Dizon D.S. Phase II activity of belinostat (PXD-101), carboplatin, and paclitaxel in women with previously treated ovarian cancer. Int. J. Gynecol. Cancer. 2012;22(6):979–986. doi: 10.1097/IGC.0b013e31825736fd. [DOI] [PubMed] [Google Scholar]
- Varga A. ASCO Annual Meeting Proceedings. 2015. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase Ib study. [Google Scholar]
- Gaillard S.L., Secord A.A., Monk B. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecol. Oncol. Res. Pract. 2016;3:11. doi: 10.1186/s40661-016-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappinelli K.B. Combining epigenetic and immunotherapy to combat cancer. Cancer Res. 2016;76(7):1683–1689. doi: 10.1158/0008-5472.CAN-15-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergens R.A. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011;1(7):598–607. doi: 10.1158/2159-8290.CD-11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangle J. Alterations of immune response of non-small cell lung cancer with azacytidine. Oncotarget. 2013;4(11):2067–2079. doi: 10.18632/oncotarget.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycon K.A. Histone deacetylase inhibitors activate CIITA and MHC class II antigen expression in diffuse large B-cell lymphoma. Immunology. 2013;140(2):259–272. doi: 10.1111/imm.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman G.G. Turning tumor cells in situ into T-helper cell-stimulating, MHC class II tumor epitope-presenters: immuno-curing and immuno-consolidation. Cancer Treat. Rev. 2004;30(3):281–290. doi: 10.1016/j.ctrv.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Mortara L. CIITA-induced MHC class II expression in mammary adenocarcinoma leads to a Th1 polarization of the tumor microenvironment, tumor rejection, and specific antitumor memory. Clin. Cancer Res. 2006;12(11 Pt 1):3435–3443. doi: 10.1158/1078-0432.CCR-06-0165. [DOI] [PubMed] [Google Scholar]
- Forero A. Expression of the MHC class II pathway in triple-negative breast cancer tumor cells is associated with a good prognosis and infiltrating lymphocytes. Cancer Immunol. Res. 2016;4(5):390–399. doi: 10.1158/2326-6066.CIR-15-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc. Natl. Acad. Sci. U. S. A. 2014;111(32):11774–11779. doi: 10.1073/pnas.1410626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Decitabine enhances lymphocyte migration and function and synergizes with CTLA-4 blockade in a murine ovarian cancer model. Cancer Immunol. Res. 2015;3(9):1030–1041. doi: 10.1158/2326-6066.CIR-15-0073. [DOI] [PubMed] [Google Scholar]
- Odunsi K. Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer. Cancer Immunol. Res. 2014;2(1):37–49. doi: 10.1158/2326-6066.CIR-13-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]