Abstract
Mossy cells comprise a large fraction of the cells in the hippocampal dentate gyrus, suggesting that their function in this region is important. They are vulnerable to ischaemia, traumatic brain injury and seizures, and their loss could contribute to dentate gyrus dysfunction in such conditions. Mossy cell function has been unclear because these cells innervate both glutamatergic and GABAergic neurons within the dentate gyrus, contributing to a complex circuitry. It has also been difficult to directly and selectively manipulate mossy cells to study their function. In light of the new data generated using methods to preferentially eliminate or activate mossy cells in mice, it is timely to ask whether mossy cells have become any less enigmatic than they were in the past.
The mammalian hippocampus is typically divided into areas CA1, CA2 and CA3, and the dentate gyrus. As in most cortical circuits, hippocampal neurons are classified as either glutamatergic principal cells or GABAergic interneurons. The dentate gyrus contains a primary glutamatergic principal cell type, the granule cells, and is distinct from other hippocampal regions in having an additional glutamatergic principal cell type, the mossy cells. Mossy cells are named for their characteristic ‘mossy’ appearance when stained using the Golgi technique, which reveals clusters of complex spines, known as thorny excrescences, on their proximal dendrites. Mossy cells comprise a large subset of neurons of the dentate gyrus and are implicated in several pathological conditions, but these cells have been hard to define functionally. Many hypotheses about their functions have been proposed, but mossy cells have remained ‘enigmatic’ because of the persistent uncertainty about their functions.
Recent technical advances that enable the selective manipulation of mossy cells1,2 mean that this enigmatic past is giving way to a clearer understanding. For example, it is now evident that mossy cells can both excite granule cells and indirectly inhibit them by activating GABAergic interneurons. However, the new results raise more questions than they seem to answer. Therefore, it is timely to consider the new data in the context of the pre-existing hypotheses about mossy cells. In this Review, I first provide an overview of the dentate gyrus circuitry and describe the existing hypotheses about mossy cell function. I then explain how the new data using transgenic mice and optogenetics have shed light on these hypotheses, and I suggest additional experiments that are necessary to resolve the outstanding questions.
The dentate gyrus
Structure, circuitry and cell types
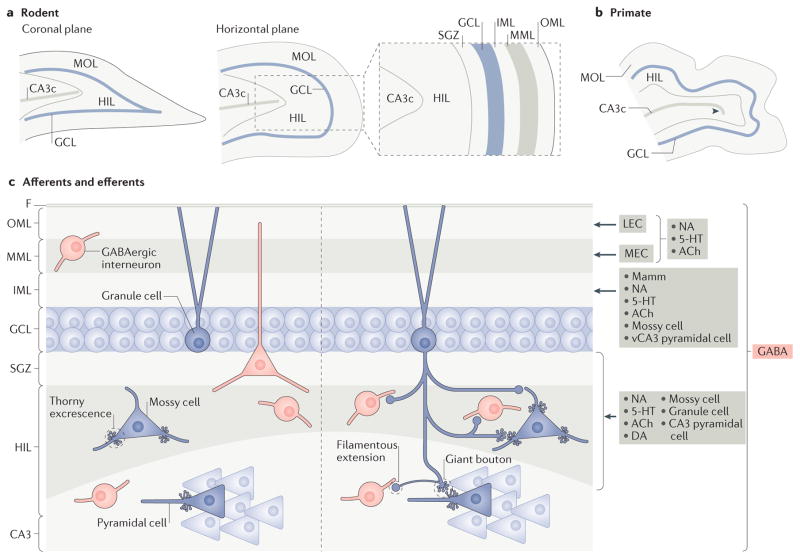
The basic structure of the dentate gyrus is discussed in detail elsewhere3 and summarized in FIG. 1a,b. The dentate gyrus is composed primarily of granule cells, which are oriented in a stereotypical manner. The dendrites of granule cells are present in the molecular layer, and their cell bodies form the adjacent granule cell layer (GCL). Between the GCL and area CA3, there is a polymorphic layer called the hilus (FIG. 1) that contains the granule cell axons, which are called mossy fibres (FIG. 1c). The molecular layer is divided into the outer molecular layer (OML), the middle molecular layer (MML) and the inner molecular layer (IML). This anatomical organization is similar in rodents and primates (FIG. 1a,b).
Figure 1. The organization of the dentate gyrus of rodents and primates.
a | The general organization of the dentate gyrus in the coronal and horizontal planes of the rodent brain. The area surrounded by the dashed box is expanded in the inset figure to show the laminar organization of the dentate gyrus, which is composed of a molecular layer (MOL), granule cell layer (GCL) and hilus (HIL). The GCL contains the principal cells of the dentate gyrus, the granule cells. The dendrites of the granule cells extend into the MOL, and their axons traverse the HIL and terminate in area CA3c. b | A schematic of the primate dentate gyrus shows that it is similar to that of the rodents, but that there is gyrification. In addition, CA3c is larger in primates than in rodents and includes a reflected blade (indicated by the arrowhead). c | The layers of the dentate gyrus are shown. The sources of major afferent inputs are shown on the right (red box indicates the GABAergic input and grey boxes indicate inputs from other neurotransmitters). GABAergic interneurons innervate all layers. The lateral entorhinal cortex (LEC) and the medial entorhinal cortex (MEC) innervate the outer molecular layer (OML) and the middle molecular layer (MML), respectively. Supramammillary (Mamm), cholinergic, mossy cell and ventral CA3 (vCA3) pyramidal cell axons innervate the inner molecular layer (IML). The OML and MML also receive inputs from the brainstem (including noradrenergic and 5-hydroxytryptamine (5-HT; also known as serotonin) inputs) and from basal forebrain cholinergic neurons. The HIL receives diverse inputs, including the axons of granule cells, dentate gyrus GABAergic neurons, mossy cells, CA3 pyramidal cells, neuromodulatory inputs from the brainstem (such as noradrenaline (NA), 5-HT and dopamine (DA)) and basal forebrain cholinergic neurons76,91,92,132,133. The efferents from the dentate gyrus to other areas arise mainly from granule cells that project to the HIL and CA3. The granule cell axon, called a mossy fibre, is complex. It makes giant boutons that innervate thorny excrescences of mossy cells and pyramidal cells and small boutons that arise from hilar collaterals and filamentous extensions from the giant boutons25,29,30,33,34. The small boutons primarily contact interneurons but also form contacts on distal dendrites of mossy cells7,34. ACh, acetylcholine; F, fissure; SGZ, subgranular zone.
Afferents to the dentate gyrus come from many sources (FIG. 1c; TABLE 1). The primary cortical input to the dentate gyrus is the glutamatergic projection from layer II of the entorhinal cortex (also known as the perforant path), which is responsible for most of the afferent inputs to the OML and MML3–5. Mossy cell axons are a major afferent input to the IML6–8. The outputs of the dentate gyrus are from the granule cell mossy fibres that project to area CA3 (REF. 3) (FIG. 1c; TABLE 1).
Table 1.
Afferents and efferents of the dentate gyrus
| Hippocampal afferents | Extra-hippocampal afferents | Efferents to dentate gyrus | Efferents to CA3 | ||
|---|---|---|---|---|---|
| Glutamatergic | GABAergic | Glutamate | GABA | ||
| Outer molecular layer | |||||
| Not applicable | Not applicable | Not applicable | |||
| Middle molecular layer | |||||
| Not applicable | Not applicable | Not applicable | |||
| Inner molecular layer | |||||
| Multiple16 | |||||
| Granule cell layer | |||||
| Not applicable |
|
||||
| Hilus | |||||
|
|
||||
The table lists the neuronal subtypes that innervate each layer of the dentate gyrus. 5-HT, 5-hydroxytryptamine; AA, axo-axonic; abGC, adult-born granule cell; ACh, acetylcholine; BC, basket cell; DA, dopamine; eGC, ectopic granule cell; GC, granule cell; HICAP, interneurons with a hilar cell body with axon targeting the commissural/associational pathway; HIPP, hilar cells that project to the terminal zone of the perforant path; LPP, lateral perforant path; Mamm, supramammillary; MC, mossy cell; mGC, mature granule cell; MOPP, interneurons with a molecular layer cell body that project to the terminal zone of the perforant path; MPP, medial perforant path; NA, noradrenaline; NG, neurogliaform; smGC, semilunar granule cell; vCA3, ventral CA3.
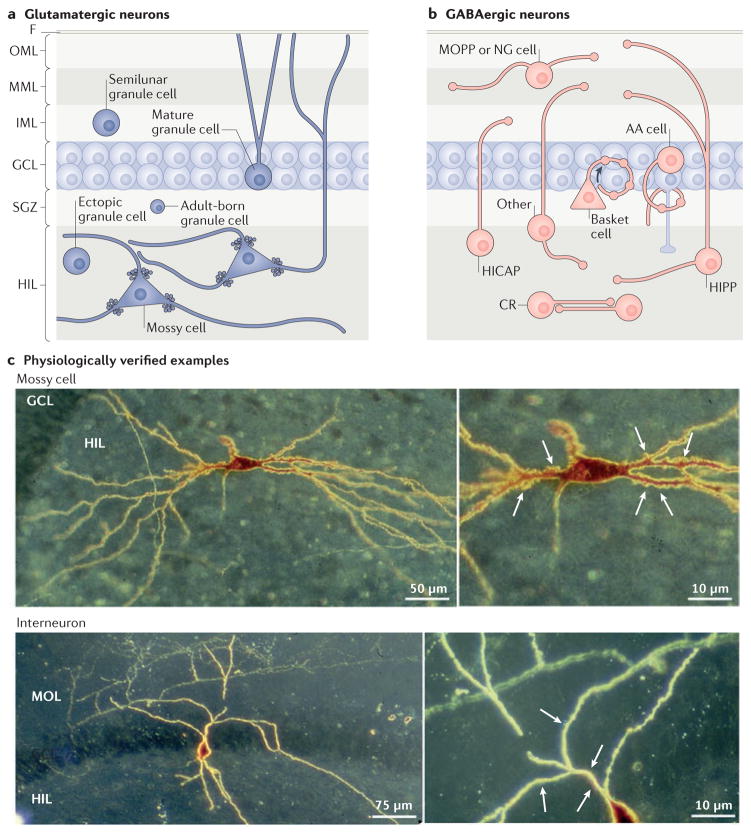
The major cell types of the dentate gyrus include the granule cells, mossy cells and GABAergic interneurons3 (FIG. 2). Most granule cells are located in the GCL, but there are small subsets in the IML (known as semilunar granule cells9,10) and hilus (known as ectopic granule cells11) (FIG. 2a). Stem cells are located in the subgranular zone12. These subgranular zone progenitors divide throughout life and migrate primarily to the GCL where they become granule cells and integrate into the dentate gyrus circuitry in a similar manner to granule cells born in early life12.
Figure 2. The cell types of the dentate gyrus.
a | Glutamatergic cells of the dentate gyrus include granule cells and mossy cells. Granule cells are not only located in the granule cell layer (GCL); there are small subsets in the inner molecular layer (IML) and hilus (HIL), and precursors to granule cells are located in the subgranular zone (SGZ). Mossy cells have long dendrites, some of which extend into the molecular layer (MOL; comprised of the IML, middle molecular layer (MML) and outer molecular layer (OML))27,28. b | GABAergic neurons of the dentate gyrus are heterogeneous. Their nomenclature is based on the location of the cell body and the axon terminal field13–15. For example, MOPP cells have a cell body in the MOL and terminals in the OML and MML, where the terminals of the perforant path are located13–15. HICAP cells (interneurons with a hilar cell body with axon targeting the commissural/associational pathway) innervate the IML, where the commissural/associational projection from mossy cells is located13–15. The neurons that innervate the granule cell somata or the axon initial segments are called perisomatic-targeting cells. Two of the most common cell types in this group are basket cells, which make basket-like endings around the granule cells and often have a pyramidal-shaped soma that is located at the border of the GCL and HIL134, and axo-axonic (AA) cells13–15. AA cells are often present near or in the GCL, as shown, and innervate granule cell axon initial segments13–15. Several GABAergic neuron subtypes innervate granule cell dendrites. The most common of these are cells in the HIL that innervate the OML and MML (HIPP cells, hilar cells that project to the terminal zone of the perforant path). An example of a neurogliaform cell (NG) that innervates the molecular layer is shown135. There are some types of GABAergic neurons that have an axon that innervates more than one layer (here labelled ‘other’)16,17, and some interneurons innervate each other, such as calretinin-expressing hilar cells (CR)136. c | The upper image shows an example of a biocytin-filled mossy cell with thorny excrescences labelled in the higher magnification inset (indicated by the arrows). The lower image shows an interneuron that was filled with biocytin in a rat hippocampal slice. Arrows point to primary dendrites that are smooth relative to the mossy cell. F, fissure.
The interneurons in the dentate gyrus are diverse, with similarities to the interneurons in other hippocampal subfields13 (FIG. 2b). They are commonly classified by the location of their cell body and axon projection, a classification scheme that emphasizes the specificity of many interneuron terminal fields for a sublayer of the dentate gyrus13–15. This is similar to the way in which the interneurons in areas CA1–CA3 are classified13, but the nomenclature is distinct13–15 (FIG. 2b). For example, a major population of hilar neurons have a hilar cell body and project to the outer two-thirds of the molecular layer. They are called HIPP cells (hilar cells that project to the terminal zone of the perforant path13–15) (FIG. 2b). However, the dentate gyrus interneuron axons do not always terminate precisely in one layer16,17, particularly during development, before pruning of dendrites and axons18. In adulthood, the lack of specificity of some axons may be important for the maintenance of inhibition in the event that a subset of interneurons is damaged. Indeed, some interneuron subtypes are more vulnerable to insults and injury than others, and the dentate gyrus interneurons can also be classified according to their relative resistance to injury. A common example of a relatively resistant cell type is the parvalbumin (PV)-expressing interneuron, which has an axon that forms a plexus around the granule cell somata. These interneurons are often called basket cells, or are included in the larger group of perisomatic-targeting neurons. Basket cells in the dentate gyrus are considered to be resistant to seizures because they survive the excitotoxicity that kills other cell types of the dentate gyrus, such as the HIPP cells19–21. The dentate gyrus interneurons have also been classified according to their expression of calcium-binding proteins (such as PV or calbindin20) or neuropeptides (including somatostatin, neuropeptide Y and cholecystokinin22). Although most dentate gyrus interneurons project locally (in the vicinity of their cell body), basket cells and some neuropeptide Y-expressing cells project to distant sites, such as the contralateral dentate gyrus23,24.
Mossy cells are located only in the hilus3,7,25. Their dendrites usually span the hilus and sometimes extend into the molecular layer6,7,25–28 (FIG. 2a). One hallmark of mossy cells is the presence of thorny excrescences, which do not exist on interneurons or granule cells6,25,26. These large spine complexes are postsynaptic to extremely large synaptic boutons of granule cells that are densely packed with glutamatergic vesicles (the so-called massive or giant mossy fibre boutons29–32). The term ‘mossy’, used for both mossy cells and granule cell mossy fibres, can be confusing but is appropriate, given the ‘mossy’ appearance of both. The general arrangement of the granule cell boutons and the thorny excrescences is similar in the hilus and CA3: massive boutons are opposed to thorny excrescences on mossy cells or pyramidal cells, whereas smaller boutons contact interneurons33,34 (FIG. 1c). The small boutons of granule cells are located on many hilar collaterals of mossy fibres, where they contact hilar interneurons and distal dendrites of mossy cells7. Small boutons are also found on filamentous extensions from giant boutons33,34.
Many investigators assume that thorny excrescences define mossy cells, but there are spiny hilar cells without thorns that have the same physiological characteristics as ‘thorny’ mossy cells7,35,36. Indeed, mossy cells vary in the degree to which they have thorny excrescences both within a given species and across species7,28,35,37. They also vary in their expression of neurochemical markers; for example, calretinin is expressed in ventral but not dorsal mossy cells in mice38,39. Thus, mossy cells are best defined using more criteria than only the presence of thorny excrescences7 (TABLE 2).
Table 2.
Characteristics of mossy cells
| General characteristic | Specific characteristic | Granule cell | Mossy cell | Dentate gyrus interneuron | Refs |
|---|---|---|---|---|---|
| Anatomical characteristics | |||||
| Cell body | Shape | Oval or circle | Diverse | Diverse | 3,6–8,25,26 |
| Size | Small | Usually large | Usually small | ||
| Axon projections and targets | Local | Hilus and CA3 | Hilus | All layers, usually subtype specific | 3,6–8,25,26 |
| Distal | None | Inner molecular layer | Weak | ||
| Contralateral | None | Inner molecular layer | Weak (BCs and HIPP cells) | ||
| Dendrites | Location | Molecular layer | Mostly hilar | Diverse | 3,6–8,25,26 |
| Spines | Dense | Dense, usually thorny | Usually few | ||
| Immunohistochemistry | GluR2 or GluR3 | Yes | Yes | No | 96,142 |
| Calretinin | Yes, in young GCs | Yes, in mice | Yes, but not in all interneurons | 38,39,70,141,143 | |
| CGRP | No | Yes | No | 144 | |
| CART | No | Yes, in human | No | 37 | |
| D2R | No | Yes | Yes, in a rare subtype of interneurons | 59 | |
| CB1R | No | Yes | Yes | 67 | |
| p11 | No | Yes | Yes, in BCs | 119 | |
| Dysbindin 1C | No | Yes | Unclear | 120 | |
| Glucocorticoid type 2 receptor | Yes | Yes | No | 145 | |
| Calbindin | Yes | No | Yes, in a subtype of interneurons | 13,20,126 | |
| Parvalbumin | No | No | Yes, in BCs | 13,20,126 | |
| STEP | No | Yes | Yes, in HIPP cells | 128 | |
| Electrophysiological characteristics | |||||
| Intrinsic | RS, FS or SS? | RS | RS | FS or SS | 7,13,16,17,146,147 |
| Action potential dv/dt | >1 | >1 | ~1 | ||
| AHP | Small | Small | Large | ||
| Time constant | Short | Long | Short | ||
| Sag | No | Some | Some | ||
| SFA | Robust | Some | Weak | ||
| Synaptic | Spontaneous EPSCs | Infrequent, relative to hilar cells | Frequent, large | Variable, usually small and fast | 35,36,55,148,149 |
| Response to trains of >2 Hz PP stimuli | One response occurs per stimulus | Depolarization of the cell occurs during the train and outlasts the train | Variable | 112,150,151 | |
| Response to ACh or DA | Hyperpolarization | Depolarization | Variable (unclear for DA) | 94,132,137 | |
| Other characteristics | |||||
| Developmental origin | Similar to MCs | Similar to GCs | Distinct from MCs and GCs | 152 | |
| Relative vulnerability | Resistant | Vulnerable | Resistant (BCs) or sensitive (HIPP cells) | 7,20,107,126 | |
ACh, acetylcholine; AHP, afterhyperpolarization; BCs, basket cells; CART, cocaine-amphetamine regulating transcript; CB1R; cannabinoid type 1 receptor; CGRP, calcitonin gene-related peptide; DA, dopamine; dv/dt, rate of rise (ratio of decay of an action potential at threshold for spike generation); D2R, dopamine D2 receptor; EPSCs, excitatory postsynaptic currents; FS, fast spiking; GCs, granule cells; GluR2, glutamate receptor type 2; HIPP, hilar cells that project to the terminal zone of the PP; MC, mossy cell; PP, perforant path; RS, regular spiking; Sag, a reduction in a voltage response to a persistent current command; SFA, spike frequency adaptation; SS, slow spiking; STEP, striatum-enriched protein-tyrosine phosphatase.
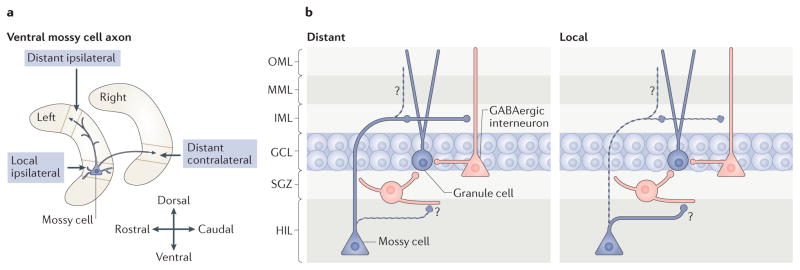
The mossy cell axon projection is complex (FIG. 3). A large projection (known as a ‘distant’ or ‘intralamellar’ projection) terminates away from the cell body in both the ipsilateral and contralateral dentate gyrus6–8,26,40,41. The terminals of this distant projection primarily synapse in the IML, and electron microscopy shows that they primarily innervate spines8. Because interneurons with dendrites in the IML rarely have spines, it is likely that the distant mossy cell axon projection primarily innervates granule cells8. Mossy cell axons also collateralize in the hilus and have extensions in the MML and OML7,8,35. Near the cell body, the hilar collaterals are robust, and there is also innervation of the IML35,42. In summary, a mossy cell innervates more than one dentate gyrus layer as well as both local and distant parts of the dentate gyrus, making it hard to predict its functions in vivo.
Figure 3. Organization of the mossy cell axon.
a | The axon of a single ventral mossy cell is illustrated schematically. Near the soma, the local ipsilateral branches of the mossy cell axon make synapses in the hilus (HIL) and the inner molecular layer (IML)7,35,42. Far from the soma, the distant ipsilateral or distant contralateral branches of the axon project primarily to the IML6–8,25,40,41. b | Distant and local ipsilateral circuitry. At distant ipsilateral locations, the axon primarily makes synapses on spines; because there are few spines on dentate gyrus interneurons, it is likely that the mossy cell axon in the IML innervates granule cells. In addition, the mossy cell axon extends hilar, outer molecular layer (OML) and middle molecular layer (MML) collaterals7,8,35. It is not clear whether mossy cell terminals in the OML and MML make synapses, or what cell types mossy cells target in the HIL (indicated by question marks). At local ipsilateral locations, the mossy cell axons collateralize in the HIL, especially near the soma. There, they are likely to contact interneurons because of the numerous interneuron dendrites present in this region and the absence of granule cell dendrites. Mossy cells also make local projections to the IML, but these are not as numerous as those to distant sites8. GCL, granule cell layer; SGZ, subgranular zone.
The role of the dentate gyrus in behaviour
To date, the dentate gyrus has been implicated in several important behavioural functions. These functions have been segregated into those that are often called ‘cognitive’, which are dependent on the more dorsal or septal half of the dentate gyrus, and those that are more ‘emotional’, which depend on the ventral or temporal half of the dentate gyrus43. The cognitive functions include those related to spatial memory, whereas the emotional functions include those associated with the regulation of mood44 and anxiety or with behaviours that have a component of stress or fear (such as contextual fear conditioning45). Additional functions have also been attributed to the dentate gyrus44,46. These divisions assume that neurons of the dorsal dentate gyrus are the most important for cognitive functions; however, it is important to note that ventral mossy cells may also contribute to these functions through their major afferent inputs to the dorsal IML (FIG. 3). Indeed, because the IML is proximal to the granule cell somata, mossy cell terminals in this region are in an ideal position to influence the activity of granule cells.
A function of the dentate gyrus that has been discussed a great deal is pattern separation, which is the ability of the dentate gyrus network to receive a pattern of afferent inputs and ‘separate’ them so that the outputs are less similar than the inputs46–50. It has been suggested that pattern separation in the dentate gyrus is important for memory storage in area CA3 because it allows similar experiences to be stored in different subsets of CA3 pyramidal cells, thus facilitating accurate memory retrieval48. This theory assumes that the afferent input patterns that are subject to pattern separation arrive at the dentate gyrus via the perforant path. However, mossy cells provide a major glutamatergic input to granule cells (see below), suggesting that they could send input patterns to granule cells in addition to, or instead of, the perforant path.
Mossy cell function in the dentate gyrus
Do mossy cells excite or inhibit granule cells?
Experiments to address the question of whether mossy cells are excitatory or inhibitory started in the 1980s51,52; however, it was not until the mid-1990s that conclusive evidence was provided that mossy cells are glutamatergic53 and can excite granule cells through direct inputs54. The net effect of mossy cell input on granule cells remains unclear because mossy cells also activate dentate gyrus interneurons that inhibit granule cells54,55.
One problem faced by researchers attempting to address this question is that it is difficult to selectively activate mossy cells using stimulating electrodes: mossy cell axons and dendrites are spatially close to other cells of the dentate gyrus and to axonal projections that influence the dentate gyrus. One strategy to circumvent this problem has been to record from pairs of monosynaptically connected mossy cells and granule cells54. This approach showed that the monosynaptic mossy cell input to a granule cell generated an excitatory postsynaptic potential (EPSP) that could only be detected when GABAergic inhibition was blocked. The mossy cell input seemed to be weakly excitatory because failures of synaptic transmission were frequent54. This suggested that the primary effect of mossy cells is the inhibition of granule cells. However, when the postsynaptic granule cell was depolarized, the effects of presynaptic mossy cell input were larger; thus, mossy cells can have a robust excitatory effect on depolarized granule cells54. Additional studies using approaches that lesioned mossy cells or the molecular layer in hippocampal slices56,57,58 provided further evidence that mossy cells excite granule cells.
Despite the value of these findings, it has become increasingly important to find a way to selectively interrogate mossy cells. Therefore, the emergence of two transgenic mouse lines that express Cre recombinase (Cre) relatively specifically in mossy cells has been an important advance. In one of these mouse lines, the Crlr promoter (Crlr encodes calcitonin receptor-like receptor) was used to drive Cre expression1. Although this approach produced selective Cre expression in mossy cells within the dentate gyrus, CA3 neurons also showed some Cre expression1. In the second mouse line, the Drd2 promoter (Drd2 encodes the dopamine D2 receptor) was used to drive Cre expression. These mice also showed strong Cre expression in mossy cells; however, an additional population of hippocampal interneurons also expressed the Drd2 promoter, showing that the Cre expression in mossy cells was not selective59,60.
To date, mossy cell function has only been addressed using the Crlr–Cre mouse line1. These mice were crossed with mice carrying a floxed diphtheria toxin receptor, and diphtheria toxin was administered in adult mice to kill mossy cells. One week after toxin injection, mossy cells were deleted and granule cells showed disinhibition in response to a perforant path stimulus, supporting the hypothesis that the effect of mossy cells is primarily inhibitory to granule cells. However, both spontaneous excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs) were decreased in patched granule cells in brain slices from these mice. Furthermore, the disinhibitory effects on granule cells driven by the deletion of mossy cells did not persist; the authors suggested that this was a result of compensatory changes1,61.
The problem of potential compensatory changes in response to cell death can now be circumvented by activating mossy cells selectively with optogenetics. To date, one study has been published in which the excitatory opsin channelrhodopsin was expressed in commissurally projecting dentate gyrus neurons2, which are mainly mossy cells. The authors showed that both EPSCs and IPSCs were evoked in granule cells in response to single light pulses focused on the commissurally projecting terminals, suggesting that mossy cells had both excitatory and inhibitory effects on granule cells. However, when the light stimulus was timed to precede perforant path stimulation, the response of the granule cells to the perforant path input was reduced. This suggested that the dominant effect of mossy cells on perforant path-evoked responses of granule cells is inhibitory. Repetitive light pulses that were more similar to patterns of activity observed in vivo were also tested and revealed that the excitation of GABAergic neurons was more persistent than the excitation of granule cells. Thus, the inhibitory effects of mossy cells might be stronger than their excitatory effects in vivo. In addition, the results showed that mossy cells are likely to activate a range of interneuron cell types, with a preference for basket cells. These experiments provided the most compelling evidence to date that the primary effect of mossy cells is the inhibition of granule cells. However, it is important to note that the commissurally projecting neurons of the dentate gyrus targeted in this study also include inhibitory neurons23,24. In addition, the studies were conducted in hippocampal slices, not in vivo.
Adult neurogenesis
Very few studies have examined the influence of mossy cells on adult neurogenesis. It has been suggested that mossy cells could be important for adult neurogenesis because they provide the first glutamatergic input to adult-born granule cells62. However, the deletion of mossy cells in Crlr–Cre mice treated with the diphtheria toxin did not appear to have a major effect on adult-born neurons1 (although many characteristics of the newborn neurons were not tested).
Contribution to plasticity
Additional insight into the potential role of mossy cells has come from studies showing that mossy cell synapses exhibit long-term potentiation (LTP). For example, granule cell input to mossy cells exhibits LTP with characteristics similar to those of mossy fibre-evoked LTP in CA3 pyramidal cells63. However, it is unclear whether mossy cell input to granule cells also shows LTP. One in vivo study showed LTP of field potentials recorded in the IML after high-frequency stimulation of the hilus64. However, another in vivo study using IML stimulation did not find LTP of the mossy cell–granule cell pathway65. Notably, high-frequency stimulation of the perforant path evoked both LTP of the perforant path–granule cell synapse and heterosynaptic LTP of the IML-evoked response, suggesting that the mossy cell input to granule cells can be potentiated65. Another in vivo study showed that LTP of the perforant path–granule cell synapse leads to LTP of the contralateral mossy cell–granule cell synapse66. This study was important because it showed that LTP of the perforant path–granule cell synapse induces plasticity of ‘downstream’ mossy cells and thus appears to be permissive for LTP of the mossy cell–granule cell synapse.
A potential explanation for the lack of LTP at the mossy cell–granule cell synapse that was observed in the earlier study65 was provided by experiments showing that the depolarization of granule cells suppresses the mossy cell input to granule cells, an effect that was mediated by the retrograde signalling of endocannabinoids67,68 through cannabinoid receptors (TABLE 2). Thus, the mossy cell input to granule cells can be suppressed by high-frequency stimulation that sufficiently depolarizes the granule cell.
Other studies of mossy cell synaptic plasticity suggested that mossy cells influence the LTP of the perforant path–granule cell synapse. For example, one study69 took advantage of the fact that in mice calretinin is expressed primarily in mossy cells38,39. LTP of the perforant path–granule cell synapse was reduced in mice lacking calretinin compared with wild-type mice69. Although intriguing, the study was limited by the constitutive nature of the knockout and the expression of calretinin in cells other than mossy cells (including a subtype of interneuron in the dentate gyrus70). However, other methods have also shown that mossy cells may facilitate or even be required for LTP of the perforant path input to granule cells. Voltage imaging in hippocampal slices showed that the granule cell–mossy cell–granule cell circuit was necessary for perforant path–granule cell LTP58. Furthermore, it was shown that the expression of growth-associated protein 43 (GAP43; also known as neuromodulin) was increased in mossy cells in response to high-frequency stimulation of the perforant path, which could support the persistence of LTP71. Thus, it has been suggested that the mossy cell and perforant pathway inputs to granule cells are cooperative65,71.
Role in hippocampal oscillations
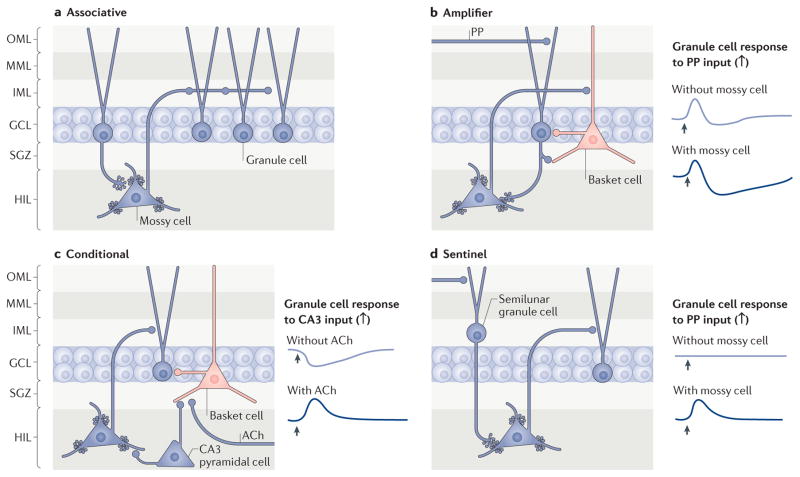
Hippocampal neurons display oscillatory behaviour at various frequencies in vivo, and these rhythms are thought to have a role in the encoding of spatial information72. Therefore, one way to better understand the role of mossy cells in the hippocampal network is to determine how variations in their activity relate to those recorded simultaneously in the hippocampal electroencephalogram (EEG). Two studies have shown that, in anaesthetized animals, mossy cells discharge in a similar manner to principal cells in relation to theta oscillations73,74. Both studies pointed out that mossy cells might act to phase-lock the activity of granule cells to the EEG throughout the hippocampal dorsoventral axis73; this is consistent with the idea that mossy cells link different subsets of granule cells75 (FIG. 4a).
Figure 4. Possible roles of mossy cells in dentate gyrus function.
a | A long-standing proposal is that mossy cells link subsets of granule cells that are spatially separated and therefore facilitate associative learning75. b | Another long-standing view is that granule cells primarily activate GABAergic interneurons, particularly basket cells106,112. This could lead to a possible circuit, as shown, in which mossy cells amplify the normal feedback inhibition of granule cells by activating GABAergic interneurons. In the figure, this circuit is exemplified by the perforant path (PP)-mediated depolarization of a granule cell. If the depolarization evokes an action potential, the granule cell would subsequently depolarize a basket cell that innervates numerous granule cells (only one granule cell is depicted), increasing the inhibition of granule cells. On the right, this physiology is schematized. Without the mossy cell in the circuit (top), a PP input (indicated by the arrow) elicits an excitatory postsynaptic potential (EPSP) in the granule cell. The EPSP is followed by an inhibitory postsynaptic potential (IPSP) that reflects the feedback inhibition (that is, the activation of the basket cell by the granule cell, which in turn hyperpolarizes the granule cell). Notably, it is unclear whether basket cells innervate the same granule cells that activate them; the circuit in the diagram is simplified for clarity. With the mossy cell in the circuit (bottom), the PP input elicits an EPSP followed by a larger and longer IPSP in the granule cell because mossy cells are first depolarized by granule cells and then activate basket cells. Note that if the PP directly innervates basket cells and mossy cells, this would lead to feedforward inhibition of granule cells. c | According to another hypothesis, the excitatory effects of mossy cells depolarize granule cells only under some conditions. In this example, the effects of a CA3 pyramidal cell input to a granule cell depend on the presence or absence of concurrent cholinergic input. Without the cholinergic input, CA3 pyramidal cells will primarily hyperpolarize granule cells by activating interneurons. With cholinergic input mediated by acetylcholine (ACh), which preferentially suppresses the interneurons137, CA3 pyramidal cells will primarily depolarize granule cells by activating mossy cells80,83. The schematic includes several simplifications for clarity. For example, the cholinergic input to pyramidal cells and mossy cells is not shown. In addition, the cholinergic input depolarizes mossy cells94, which would lead to a greater potential to excite granule cells in the circuit that is shown. d | Finally, mossy cells have also been proposed to act as ‘sentinels’ that inform granule cells that changes have occurred even in the absence of strong PP input. This sentinel function would be reflected by the lack of a response of a granule cell to the PP input unless the mossy cell is activated. This might occur, for example, if the PP input to granule cells is weak. Under these conditions, the PP might still activate semilunar granule cells because they are activated more readily by the PP. These cells have a robust excitatory effect on mossy cells9,10, which would then activate granule cells, as shown in the diagram. On the right, this is depicted by a lack of depolarization in a granule cell (located in the granule cell layer (GCL)) in the absence of mossy cells. HIL, hilus; IML, inner molecular layer; MML, middle molecular layer; OML, outer molecular layer; SGZ, subgranular zone.
Another study74 recorded the intracellular activity of mossy cells in anaesthetized rats. The authors found that when the tail was pinched during ongoing theta oscillations, mossy cells could, but did not always, depolarize. This variability is consistent with the idea that there are functional subtypes of mossy cells, which is also suggested by variations in their structure7 and by dorsoventral variations in the expression of calretinin (see above)38,39.
In this context, it is interesting to consider the data from Crlr–Cre mice treated with the diphtheria toxin1 that showed that, shortly after treatment with the toxin, theta power in the dentate gyrus increased. This implies that the activity of the dentate gyrus interneurons was suppressed following the loss of mossy cells, because other methods that depress the firing of the dentate gyrus interneurons also increase theta power76. These data suggest that mossy cells are likely to have a more important role in theta rhythm than previously considered.
Possible contributions to behaviour
Although our understanding of the role of mossy cells in dentate gyrus function is limited, several intriguing hypotheses about their potential contributions to behaviour have been proposed, based on the knowledge that we do have.
Associative functions
One early hypothesis for the role of mossy cells in hippocampal function is reflected in one of the names given to their axon projection, the associational projection75. Mossy cells may allow physically separate subsets of granule cells to be associated with one another, analogous to the way in which the recurrent collaterals of CA3 pyramidal cells allow different subsets of pyramidal cells to interact75 (FIG. 4a). This idea was supported by detailed electron microscopy-based studies of the mossy cell axon8,42, which showed that, because of the numerous varicosities present on a mossy cell axon in the IML, a single mossy cell could potentially link multiple granule cells75.
Pattern separation
As described above, a major question is how the dentate gyrus separates patterns of afferent input that contain elements that are identical (overlapping). An example of such overlap would be two afferent inputs that are active during two different patterns of input50; however, other characteristics could potentially also be shared. Mossy cells may contribute to the separation of two overlapping patterns in several ways. For two patterns of input from the entorhinal cortex, separation could occur if the shared (identical) element of one pattern was coincident with mossy cell input to granule cells but not coincident with the other pattern. When coincident, mossy cells could depolarize the same granule cell dendrite as the entorhinal input and do so at a similar time, causing action potentials to be elicited by one pattern of input but not the other. Alternatively, activity of mossy cells in one pattern but not the other pattern could increase GABAergic inhibition of granule cells, reducing the number of action potentials in the granule cells in response to one pattern but not the other (FIG. 4b). One might argue that granule cells are strongly inhibited regardless of the activity of the mossy cells, because their soma and dendrites are well innervated by axons of diverse interneuron subtypes13–16. However, unlike interneurons, mossy cells could activate GABAergic interneurons broadly and synchronously, and therefore increase GABAergic inhibition of granule cells in a unique way (FIG. 4b). This idea is consistent with the proposed mossy cell-mediated phase-locking of granule cells73,74.
One argument against this idea is that the projection of mossy cells to the distant ipsilateral IML targets many more granule cells than interneurons8. However, the function of these excitatory inputs might differ from that of a typical excitatory input because mossy cell input to granule cells may be excitatory only under certain conditions (FIG. 4c).
Alternatively, excitatory input of mossy cells to granule cells could contribute to pattern separation by promoting the activity and development of young adult-born granule cells, and by having a weakened effect as the granule cells age. This idea is consistent with the evidence that behavioural tasks that are suggested to involve pattern separation are impaired when young adult-born granule cells are ablated, an effect that is not evident after destruction of older granule cells77,78. Whether such a correlation between changes in mossy cell input and granule cell ageing occurs is unknown, but it would be consistent with the suggestion that adult-born granule cells become less active as they age79.
Pattern completion refers to the retrieval of a stored memory when presented with a subset of the initial input that was used to produce the memory. Pattern separation and pattern completion are often discussed as if they involve the dentate gyrus and area CA3, respectively, with the granule cell mossy fibres making a one-way path to CA3. However, CA3 pyramidal cell axons also project ‘back’ to the dentate gyrus80. CA3 pyramidal cell axon collaterals project far into the hilus81,82 and, in the ventral pole of the hippocampus, their projections extend further, into the IML81. Most of the projections arise from CA3c, with fewer arising from CA3b and CA3a81,82. However, recurrent collaterals between CA3a–CA3b pyramidal cells and CA3c pyramidal cells can make it possible for CA3a–CA3b to influence the dentate gyrus via the CA3c80. In the hilus, CA3 pyramidal cells innervate mossy cells and interneurons (most dentate gyrus interneurons have a soma or dendrites in the hilus)83–85. In hippocampal slices, the major effect of CA3 firing is GABAergic inhibition of granule cells83. However, the delay between a pyramidal cell action potential and the onset of granule cell inhibition (10–20 ms) suggests that a simple pyramidal cell–interneuron–granule cell pathway is not responsible; instead, a pyramidal cell–mossy cell–interneuron–granule cell pathway or a pyramidal cell–pyramidal cell–interneuron–granule cell route seems more likely to be responsible83. Notably, a robust pyramidal cell–mossy cell–granule cell excitatory pathway is revealed when GABAergic inhibition is blocked83.
The backprojection from CA3 to the dentate gyrus might affect circuitry in the dentate gyrus and CA3 (REFS 86,87), and the existence of this pathway might add to the potential ways in which information may be processed. Although the recurrent collateral network of CA3 is often thought to provide the only robust excitatory recurrent circuitry, synapses made by the CA3–dentate gyrus backprojection also provide a recurrent circuitry. Indeed, a study using computational modelling found that this backprojection has a crucial role in the ability of the computational model to perform simulated pattern separation and completion49.
Novelty
It has been suggested that the dentate gyrus contributes to the ability to define what is familiar and what is novel in the environment88,89. Mossy cells may contribute to this function by exciting granule cells when a novel sensory input is processed in the lateral entorhinal cortex and is sent to the dentate gyrus by the lateral perforant path. Such a pathway is suggested by recordings from a subset of mossy cells that have a low action potential threshold in response to a perforant path stimulus27. These mossy cells had dendrites in the molecular layer, where they could receive direct perforant path inputs27. Whether a direct entorhinal cortex–mossy cell pathway exists has not been proven, but electrophysiological data suggest that perforant path axons make monosynaptic inputs onto mossy cells27. Mossy cell dendrites in the molecular layer have been shown in diverse species28, and in primates there are also dendrites of CA3c pyramidal cells in the molecular layer90. Thus, mossy cells might act as sentinels that signal to granule cells that a change has occurred. This could ensure that the input from the entorhinal cortex always depolarizes some granule cells, even when it is too weak to reach the threshold for a granule cell action potential by itself.
Another possible path from the entorhinal cortex to mossy cells involves the semilunar granule cells in the IML, which receive robust inputs from the entorhinal cortex and activate the mossy cells in a robust manner9,10. Thus, an entorhinal cortex–semilunar granule cell–mossy cell pathway could explain the low action potential threshold of some mossy cells (FIG. 4d).
It is notable that the hilus receives afferents from the brainstem, such as inputs from the locus coeruleus76,91,92. Many of these brainstem inputs are part of the reticular activating system and, therefore, would act in concert with entorhinal inputs to ‘inform’ the dentate gyrus about a changing environment. Mossy cells also receive cholinergic inputs93; this is important because the activation of muscarinic cholinergic receptors depolarizes the mossy cells and elicits an afterdepolarization that does not occur in other hilar cell types94. For these neuromodulatory inputs, mossy cells would potentially be activated even when other dentate gyrus neurons are not. Thus, the extrinsic afferents that preferentially activate the mossy cells may provide an alternative mechanism for a sentinel function.
In vivo support for the idea that mossy cells act as a type of sentinel comes from immunohistochemical studies of Fos, a marker of neuronal activity95. When rats were removed from their home cage, brought to a laboratory and rapidly perfused, a subset of mossy cells in the ventral hippocampus expressed FOS protein, as did a subset of granule cells in the dorsal dentate gyrus (the target of ventral mossy cell projections)95. Presumably, the expression of FOS in mossy cells occurred in response to small changes in the environment when the animals were in the home cage, or to rapid changes as the animals experienced novelty of the laboratory where they were brought to be perfused95.
Anxiety
Behavioural studies using Crlr–Cre mice demonstrated that the diphtheria toxin-mediated deletion of mossy cells led to behaviours that suggested increased anxiety compared with controls1. This might be expected, given that there are more mossy cells in the ventral dentate gyrus than in the dorsal dentate gyrus96–98 and given that lesions of the ventral dentate gyrus lead to anxiety99. One mechanism by which mossy cells might contribute to innate anxiety is related to the idea that mossy cells can signal changes in the environment (see above). Without such ‘cues’, there may be less certainty in the environment, such as certainty of safety from predators, leading to anxiety. Mossy cells may also play a part in other types of anxiety (as well as in fear and stress); indeed, transgenic mice in which mossy cells were ablated spent less time in the open arm of the elevated plus maze than control mice1, a task that does not necessarily reflect innate anxiety.
Mossy cell vulnerability
A distinguishing feature of mossy cells is their vulnerability to insults or injury. Pathological studies show that the hilus is a common site of neuronal loss in temporal lobe epilepsy (TLE)100, a subtype of epilepsy in which seizures involve the temporal lobe of the cortex. Although some mossy cells survive in TLE97, many are vulnerable101. As animal models of TLE were developed, detailed comparisons of the cell types in the hilus showed that two hilar cell types were especially vulnerable: the mossy cells and the somatostatin-expressing hilar interneurons (HIPP cells); in comparison, granule cells were more resistant7,19,21,101. In rats, mossy cells die after many types of insults and injuries that are risk factors for TLE, including forebrain ischaemia7,102, status epilepticus7,96–98 (with exceptions103) and traumatic brain injury104,105. This led to the hypothesis that a loss of hilar cells — and possibly a loss of mossy cells specifically — causes TLE106. Some of the potential causes of mossy cell vulnerability75,107–109 are shown in BOX 1.
Box 1. Potential mechanisms of mossy cell vulnerability.
Mossy cells may be vulnerable to insults or injury as a result of presynaptic mechanisms. For example, as shown in part a of the figure, the giant boutons of granule cells can release large concentrations of glutamate onto mossy cell thorny excrescences, resulting in excitotoxicity7,75,106,107,109. Peptides such as brain-derived neurotrophic factor that are located in dense core vesicles within the giant boutons can facilitate the release of glutamate, exacerbating this excitotoxicity125.
The vulnerability of mossy cells has also been suggested to arise owing to their weak expression of the calcium-binding proteins parvalbumin and calbindin, which results in poor buffering of intracellular calcium when it enters the cell20. However, neurons outside the hippocampus that lack calcium-binding proteins are not necessarily vulnerable126. Moreover, in a mouse model of epilepsy, mice that lack calcium-binding proteins have a phenotype similar to that of wild-type mice127.
Mossy cells also express striatum-enriched protein-tyrosine phosphatase (STEP), the expression of which has been suggested to explain the vulnerability of HIPP cells (hilar cells that project to the terminal zone of the perforant path)128. Mossy cells express lower levels of STEP than HIPP cells (based on immunohisto-chemistry128), but even a low expression level of STEP could contribute to their vulnerability given its importance for the vulnerability of HIPP cells128. HIPP and other hilar cells, including mossy cells, also have low levels of the δ-subunit of type A GABA receptors, which normally contributes to GABAergic inhibition, and therefore low levels of this subunit could make a cell vulnerable to excitotoxicity129.
It has been suggested that the vulnerability of mossy cells may be due to low levels of autophagy130, the process involved in waste removal from neurons. In the absence of strong autophagy, it is possible that mossy cells may not be able to keep up with metabolic demands.
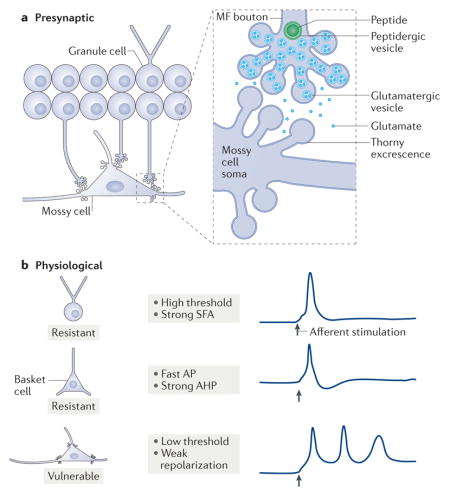
Finally, it has been suggested that the vulnerability of mossy cells may be due to their physiological properties. In the example shown in part b of the figure, a typical action potential (AP) in a granule cell that is evoked by an afferent input (arrow) gives rise to only one AP. This limited firing, attributed to the high threshold and strong spike frequency adaptation (SFA) of granule cells131, limits excitotoxicity. As shown, the AP generated in a basket cell is faster and is followed by strong after hyperpolarization (AHP)13,16,17, which also limits firing and excitotoxicity. However, an afferent input to a mossy cell leads more readily to APs, and the APs are of longer duration with relatively weak repolarization35. Thus, excitotoxicity is more likely to occur in mossy cells. MF, mossy fibre.
The hypothesis that a loss of mossy cells causes TLE remains a subject of debate. One argument for this hypothesis notes that mossy cells can inhibit granule cells, which may normally prevent seizure activity from passing from the entorhinal cortex to the hippocampus110. Entry of seizure activity into the hippocampus could be important to sustain and amplify seizures. It has also been proposed that mossy cell-mediated excitation of granule cells is increased after insults that lead to TLE, causing hyperexcitability1,104–106,111,112. However, hyperexcitability could also be caused by a loss of HIPP cells97 or calretinin-expressing interneurons70. Diverse changes in other aspects of the inhibitory control of granule cells113, including changes in the subunit composition of type A GABA receptors114–116, have led to the current consensus that a loss of mossy cells may contribute to TLE but is unlikely to be fully responsible for it.
One limitation of animal models of TLE is that many changes occur in response to the induction of epilepsy, not only a loss of mossy cells. Because of the relative specificity of the deletion of mossy cells in Crlr–Cre mice treated with the diptheria toxin, it was important to note that the mice showed no spontaneous seizures1, supporting the idea that a loss of mossy cells is insufficient to cause TLE. However, the mice did exhibit transient disinhibition in response to perforant path stimulation, showing that the loss of mossy cells did have a marked effect in these mice. In addition, the mouse strain used to generate the Crlr–Cre mice was seizure resistant, as noted by the authors61.
How a loss of mossy cells would contribute to TLE is unknown. One possibility is that the loss of mossy cells could make seizures more severe. In support of this idea, mice lacking mossy cells had more severe seizures than controls1. Interestingly, the deletion of adult-born granule cells also increases the severity of seizures117. Thus, mossy cells could have an important role in TLE through their activation of immature adult-born granule cells, which innervate GABAergic neurons to a greater extent than excitatory neurons until their giant boutons develop118.
Mossy cells may also influence other diseases. For example, mossy cells and basket cells express p11 and helicase-like transcription factor (SMARCA3), proteins that are potentially important in major depressive disorder119. Epigenetic changes mediated by p11 through its actions on SMARCA3 (REF. 119) could change the physiological properties of mossy cells and basket cells, making their contribution to dentate gyrus function more robust or weaker. Mossy cells also express dysbindin 1C120 and dopamine D2 receptors59,60, both of which are implicated in schizophrenia. The fact that a loss of mossy cells causes impaired contextual discrimination1, and that mice without mossy cells show behaviours that are consistent with increased anxiety (described above), suggests that mossy cells may have roles in psychiatric disorders. Precisely what these roles are remains to be determined.
Conclusions and perspectives
Experiments that have preferentially deleted or activated mossy cells have supported the long-standing hypothesis that the inhibition of granule cells is the major functional role of mossy cells. However, experiments in awake behaving animals are still lacking. Without such physiological data one wonders whether the conditions for detection of mossy cell-mediated excitation of granule cells have simply not been met. There is a gap between studies of dentate gyrus circuitry and studies of dentate gyrus-dependent behaviour, and a major goal for the future would be to fill this void. Current approaches to address this issue are labour intensive and expensive, but rewarding. For example, patch clamp recordings in the dentate gyrus of awake, head-fixed mice have been made121; if mossy cells can be patched, a great deal of information could be gleaned. A more feasible alternative may be to use juxtacellular recording122, which allows many questions to be addressed, particularly when it is combined with electrodes to monitor other hippocampal subfields or oscillatory behaviour. Microendoscopy to image the hilus in vivo in awake behaving mice is becoming feasible123. Using dyes such as GCaMP6 (REF. 124), expressed in cells under the control of Cre, would make selective imaging of mossy cells in vivo possible. However, these techniques require significant trauma to overlying tissues, to which mossy cells are particularly sensitive. While investigators attempt to circumvent this and other difficulties, other fundamental questions also need to be addressed. For example, quantitative data about mossy cell inputs and their outputs are surprisingly incomplete for rodents and especially for primates, for both sexes and for diverse ages. Such data would help to refine hypotheses, fuel the development of approaches such as computational modelling and ultimately make mossy cells much less enigmatic than they are now.
Acknowledgments
This work is supported by the US National Institutes of Health, the Alzheimer’s Association and the New York State Office of Mental Health.
Glossary
- Golgi technique
A method established by Camillo Golgi that stains many neurons almost completely (except for their axons) so they can be visualized in detail.
- Optogenetics
The use of light to activate opsins (located in the plasma membrane), which open channels for cations or anions to flow. After opsins are expressed in one cell type, they can be activated selectively by light. Targeting opsins to specific cell types is done after identifying unique genes in the cell type, so the combination of light (opto-) and genetics (optogenetics) is fundamental to the approach.
- Electron microscopy
The use of microscopes with very high (nanometre) resolution, made possible by accelerating the electrons through a specialized microscope. Electron microscopy can be used with very thin brain sections, allowing parts of neurons (such as synapses) to be detected.
- Contextual fear conditioning
A behavioural test that examines the response to an environment or context after a prior exposure to the context and a painful stimulus.
- Cre recombinase
(Cre). Part of a site-specific recombination system derived from Escherichia coli bacteriophage P1. Two short DNA sequences (loxP sites) are engineered to flank the target DNA. Activation of the Cre recombinase enzyme catalyses the recombination between the loxP sites, leading to excision of the intervening sequence.
- Disinhibition
A decrease in inhibition (usually GABAergic). For example, blockade of the release of GABA from GABAergic neurons would result in a decrease in inhibition of the neuron that is postsynaptic to the GABAergic neuron.
- Long-term potentiation
(LTP). A lasting increase in synaptic transmission. LTP is often elicited by a brief period of high-frequency presynaptic firing. However, other types of stimulation can elicit LTP, such as exposure to neuromodulators.
- Field potentials
The changes in the extracellular potential that reflect changes in the flow of cations and anions in the extracellular space.
- Retrograde signalling
The changes induced in a presynaptic terminal, usually mediated by a neuromodulator acting on its presynaptic receptors, which are evoked by release of the neuromodulator from the postsynaptic site.
- Voltage imaging
The identification of neuronal activity by capturing changes in fluorescence that are proportional to changes in membrane potential. Typically, a voltage-sensitive dye is applied to the preparation of neurons so that voltage imaging can be conducted.
- Electroencephalogram
(EEG). Recordings of the electrical activity of the brain with electrodes that are not inside the neurons (intracellular), but outside (extracellular) or remote (on the brain surface or skull).
- Oscillations
The intermittent activity of neurons that is sufficiently synchronous to induce rhythmic fluctuations in the extracellular potential.
- Recurrent collaterals
The branches of the axons of a population of neurons that innervate the dendrites of the same population of neurons. In area CA3, the pyramidal cells do not innervate their own dendrites but the dendrites of other CA3 pyramidal neurons.
- Afterdepolarization
A depolarization occurring after an event, typically after an action potential.
- Spike frequency adaptation
(SFA). A reduction in frequency of action potential discharge during a constant depolarizing input. Most neurons have a high firing frequency after the beginning of a strong depolarization, and the firing frequency decays if the depolarization continues.
- Contextual discrimination
A behavioural task that tests the ability to distinguish two environments or contexts.
Footnotes
Competing interests statement
The author declares no competing interests.
References
- 1.Jinde S, et al. Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation. Neuron. 2012;76:1189–1200. doi: 10.1016/j.neuron.2012.10.036. This study examined the consequence of ablating mossy cells with the most selective approach to date. Although some CA3 pyramidal cells were also affected, the results showed that mossy cells have an important role in the dentate gyrus network. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu TT, Lee CT, Tai MH, Lien CC. Differential recruitment of dentate gyrus interneuron types by commissural versus perforant pathways. Cereb Cortex. 2016;26:2715–2727. doi: 10.1093/cercor/bhv127. This study used optogenetics in hippocampal slices to show that mossy cells could excite or inhibit granule cells; the authors found that the major effect was inhibition. [DOI] [PubMed] [Google Scholar]
- 3.Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- 5.Witter MP. The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog Brain Res. 2007;163:43–61. doi: 10.1016/S0079-6123(07)63003-9. [DOI] [PubMed] [Google Scholar]
- 6.Ribak CE, Seress L, Amaral DG. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- 7.Scharfman HE, Myers CE. Hilar mossy cells of the dentate gyrus: a historical perspective. Front Neural Circuits. 2012;6:106. doi: 10.3389/fncir.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckmaster PS, Wenzel HJ, Kunkel DD, Schwartzkroin PA. Axon arbors and synaptic connections of hippocampal mossy cells in the rat in vivo. J Comp Neurol. 1996;366:271–292. doi: 10.1002/(sici)1096-9861(19960304)366:2<270::aid-cne7>3.0.co;2-2. This paper is an excellent quantitative study of the mossy cell projection in vivo. [DOI] [PubMed] [Google Scholar]
- 9.Larimer P, Strowbridge BW. Representing information in cell assemblies: persistent activity mediated by semilunar granule cells. Nat Neurosci. 2010;13:213–222. doi: 10.1038/nn.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams PA, Larimer P, Gao Y, Strowbridge BW. Semilunar granule cells: glutamatergic neurons in the rat dentate gyrus with axon collaterals in the inner molecular layer. J Neurosci. 2007;27:13756–13761. doi: 10.1523/JNEUROSCI.4053-07.2007. This study was the first to characterize semilunar granule cells with electrophysiology, and it showed their potential importance in the network of the dentate gyrus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scharfman HE, Goodman J, McCloskey D. Ectopic granule cells of the rat dentate gyrus. Dev Neurosci. 2007;29:14–27. doi: 10.1159/000096208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015;7:a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Halasy K, Somogyi P. Subdivisions in the multiple GABAergic innervation of granule cells in the dentate gyrus of the rat hippocampus. Eur J Neurosci. 1993;5:411–429. doi: 10.1111/j.1460-9568.1993.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 15.Han ZS, Buhl EH, Lorinczi Z, Somogyi P. A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci. 1993;5:395–410. doi: 10.1111/j.1460-9568.1993.tb00507.x. References 14 and 15 proposed an organization of the dentate gyrus interneurons according to the location of the cell body and terminal field of the axon. [DOI] [PubMed] [Google Scholar]
- 16.Hosp JA, et al. Morpho-physiological criteria divide dentate gyrus interneurons into classes. Hippocampus. 2014;24:189–203. doi: 10.1002/hipo.22214. This paper suggested an alternative nomenclature to the organization of interneurons proposed in references 14 and 15 that reconciled discrepancies in the literature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scharfman HE. Electrophysiological diversity of pyramidal-shaped neurons at the granule cell layer/hilus border of the rat dentate gyrus recorded in vitro. Hippocampus. 1995;5:287–305. doi: 10.1002/hipo.450050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seay-Lowe SL, Claiborne BJ. Morphology of intracellularly labeled interneurons in the dentate gyrus of the immature rat. J Comp Neurol. 1992;324:23–36. doi: 10.1002/cne.903240104. [DOI] [PubMed] [Google Scholar]
- 19.Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. This paper showed that mossy cells were vulnerable to seizures but that many GABAergic neurons were not, which was surprising because a loss of GABAergic neurons was considered to be a cause of epilepsy. [DOI] [PubMed] [Google Scholar]
- 20.Sloviter RS. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol. 1989;280:183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- 21.Schwarzer C, Williamson JM, Lothman EW, Vezzani A, Sperk G. Somatostatin, neuropeptide Y, neurokinin B and cholecystokinin immunoreactivity in two chronic models of temporal lobe epilepsy. Neuroscience. 1995;69:831–845. doi: 10.1016/0306-4522(95)00268-n. [DOI] [PubMed] [Google Scholar]
- 22.Houser CR. Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog Brain Res. 2007;163:217–232. doi: 10.1016/S0079-6123(07)63013-1. [DOI] [PubMed] [Google Scholar]
- 23.Goodman JH, Sloviter RS. Evidence for commissurally projecting parvalbumin-immunoreactive basket cells in the dentate gyrus of the rat. Hippocampus. 1992;2:13–21. doi: 10.1002/hipo.450020103. [DOI] [PubMed] [Google Scholar]
- 24.Deller T, Nitsch R, Frotscher M. Phaseolus vulgaris–leucoagglutinin tracing of commissural fibers to the rat dentate gyrus: evidence for a previously unknown commissural projection to the outer molecular layer. J Comp Neurol. 1995;352:55–68. doi: 10.1002/cne.903520105. [DOI] [PubMed] [Google Scholar]
- 25.Amaral DG. A golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;15:851–914. doi: 10.1002/cne.901820508. This paper provided the first detailed description of hilar cells and mossy cells. [DOI] [PubMed] [Google Scholar]
- 26.Frotscher M, Seress L, Schwerdtfeger WK, Buhl E. The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. J Comp Neurol. 1991;312:145–163. doi: 10.1002/cne.903120111. [DOI] [PubMed] [Google Scholar]
- 27.Scharfman HE. Dentate hilar cells with dendrites in the molecular layer have lower thresholds for synaptic activation by perforant path than granule cells. J Neurosci. 1991;11:1660–1673. doi: 10.1523/JNEUROSCI.11-06-01660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blackstad JB, et al. Observations on hippocampal mossy cells in mink (neovison vison) with special reference to dendrites ascending to the granular and molecular layers. Hippocampus. 2016;26:229–245. doi: 10.1002/hipo.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chicurel ME, Harris KM. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J Comp Neurol. 1992;325:169–182. doi: 10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- 30.Blackstad TW, Kjaerheim A. Special axo-dendritic synapses in the hippocampal cortex: electron and light microscopic studies on the layer of mossy fibers. J Comp Neurol. 1961;117:133–159. doi: 10.1002/cne.901170202. [DOI] [PubMed] [Google Scholar]
- 31.Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 32.Laatsch RH, Cowan WM. Electron microscopic studies of the dentate gyrus of the rat. I Normal structure with special reference to synaptic organization. J Comp Neurol. 1966;128:359–395. doi: 10.1002/cne.901280305. [DOI] [PubMed] [Google Scholar]
- 33.Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- 34.Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. Using quantitative anatomical methods, this paper identified that mossy fibre synapses on GABAergic neurons outnumber those on CA3 pyramidal cells, suggesting that the pathway has strong inhibitory effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scharfman HE, Schwartzkroin PA. Electrophysiology of morphologically identified mossy cells of the dentate hilus recorded in guinea pig hippocampal slices. J Neurosci. 1988;8:3812–3821. doi: 10.1523/JNEUROSCI.08-10-03812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scharfman HE. Characteristics of spontaneous and evoked EPSPs recorded from dentate spiny hilar cells in rat hippocampal slices. J Neurophysiol. 1993;70:742–757. doi: 10.1152/jn.1993.70.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seress L, Abraham H, Doczi T, Lazar G, Kozicz T. Cocaine- and amphetamine-regulated transcript peptide (CART) is a selective marker of rat granule cells and of human mossy cells in the hippocampal dentate gyrus. Neuroscience. 2004;125:13–24. doi: 10.1016/j.neuroscience.2003.12.035. This paper showed that there are important exceptions to the idea that mossy cells are always lost in TLE. [DOI] [PubMed] [Google Scholar]
- 38.Blasco-Ibanez JM, Freund TF. Distribution, ultrastructure, and connectivity of calretinin-immunoreactive mossy cells of the mouse dentate gyrus. Hippocampus. 1997;7:307–320. doi: 10.1002/(SICI)1098-1063(1997)7:3<307::AID-HIPO6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 39.Fujise N, Liu Y, Hori N, Kosaka T. Distribution of calretinin immunoreactivity in the mouse dentate gyrus: II. Mossy cells, with special reference to their dorsoventral difference in calretinin immunoreactivity. Neuroscience. 1998;82:181–200. doi: 10.1016/s0306-4522(97)00261-3. [DOI] [PubMed] [Google Scholar]
- 40.Zimmer J. Ipsilateral afferents to the commissural zone of the fascia dentata, demonstrated in decommissurated rats by silver impregnation. J Comp Neurol. 1971;142:393–416. doi: 10.1002/cne.901420402. [DOI] [PubMed] [Google Scholar]
- 41.Berger TW, Semple-Rowland S, Bassett JL. Hippocampal polymorph neurons are the cells of origin for ipsilateral association and commissural afferents to the dentate gyrus. Brain Res. 1981;224:329–336. doi: 10.1016/0006-8993(81)91138-0. [DOI] [PubMed] [Google Scholar]
- 42.Buckmaster PS, Strowbridge BW, Kunkel DD, Schmiege DL, Schwartzkroin PA. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus. 1992;2:349–362. doi: 10.1002/hipo.450020403. [DOI] [PubMed] [Google Scholar]
- 43.Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Sahay A, Drew MR, Hen R. Dentate gyrus neurogenesis and depression. Prog Brain Res. 2007;163:697–722. doi: 10.1016/S0079-6123(07)63038-6. [DOI] [PubMed] [Google Scholar]
- 45.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 46.Kesner RP. A behavioral analysis of dentate gyrus function. Prog Brain Res. 2007;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- 47.Rolls ET. Pattern separation, completion, and categorisation in the hippocampus and neocortex. Neurobiol Learn Mem. 2016;129:4–28. doi: 10.1016/j.nlm.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers CE, Scharfman HE. Pattern separation in the dentate gyrus: a role for the CA3 backprojection. Hippocampus. 2011;21:1190–1215. doi: 10.1002/hipo.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myers CE, Scharfman HE. A role for hilar cells in pattern separation in the dentate gyrus: a computational approach. Hippocampus. 2009;19:321–337. doi: 10.1002/hipo.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buzsaki G, Eidelberg E. Commissural projection to the dentate gyrus of the rat: evidence for feed-forward inhibition. Brain Res. 1981;230:346–350. doi: 10.1016/0006-8993(81)90413-3. [DOI] [PubMed] [Google Scholar]
- 52.Douglas RM, McNaughton BL, Goddard GV. Commissural inhibition and facilitation of granule cell discharge in fascia dentata. J Comp Neurol. 1983;219:285–294. doi: 10.1002/cne.902190304. [DOI] [PubMed] [Google Scholar]
- 53.Soriano E, Frotscher M. Mossy cells of the rat fascia dentata are glutamate-immunoreactive. Hippocampus. 1994;4:65–69. doi: 10.1002/hipo.450040108. This paper provided the first evidence that mossy cells were glutamatergic. [DOI] [PubMed] [Google Scholar]
- 54.Scharfman HE. Electrophysiological evidence that dentate hilar mossy cells are excitatory and innervate both granule cells and interneurons. J Neurophysiol. 1995;74:179–194. doi: 10.1152/jn.1995.74.1.179. This paper showed that mossy cells directly excite granule cells and dentate gyrus interneurons. [DOI] [PubMed] [Google Scholar]
- 55.Larimer P, Strowbridge BW. Nonrandom local circuits in the dentate gyrus. J Neurosci. 2008;28:12212–12223. doi: 10.1523/JNEUROSCI.3612-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ratzliff AdH, Howard AL, Santhakumar V, Osapay I, Soltesz I. Rapid deletion of mossy cells does not result in a hyperexcitable dentate gyrus: implications for epileptogenesis. J Neurosci. 2004;24:2259–2269. doi: 10.1523/JNEUROSCI.5191-03.2004. This paper used a method to delete mossy cells in a hippocampal slice and did not find any evidence for hyperexcitability, thus arguing against the idea that mossy cells normally activate interneurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jackson MB, Scharfman HE. Positive feedback from hilar mossy cells to granule cells in the dentate gyrus revealed by voltage-sensitive dye and microelectrode recording. J Neurophysiol. 1996;76:601–616. doi: 10.1152/jn.1996.76.1.601. [DOI] [PubMed] [Google Scholar]
- 58.Wright BJ, Jackson MB. Long-term potentiation in hilar circuitry modulates gating by the dentate gyrus. J Neurosci. 2014;34:9743–9753. doi: 10.1523/JNEUROSCI.0814-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gangarossa G, et al. Characterization of dopamine D1 and D2 receptor-expressing neurons in the mouse hippocampus. Hippocampus. 2012;22:2199–2207. doi: 10.1002/hipo.22044. [DOI] [PubMed] [Google Scholar]
- 60.Puighermanal E, et al. drd2-cre:ribotag mouse line unravels the possible diversity of dopamine D2 receptor-expressing cells of the dorsal mouse hippocampus. Hippocampus. 2015;25:858–875. doi: 10.1002/hipo.22408. [DOI] [PubMed] [Google Scholar]
- 61.Jinde S, Zsiros V, Nakazawa K. Hilar mossy cell circuitry controlling dentate granule cell excitability. Front Neural Circuits. 2013;7:14. doi: 10.3389/fncir.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chancey JH, Poulsen DJ, Wadiche JI, Overstreet-Wadiche L. Hilar mossy cells provide the first glutamatergic synapses to adult-born dentate granule cells. J Neurosci. 2014;34:2349–2354. doi: 10.1523/JNEUROSCI.3620-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lysetskiy M, Foldy C, Soltesz I. Long- and short-term plasticity at mossy fiber synapses on mossy cells in the rat dentate gyrus. Hippocampus. 2005;15:691–696. doi: 10.1002/hipo.20096. [DOI] [PubMed] [Google Scholar]
- 64.Hetherington PA, Austin KB, Shapiro ML. Ipsilateral associational pathway in the dentate gyrus: an excitatory feedback system that supports N-methyl-D-aspartate–dependent long-term potentiation. Hippocampus. 1994;4:422–438. doi: 10.1002/hipo.450040405. [DOI] [PubMed] [Google Scholar]
- 65.Kleschevnikov AM, Routtenberg A. Long-term potentiation recruits a trisynaptic excitatory associative network within the mouse dentate gyrus. Eur J Neurosci. 2003;17:2690–2702. doi: 10.1046/j.1460-9568.2003.02709.x. [DOI] [PubMed] [Google Scholar]
- 66.Alvarez-Salvado E, Pallares V, Moreno A, Canals S. Functional MRI of long-term potentiation: imaging network plasticity. Phil Trans R Soc B. 2014;369:20130152. doi: 10.1098/rstb.2013.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiu CQ, Castillo PE. Input-specific plasticity at excitatory synapses mediated by endocannabinoids in the dentate gyrus. Neuropharmacology. 2008;54:68–78. doi: 10.1016/j.neuropharm.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hofmann ME, Nahir B, Frazier CJ. Endocannabinoid-mediated depolarization-induced suppression of inhibition in hilar mossy cells of the rat dentate gyrus. J Neurophysiol. 2006;96:2501–2512. doi: 10.1152/jn.00310.2006. [DOI] [PubMed] [Google Scholar]
- 69.Schurmans S, et al. Impaired long-term potentiation induction in dentate gyrus of calretinin-deficient mice. Proc Natl Acad Sci USA. 1997;94:10415–10420. doi: 10.1073/pnas.94.19.10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tóth K, Maglóczky Z. The vulnerability of calretinin-containing hippocampal interneurons to temporal lobe epilepsy. Front Neuroanat. 2014;8:100. doi: 10.3389/fnana.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Namgung U, Matsuyama S, Routtenberg A. Long-term potentiation activates the GAP-43 promoter: selective participation of hippocampal mossy cells. Proc Natl Acad Sci USA. 1997;94:11675–11680. doi: 10.1073/pnas.94.21.11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soltesz I, Bourassa J, Deschenes M. The behavior of mossy cells of the rat dentate gyrus during theta oscillations in vivo. Neuroscience. 1993;57:555–564. doi: 10.1016/0306-4522(93)90005-z. [DOI] [PubMed] [Google Scholar]
- 74.Henze DA, Buzsáki G. Hilar mossy cells: functional identification and activity in vivo. Prog Brain Res. 2007;163:199–216. doi: 10.1016/S0079-6123(07)63012-X. [DOI] [PubMed] [Google Scholar]
- 75.Buckmaster PS, Schwartzkroin PA. Hippocampal mossy cell function: a speculative view. Hippocampus. 1994;4:393–402. doi: 10.1002/hipo.450040402. [DOI] [PubMed] [Google Scholar]
- 76.Brown RA, Walling SG, Milway JS, Harley CW. Locus ceruleus activation suppresses feedforward interneurons and reduces β-γ electroencephalogram frequencies while it enhances θ frequencies in rat dentate gyrus. J Neurosci. 2005;25:1985–1991. doi: 10.1523/JNEUROSCI.4307-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alme CB, et al. Hippocampal granule cells opt for early retirement. Hippocampus. 2010;20:1109–1123. doi: 10.1002/hipo.20810. [DOI] [PubMed] [Google Scholar]
- 80.Scharfman HE. The CA 3 “backprojection” to the dentate gyrus. Prog Brain Res. 2007;163:627–637. doi: 10.1016/S0079-6123(07)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li X, Somogyi P, Ylinen A, Buzsáki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- 82.Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. References 81 and 82 showed that there was a pattern of innervation ‘back’ to the dentate gyrus, opposite to the direction of the trisynaptic circuitry. [DOI] [PubMed] [Google Scholar]
- 83.Scharfman HE. EPSPs of dentate gyrus granule cells during epileptiform bursts of dentate hilar “mossy” cells and area CA3 pyramidal cells in disinhibited rat hippocampal slices. J Neurosci. 1994;14:6041–6057. doi: 10.1523/JNEUROSCI.14-10-06041.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scharfman HE. Evidence from simultaneous intracellular recordings in rat hippocampal slices that area CA3 pyramidal cells innervate dentate hilar mossy cells. J Neurophysiol. 1994;72:2167–2180. doi: 10.1152/jn.1994.72.5.2167. References 83 and 84 showed the physiological effects that supported the idea that pyramidal cells innervate hilar neurons such as mossy cells. [DOI] [PubMed] [Google Scholar]
- 85.Kneisler TB, Dingledine R. Synaptic input from CA3 pyramidal cells to dentate basket cells in rat hippocampus. J Physiol. 1995;487:125–146. doi: 10.1113/jphysiol.1995.sp020866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lisman JE, Talamini LM, Raffone A. Recall of memory sequences by interaction of the dentate and CA3: a revised model of the phase precession. Neural Netw. 2005;18:1191–1201. doi: 10.1016/j.neunet.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 87.Penttonen M, Kamondi A, Sik A, Acsady L, Buzsaki G. Feed-forward and feed-back activation of the dentate gyrus in vivo during dentate spikes and sharp wave bursts. Hippocampus. 1997;7:437–450. doi: 10.1002/(SICI)1098-1063(1997)7:4<437::AID-HIPO9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 88.Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- 89.Aggleton JP, Brown MW, Albasser MM. Contrasting brain activity patterns for item recognition memory and associative recognition memory: insights from immediate-early gene functional imaging. Neuropsychologia. 2013;50:3141–3155. doi: 10.1016/j.neuropsychologia.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 90.Buckmaster PS, Amaral DG. Intracellular recording and labeling of mossy cells and proximal CA3 pyramidal cells in macaque monkeys. J Comp Neurol. 2001;430:264–281. doi: 10.1002/1096-9861(20010205)430:2<264::aid-cne1030>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 91.Amaral DG, Campbell MJ. Transmitter systems in the primate dentate gyrus. Hum Neurobiol. 1986;5:169–180. [PubMed] [Google Scholar]
- 92.Swanson LW, Kohler C, Bjorklund A. In: Handbook of Chemical Neuroanatomy. Hokfelt T, Bjorklund A, Swanson LW, editors. Vol. 5. Elsevier; 1987. pp. 125–277. [Google Scholar]
- 93.Deller T, Katona I, Cozzari C, Frotscher M, Freund TF. Cholinergic innervation of mossy cells in the rat fascia dentata. Hippocampus. 1999;9:314–320. doi: 10.1002/(SICI)1098-1063(1999)9:3<314::AID-HIPO10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 94.Hofmann ME, Frazier CJ. Muscarinic receptor activation modulates the excitability of hilar mossy cells through the induction of an afterdepolarization. Brain Res. 2010;1318:42–51. doi: 10.1016/j.brainres.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duffy AM, Schaner MJ, Chin J, Scharfman HE. Expression of c-fos in hilar mossy cells of the dentate gyrus in vivo. Hippocampus. 2013;23:649–655. doi: 10.1002/hipo.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiao Y, Nadler JV. Stereological analysis of GluR2-immunoreactive hilar neurons in the pilocarpine model of temporal lobe epilepsy: correlation of cell loss with mossy fiber sprouting. Exp Neurol. 2007;205:569–582. doi: 10.1016/j.expneurol.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buckmaster PS, Jongen-Relo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci. 1999;19:9519–9529. doi: 10.1523/JNEUROSCI.19-21-09519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Volz F, et al. Stereologic estimation of hippocampal GluR2/3- and calretinin-immunoreactive hilar neurons (presumptive mossy cells) in two mouse models of temporal lobe epilepsy. Epilepsia. 2011;52:1579–1589. doi: 10.1111/j.1528-1167.2011.03086.x. [DOI] [PubMed] [Google Scholar]
- 99.Bannerman DM, et al. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- 100.Margerison J, Corsellis J. Epilepsy and the temporal lobes: a clinical, electrographic and neuropathological study of the brain in epilepsy with particular reference to the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- 101.de Lanerolle NC, Lee TS, Spencer DD. In: Jasper’s Basic Mechanisms of the Epilepsies. 4. Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Oxford Univ. Press; 2012. pp. 387–404. [PubMed] [Google Scholar]
- 102.Crain BJ, Westerkam WD, Harrison AH, Nadler JV. Selective neuronal death after transient forebrain ischemia in the mongolian gerbil: a silver impregnation study. Neuroscience. 1988;27:387–402. doi: 10.1016/0306-4522(88)90276-x. [DOI] [PubMed] [Google Scholar]
- 103.Kotti T, Tapiola T, Riekkinen PJ, Sr, Miettinen R. The calretinin-containing mossy cells survive excitotoxic insult in the gerbil dentate gyrus Comparison of excitotoxicity-induced neuropathological changes in the gerbil and rat. Eur J Neurosci. 1996;8:2371–2378. doi: 10.1111/j.1460-9568.1996.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 104.Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Santhakumar V, et al. Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: the ‘irritable mossy cell’ hypothesis. J Physiol. 2000;524:117–134. doi: 10.1111/j.1469-7793.2000.00117.x. This paper showed that after head trauma there is hyperexcitability in the dentate gyrus and that mossy cells may contribute to the hyperexcitability because they become activated or ‘irritable’ and therefore excite granule cells more than normal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sloviter RS. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol. 1994;35:640–654. doi: 10.1002/ana.410350604. [DOI] [PubMed] [Google Scholar]
- 107.Scharfman HE. The role of nonprincipal cells in dentate gyrus excitability and its relevance to animal models of epilepsy and temporal lobe epilepsy. Adv Neurol. 1999;79:805–820. [PubMed] [Google Scholar]
- 108.Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwartzkroin PA, et al. Possible mechanisms of seizure-related cell damage in the dentate hilus. Epilepsy Res Suppl. 1996;12:317–324. [PubMed] [Google Scholar]
- 110.Hsu D. The dentate gyrus as a filter or gate: a look back and a look ahead. Prog Brain Res. 2007;163:601–613. doi: 10.1016/S0079-6123(07)63032-5. [DOI] [PubMed] [Google Scholar]
- 111.Ratzliff A, Santhakumar V, Howard A, Soltesz I. Mossy cells in epilepsy: rigor mortis or vigor mortis? Trends Neurosci. 2002;25:140–144. doi: 10.1016/s0166-2236(00)02122-6. [DOI] [PubMed] [Google Scholar]
- 112.Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- 113.Zhang W, Buckmaster PS. Dysfunction of the dentate basket cell circuit in a rat model of temporal lobe epilepsy. J Neurosci. 2009;29:7846–7856. doi: 10.1523/JNEUROSCI.6199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 115.Coulter DA. Epilepsy-associated plasticity in γ-aminobutyric acid receptor expression, function, and inhibitory synaptic properties. Int Rev Neurobiol. 2001;45:237–252. doi: 10.1016/s0074-7742(01)45013-6. [DOI] [PubMed] [Google Scholar]
- 116.Sperk G, Furtinger S, Schwarzer C, Pirker S. GABA and its receptors in epilepsy. Adv Exp Med Biol. 2004;548:92–103. doi: 10.1007/978-1-4757-6376-8_7. References 113–116 describe complex changes to GABAergic inhibition in the dentate gyrus in models of TLE and that one mechanism, such as a loss of mossy cell input to interneurons, is unlikely to explain the pathophysiology of TLE. [DOI] [PubMed] [Google Scholar]
- 117.Iyengar SS, et al. Suppression of adult neurogenesis increases the acute effects of kainic acid. Exp Neurol. 2015;264:135–149. doi: 10.1016/j.expneurol.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scharfman HE, Bernstein HL. Potential implications of a monosynaptic pathway from mossy cells to adult-born granule cells of the dentate gyrus. Front Syst Neurosci. 2015;9:112. doi: 10.3389/fnsys.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oh YS, et al. SMARCA3, a chromatin-remodeling factor, is required for p11-dependent antidepressant action. Cell. 2013;152:831–843. doi: 10.1016/j.cell.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang H, et al. Dysbindin-1C is required for the survival of hilar mossy cells and the maturation of adult newborn neurons in dentate gyrus. J Biol Chem. 2014;289:29060–29072. doi: 10.1074/jbc.M114.590927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Anderson RW, Strowbridge BW. α-Band oscillations in intracellular membrane potentials of dentate gyrus neurons in awake rodents. Learn Mem. 2014;21:656–661. doi: 10.1101/lm.036269.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang Q, Brecht M, Burgalossi A. Juxtacellular recording and morphological identification of single neurons in freely moving rats. Nat Protoc. 2014;9:2369–2381. doi: 10.1038/nprot.2014.161. [DOI] [PubMed] [Google Scholar]
- 123.Barretto RP, Schnitzer MJ. In vivo microendoscopy of the hippocampus. Cold Spring Harb Protoc. 2012;2012:1092–1099. doi: 10.1101/pdb.prot071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scharfman HE. In: Synaptic Plasticity and Transsynaptic Signalling. Stanton PK, Bramham CR, Scharfman HE, editors. Springer; 2005. pp. 201–220. [Google Scholar]
- 126.Freund TF, Buzsaki G, Leon A, Baimbridge KG, Somogyi P. Relationship of neuronal vulnerability and calcium binding protein immunoreactivity in ischemia. Exp Brain Res. 1990;83:55–66. doi: 10.1007/BF00232193. [DOI] [PubMed] [Google Scholar]
- 127.Bouilleret V, Schwaller B, Schurmans S, Celio MR, Fritschy JM. Neurodegenerative and morphogenic changes in a mouse model of temporal lobe epilepsy do not depend on the expression of the calcium-binding proteins parvalbumin, calbindin, or calretinin. Neuroscience. 2000;97:47–58. doi: 10.1016/s0306-4522(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 128.Choi YS, et al. Status epilepticus-induced somatostatinergic hilar interneuron degeneration is regulated by striatal enriched protein tyrosine phosphatase. J Neurosci. 2007;27:2999–3009. doi: 10.1523/JNEUROSCI.4913-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tong X, et al. Ectopic expression of α6 and δ GABAA receptor subunits in hilar somatostatin neurons increases tonic inhibition and alters network activity in the dentate gyrus. J Neurosci. 2015;35:16142–16158. doi: 10.1523/JNEUROSCI.2853-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yuan Y, Wang H, Wei Z, Li W. Impaired autophagy in hilar mossy cells of the dentate gyrus and its implication in schizophrenia. J Genet Genom. 2015;42:1–8. doi: 10.1016/j.jgg.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 131.Staley KJ, Otis TS, Mody I. Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings. J Neurophysiol. 1992;67:1346–1358. doi: 10.1152/jn.1992.67.5.1346. [DOI] [PubMed] [Google Scholar]
- 132.Etter G, Krezel W. Dopamine D2 receptor controls hilar mossy cells excitability. Hippocampus. 2014;24:725–732. doi: 10.1002/hipo.22280. [DOI] [PubMed] [Google Scholar]
- 133.Leranth C, Frotscher M. Cholinergic innervation of hippocampal GAD- and somatostatin-immunoreactive commissural neurons. J Comp Neurol. 1987;261:33–47. doi: 10.1002/cne.902610104. [DOI] [PubMed] [Google Scholar]
- 134.Ribak CE. Local circuitry of GABAergic basket cells in the dentate gyrus. Epilepsy Res Suppl. 1992;7:29–47. [PubMed] [Google Scholar]
- 135.Armstrong C, Szabadics J, Tamas G, Soltesz I. Neurogliaform cells in the molecular layer of the dentate gyrus as feed-forward γ-aminobutyric acidergic modulators of entorhinal-hippocampal interplay. J Comp Neurol. 2011;519:1476–1491. doi: 10.1002/cne.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gulyas AI, Hajos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16:3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Freund TF. GABAergic septal and serotonergic median raphe afferents preferentially innervate inhibitory interneurons in the hippocampus and dentate gyrus. Epilepsy Res Suppl. 1992;7:79–91. [PubMed] [Google Scholar]
- 138.Leranth C, Hajszan T. Extrinsic afferent systems to the dentate gyrus. Prog Brain Res. 2007;163:63–84. doi: 10.1016/S0079-6123(07)63004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wyss JM, Swanson LW, Cowan WM. Evidence for an input to the molecular layer and the stratum granulosum of the dentate gyrus from the supramammillary region of the hypothalamus. Anat Embryol (Berl) 1979;156:165–176. doi: 10.1007/BF00300012. [DOI] [PubMed] [Google Scholar]
- 140.Toni N, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brandt MD, et al. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 2003;24:603–613. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 142.Leranth C, Szeidemann Z, Hsu M, Buzsáki G. AMPA receptors in the rat and primate hippocampus: a possible absence of GluR2/3 subunits in most interneurons. Neuroscience. 1996;70:631–652. doi: 10.1016/s0306-4522(96)83003-x. [DOI] [PubMed] [Google Scholar]
- 143.Liu Y, Fujise N, Kosaka T. Distribution of calretinin immunoreactivity in the mouse dentate gyrus. I General description. Exp Brain Res. 1996;108:389–403. doi: 10.1007/BF00227262. [DOI] [PubMed] [Google Scholar]
- 144.Freund TF, Hajos N, Acsady L, Gorcs TJ, Katona I. Mossy cells of the rat dentate gyrus are immunoreactive for calcitonin gene-related peptide (CGRP) Eur J Neurosci. 1997;9:1815–1830. doi: 10.1111/j.1460-9568.1997.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 145.Patel A, Bulloch K. Type II glucocorticoid receptor immunoreactivity in the mossy cells of the rat and the mouse hippocampus. Hippocampus. 2003;13:59–66. doi: 10.1002/hipo.10045. [DOI] [PubMed] [Google Scholar]
- 146.Lubke J, Frotscher M, Spruston N. Specialized electrophysiological properties of anatomically identified neurons in the hilar region of the rat fascia dentata. J Neurophysiol. 1998;79:1518–1534. doi: 10.1152/jn.1998.79.3.1518. [DOI] [PubMed] [Google Scholar]
- 147.Buckmaster PS, Strowbridge BW, Schwartzkroin PA. A comparison of rat hippocampal mossy cells and CA3c pyramidal cells. J Neurophysiol. 1993;70:1281–1299. doi: 10.1152/jn.1993.70.4.1281. [DOI] [PubMed] [Google Scholar]
- 148.Livsey CT, Vicini S. Slower spontaneous excitatory postsynaptic currents in spiny versus aspiny hilar neurons. Neuron. 1992;8:745–755. doi: 10.1016/0896-6273(92)90095-u. [DOI] [PubMed] [Google Scholar]
- 149.Strowbridge BW, Buckmaster PS, Schwartzkroin PA. Potentiation of spontaneous synaptic activity in rat mossy cells. Neurosci Lett. 1992;142:205–210. doi: 10.1016/0304-3940(92)90374-g. [DOI] [PubMed] [Google Scholar]
- 150.Scharfman HE, Schwartzkroin PA. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science. 1989;246:257–260. doi: 10.1126/science.2508225. [DOI] [PubMed] [Google Scholar]
- 151.Scharfman HE, Schwartzkroin PA. Responses of cells of the rat fascia dentata to prolonged stimulation of the perforant path: sensitivity of hilar cells and changes in granule cell excitability. Neuroscience. 1990;35:491–504. doi: 10.1016/0306-4522(90)90324-w. [DOI] [PubMed] [Google Scholar]
- 152.Li G, et al. Hilar mossy cells share developmental influences with dentate granule neurons. Dev Neurosci. 2007;30:255–261. doi: 10.1159/000110347. [DOI] [PubMed] [Google Scholar]