Hypomyelinating leukodystrophy (HLD) is a genetic demyelinating and dismyelinating disease in the oligodendrocyte, the central nervous system (CNS) myelin-forming glia [1]. Pelizaeus-Merzbacher disease is a prototypic HLD and is now called HLD1. HLD1 is caused by mutations of the gene encoding proteolipid protein 1 (PLP1). HLD4 (OMIM No. 612233) is associated with a missense mutation of mitochondrial heat shock protein HSPD1 (also called Hsp60) [2]. HSPD1 is a member of the chaperonin ATPase family and participates in biosynthesis of a series of mitochondrial proteins involved in metabolic and redox regulation.
We have reported that changes of protein properties caused by their disease mutations are associated with their disease phenotypes of oligodendrocytes [3], [4], [5], [6], [7]. We herein report that transgenic mice expressing HLD4-associated (Asp-29-to-Gly) mutant of HSPD1 exhibit a defect in myelination in brain. We injected a DNA construct expressing HSPD1 (D29G) under the regulation of myelin-specific myelin basic protein (MBP) promoter [5] into ~ 200 of mouse fertilized eggs (Fig. S1), resulting in three lines of F0 generation's transgenic mice. The transgene of only one line was propagated into F1 and subsequent generations.
Fig. S1.
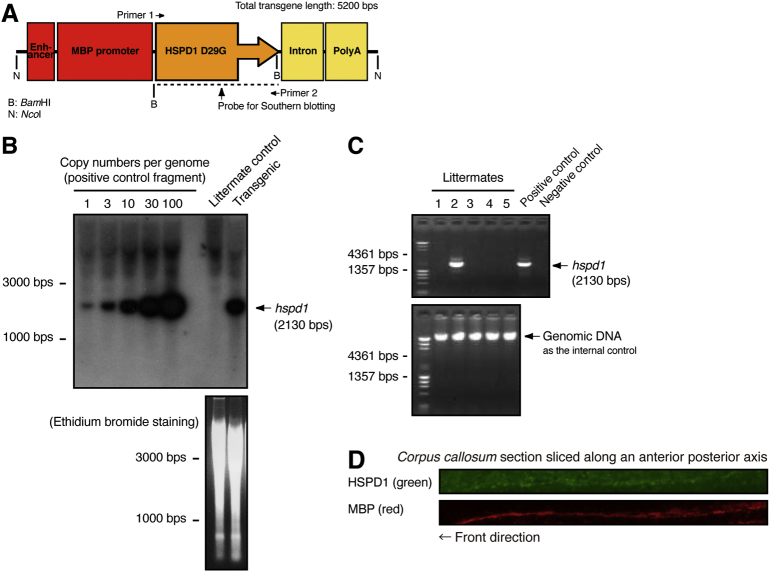
Generation of HSPD1 (D29G)-transgenic mice. (A) NcoI-digested transgene expressing HSPD1 (D29G) is composed of SV40 enhancer, mouse MBP promoter [5], human HSPD1 (D29G) tagged with FLAG at the C-terminus, artificial intron [5], and human chorionic gonadotropin polyadenylation signal. (B) Southern blotting confirmed that HSPD1 (D29G)-transgenic mice contain ~ 15 transgenes per genome. Genomic DNA in a denaturing agarose gel is also shown. (C) Presence of transgenes in transgenic mice was also confirmed by genomic PCR for HSPD1 (D29G). Genomic DNA in a non-denaturing agarose gel is also shown. (D) Immunostaining with an anti-FLAG antibody (green) confirmed that HSPD1 (D29G) is expressed along transgenic mouse corpus callosum. Despite decreased staining intensity (for transgenic mice), MBP expression (red) is also shown.
We have reported that changes of protein properties caused by their disease mutations are associated with their disease phenotypes of oligodendrocytes [3], [4], [5], [6], [7]. We herein report that transgenic mice expressing HLD4-associated (Asp-29-to-Gly) mutant of HSPD1 exhibit a defect in myelination in brain. We injected a DNA construct expressing HSPD1 (D29G) under the regulation of myelin-specific myelin basic protein (MBP) promoter [5] into ~ 200 of mouse fertilized eggs (Fig. S1), resulting in three lines of F0 generation's transgenic mice. The transgene of only one line was propagated into F1 and subsequent generations.
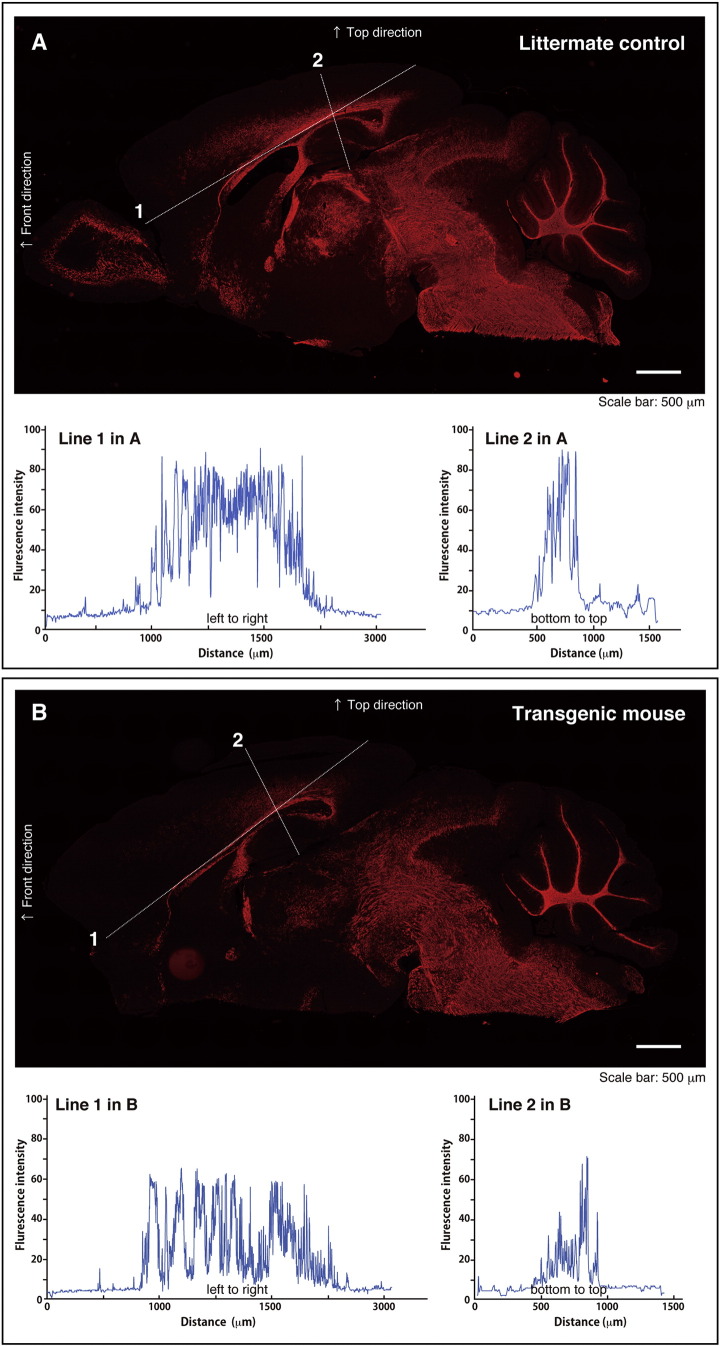
We immunostained neonatal transgenic mouse brain tissues sliced along an anterior and posterior axis with an anti-MBP antibody. Transgenic mice exhibit decreased myelin formation in corpus callosum (line 1 in panels A and B of the Fig. 1) as well as other brain regions, comparing with littermate controls. Corpus callosum typically contains a lot of axons with myelin sheaths and is one of the major portions suffering myelin defects in human and rodents. Generating mice exhibiting demyelinating or dismyelinating diseases may allow us not only to study how HLD-responsible gene mutations cause diseases but also to explore their therapeutic target molecules.
Fig. 1.
MBP staining of HSPD1 (D29G)-transgenic mice (B) and the littermate controls (A). Neonatal mouse brains were sliced along an anterior and posterior axis and immunostained with BioLegend, Inc.'s anti-MBP antibody (red), following with secondary fluorescent antibodies. Transgenic mice express the HSPD1 construct (see supplementary data) and exhibit decreased myelin formation. Scale bar indicates 500 μm. Line 1 is drawn along corpus callosum. Red color intensities along lines 1 and 2 are shown in two lower graphs. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The following is the supplementary data related to this article.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ymgmr.2017.03.003.
References
- 1.Clayton B.L., Popko B. Endoplasmic reticulum stress and the unfolded protein response in disorders of myelinating glia. Brain Res. 2016;1648:594–596. doi: 10.1016/j.brainres.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magen D., Georgopoulos C., Bross P., Ang D., Segev Y., Goldsher D., Nemirovski A., Shahar E., Ravid S., Luder A., Heno B., Gershoni-Baruch R., Skorecki K., Mandel H. Mitochondrial Hsp60 chaperonopathy causes an autosomal-recessive neurodegenerative disorder linked to brain hypomyelination and leukodystrophy. Am. J. Hum. Genet. 2008;83:30–42. doi: 10.1016/j.ajhg.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyamoto Y., Eguchi T., Kawahara K., Hasegawa N., Nakamura K., Funakoshi-Tago M., Tanoue A., Tamura H., Yamauchi J. Hypomyelinating leukodystrophy-associated missense mutation in HSPD1 blunts mitochondrial dynamics. Biochem. Biophys. Res. Commun. 2015;462:275–281. doi: 10.1016/j.bbrc.2015.04.132. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto Y., Funakoshi-Tago M., Hasegawa N., Eguchi T., Tanoue A., Tamura H., Yamauchi J. Data supporting mitochondrial morphological changes by SPG13-associated HSPD1 mutants. Data Brief. 2016;6:482–488. doi: 10.1016/j.dib.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyamoto Y., Torii T., Eguchi T., Nakamura K., Tanoue A., Yamauchi J. Hypomyelinating leukodystrophy-associated missense mutant of FAM126A/hyccin/DRCTNNB1A aggregates in the endoplasmic reticulum. J. Clin. Neurosci. 2014;21:1033–1039. doi: 10.1016/j.jocn.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Torii T., Miyamoto Y., Yamauchi J., Tanoue A. Pelizaeus-Merzbacher disease: cellular pathogenesis and pharmacologic therapy. Pediatr. Int. 2014;56:659–666. doi: 10.1111/ped.12450. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto Y., Torii T., Kawahara K., Hasegawa N., Tanoue A., Seki Y., Morimoto T., Funakoshi-Tago M., Tamura H., Homma K., Yamamoto M., Yamauchi J. Data on the effect of hypomyelinating leukodystrophy 6 (HLD6)-associated mutations on the TUBB4A properties. Data Brief. 2017;11:284–289. doi: 10.1016/j.dib.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]