Abstract
Vectors based on adeno-associated virus serotype 8 (AAV8) have been evaluated in several clinical trials of gene therapy for hemophilia B with encouraging results. In preparation for a Phase 1 clinical trial of AAV8 gene therapy for the treatment of homozygous familial hypercholesterolemia (HoFH), the safety of the clinical candidate vector, AAV8.TBG.hLDLR, was evaluated in wild-type rhesus macaques and macaques heterozygous for a nonsense mutation in the low-density lipoprotein receptor (LDLR) gene (LDLR+/−). Intravenous infusion of 1.25 × 1013 GC/kg of AAV8.TBG.hLDLR expressing the human version of LDLR was well tolerated and associated with only mild histopathology that was restricted to the liver and sporadic, low-level, and transient elevations in transaminases. Some animals developed T cells to both capsid and the hLDLR transgene, although these adaptive immune responses were most evident at the early time points from peripheral blood and in mononuclear cells derived from the liver. This toxicology study supports the safety of AAV8.TBG.hLDLR for evaluation in HoFH patients, and provides some context for evaluating previously conducted clinical trials of AAV8 in patients with hemophilia.
Keywords: : AAV, gene therapy, familial hypercholesterolemia, low density lipoprotein receptor, LDLR, toxicology
Introduction
Familial hypercholesterolemia (FH) is caused by a defect in the gene encoding low-density lipoprotein receptor (LDLR).1,2 Patients that are heterozygous for one loss-of-function mutation demonstrate elevated serum LDL and premature coronary artery disease. Massive elevations in serum LDL are observed in homozygous FH patients (HoFH), who exhibit loss-of-function mutations on both LDLR genes.3 The success of orthotropic liver transplantation in correcting hypercholesterolemia in HoFH patients has indicated that hepatic expression of LDL is an important target for controlling serum LDL, and has paved the way for liver-directed gene therapy for this disease. In fact, the authors conducted the first clinical trial of gene therapy for a metabolic liver disease using an ex vivo approach in patients with HoFH.4
The development of improved vectors for in vivo gene transfer to hepatocytes, such as those based on adeno-associated virus (AAV), shows promise for treating and potentially curing patients with HoFH. The authors' laboratory developed a highly hepatotropic vector based on AAV serotype 8 (AAV8), which has been used to treat patients with hemophilia B successfully.5,6 This vector was evaluated in several mouse models of HoFH, and it showed highly efficient and stable correction of serum LDL that led to the regression of pre-existing atherosclerosis.7,8 Herein, experiments in nonhuman primates (NHPs) are described, which were designed to assess the safety of an AAV8 vector expressing human LDLR (hLDLR) formally in preparation for a Phase 1 study in HoFH patients.
The safety assessment of in vivo gene therapy, which includes studies of immune toxicity, is an important aspect in the evaluation of AAV vectors for human applications. Experience has shown that mouse models are relatively resistant to the activation of adaptive immune responses to AAV capsid and AAV-encoded transgenes. Therefore, pharmacology experiments in mouse models of the disease are often complemented with toxicology studies in NHPs. The availability of a macaque model for FH provided a unique opportunity to evaluate toxicology in a model that simulates both the metabolic defect as well as a more authentic host immune response.9
In 1988, Scanu et al. described a family of rhesus macaques that demonstrated persistent elevations in plasma cholesterol.9 Analysis of skin fibroblasts demonstrated a 50% reduction in LDLR activity, which was subsequently shown to be due to a nonsense mutation in exon 6 of the LDLR gene that led to the expression of a dysfunctional and truncated protein (LDLR+/– NHP model).10 Unfortunately, attempts to generate a homozygous FH macaque have failed for reasons that are unclear.
The level of hypercholesterolemia present in the NHP LDLR+/– model is quite modest on a chow diet, and does not resemble that found in humans with heterozygous FH. However, feeding a high-fat diet to LDLR+/− rhesus macaques leads to levels of hypercholesterolemia comparable to those seen in severe heterozygous and HoFH patients (600–1,100 mg/dL). LDLR+/− macaques that were placed on a high-fat diet developed elevated systemic inflammatory cytokines (MCP-1 and interleukin-8 [IL-8]) and increased hepatic fat content (5–5.6%), as measured by magnetic resonance spectroscopy, suggesting that LDLR+/− macaques develop signs of hepatic steatosis when on a high-fat diet (unpublished data). Importantly, hepatic steatosis does not appear to be a manifestation of HoFH in patients, despite their persistently elevated LDL. In one published clinical trial of HoFH subjects, the average percent liver fat content at baseline was 1.0% (range 0.0–5.0%).11 Normal liver fat content is considered to be <5%.12 In addition, LDLR+/− macaques fed a high-fat diet develop similar levels of hypercholesterolemia, as observed in some high-fat diet-fed wild-type macaques.9,10 The fact that high-fat diet-fed LDLR+/− macaques do not simulate the physiology of the liver, as seen in HoFH patients, and that dyslipidemia on a chow-fed diet is virtually absent, precludes their use in assessing pharmacology of the vector. Despite the limitations of the FH macaque model, a toxicology study of the AAV8-LDLR product in both wild-type and heterozygous FH macaques was undertaken.
Materials and Methods
Vector production
AAV vectors expressing human LDLR (hLDLR) from the thyroxine binding globulin (TBG) promoter were produced by the Penn Vector Core at the University of Pennsylvania, as previously described.13 The final vector products were diluted in sterile phosphate-buffered saline (PBS). A certificate of analysis that verifies quality and purity is provided in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/humc).
NHPs
Wild-type rhesus macaques (n = 4) were obtained from Charles River Laboratories (Houston, TX). LDLR+/− rhesus macaques (n = 4) were obtained from Southwest Foundation for Biomedical Research (San Antonio, TX). NHP studies were conducted at the University of Pennsylvania School of Medicine within an USDA-registered, AAALAC-accredited, and PHS-assured facility.
Prior to the current study discussed here, LDLR+/− rhesus macaques 19269, 19498, 19499, and 2031 were fed a high-fat diet for approximately 5 months, during which cholesterol levels increased substantially and reached a maximum and stable peak. This was associated with evidence of steatohepatitis based on the development of sustained levels of pro-inflammatory cytokines in the blood. These four macaques were then taken off the high-fat diet, and serum cholesterol stabilized to levels observed before initiation of the high-fat diet after approximately 1 month. Additionally, inflammatory cytokines in serum dropped to non-detectable levels 2 months after stopping the high-fat diet. To ensure that animals had resolved all underlying steatosis, animals were injected with vector at least 4 months after they had been taken off the high-fat diet.
Analyses during the in-life phase
Rhesus macaques were anesthetized, and blood was collected via the femoral vein for complete blood counts (CBC), clinical chemistries, and coagulation panels, which were analyzed by Antech Diagnostics (Irvine, CA). The following parameters were evaluated: hemoglobin, hematocrit, white blood cell count, red blood cell count, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelet count, total protein, albumin, globulin, albumin/globulin ratio, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, gamma-glutamyl transferase, total bilirubin, urea nitrogen, creatinine, blood urea nitrogen/creatinine ratio, phosphorus, glucose, calcium, magnesium, sodium, potassium, sodium/potassium ratio, chloride, cholesterol, triglyceride, amylase, lipase, creatinine kinase, activated prothrombin time, prothrombin time, and fibrinogen.
Neutralizing antibody (NAb) titers were determined on serum samples, as previously described.14 Peripheral blood mononuclear cells (PBMCs) were isolated and cryopreserved. Lymphocytes were isolated from the spleen, bone marrow, and liver, as previously described.15 T-cell responses to AAV8 and hLDLR were analyzed by interferon-gamma (IFN-γ) enzyme-linked immunospot assay (ELISPOT), as previously described,16 using peptide libraries specific for the AAV8 capsid and hLDLR transgene, where positive response criteria are >55 spot forming units (SFU)/106 cells and three times the medium negative control value (no stimulation).
Necropsy and analysis of tissues for pathology
At the scheduled necropsy time points, rhesus macaques were euthanized and necropsied, and tissues were harvested for full pathology and biodistribution. The list of tissues collected is presented in Table 1.
Table 1.
Tissues collected at time of necropsy
| Tissues collected | ||
|---|---|---|
| Adrenal gland, left | Large intestine, colon | Ovary, right |
| Adrenal gland, right | Liver, caudate lobe | Pancreas |
| Aorta (thoracic and abdominal) | Liver, left lobe | Small intestine, duodenum |
| Ascending aorta (proximal) | Liver, middle lobe | Small intestine, jejunum |
| Bone marrow, femur | Liver, right lobe | Spinal Cord |
| Brain, cerebellum | Lung, left | Spleen |
| Brain, cerebrum | Lung, right | Stomach |
| Esophagus | Lymph node, axillary | Testicle, left |
| Eyes | Lymph node, inguinal | Testicle, right |
| Gall bladder | Lymph node, mesenteric | Thymus |
| Heart | Lymph node, popliteal | Thyroid gland (with parathyroid) |
| Kidney, left | Muscle, quadriceps femoris | Trachea |
| Kidney, right | Ovary, left | Gross lesions (if any) |
All tissues collected for histopathology were fixed using 10% neutral-buffered formalin, paraffin embedded, sectioned, and stained for histopathology using hematoxylin and eosin (H&E) stain. Slides were sent out to Antech Diagnostics, where they were examined microscopically by a pathologist.
At the time of necropsy, the liver, spleen, and bone marrow were collected, and lymphocytes were isolated from each tissue. T-cell responses to AAV8 and hLDLR were analyzed by IFN-γ ELISPOT in which positive response criteria were the same as described above.
Molecular and immunological analyses of tissues
At the time of necropsy, tissues collected for biodistribution were frozen on dry ice. DNA was extracted from tissues, and TaqMan quantitative polymerase chain reaction (qPCR) reactions were performed, as previously described.17 The qPCR assay followed the Office of Cellular, Tissue, and Gene Therapies (OCTGT) current standard with a minimum of five samples per tissue being assayed. This assay adheres to the Food and Drug Administration's guidance document regarding the tissue collection procedure and the qPCR assay methodology: Guidance for Industry: Gene Therapy Clinical Trials—Observing Subjects for Delayed Adverse Events.
Tissues were also collected for RNA expression. DNase-treated total RNA was isolated from 100 mg of liver. RNA was quantified by spectrophotometry, and aliquots were reverse transcribed to cDNA using random primers. cDNA containing the vector-derived message was quantified by qPCR designed to detect the vector specific sequence for the region spanning the transgene and poly A signal. For each sample, four reactions were conducted, including duplicate reactions with cDNA derived from 100 ng (50 ng for some samples) of RNA initially, and duplicate reactions were set up as negative controls with the same amount of RNA but were not treated with reverse transcriptase (RT). A standard curve was established ranging from 10, 102, 103, 104, 105, to 106 copies of linearized AAV cis plasmid DNA. The acceptance criteria of the assay were as follows: (1) the correlation coefficient (R2) of the standard curve must be >0.985; (2) the column flow through control (FTC) must test below the limit of detection (10 copies); (3) the no template control (NTC) must test below the assay limit of detection; (4) the copy number detected from RNA not treated with RT must be <10% of that detected from RNA treated with RT; and (5) for samples with a quantifiable number of copies of the target sequence (within the range of quantification), the difference between Ct values for the duplicate reaction must be ≤1 Ct. The sensitivity of the assay (limit of quantification) was 10–100 copies per reaction. The qPCR assay includes both positive and negative controls and is able to detect <20 copies/100 ng of RNA with 95% confidence. Ratios or averages were calculated.
Statistical analyses
Statistical analyses were performed on AST and ALT blood chemistry values. Ranges of normal values for wild type and LDLR+/− rhesus macaques were determined separately for each genotype by taking the mean of all values collected for the study animals before vector administration and by calculating the standard deviation (SD). The range was then presented as the mean ± SD. Values outside of two SDs of the mean were considered to be extreme values.
Results
Study rational and design
One male and one female rhesus macaque from each genotype (wild type or LDLR+/−) was assigned to each group. Single animals from each group were necropsied on day 28 or day 365. Table 2 summarizes the study design and key aspects of the macaques used in this study.
Table 2.
Summary of experiment groups
| Group | NHP IDs | Sex | Weight (kg) | Genotype | Vector/control | Necropsy time point |
|---|---|---|---|---|---|---|
| 1 | 090-0275 | Male | 3.65 | Wild type | AAV8.TBG.hLDLR | Day 28 |
| 090-0297 | Female | 4.00 | Wild type | AAV8.TBG.hLDLR | Day 28 | |
| 2 | 19499 | Male | 10.05 | LDLR+/− | AAV8.TBG.hLDLR | Day 28 |
| 19269 | Female | 9.15 | LDLR+/− | AAV8.TBG.hLDLR | Day 28 | |
| 3 | 090-0263 | Male | 3.90 | Wild type | AAV8.TBG.hLDLR | Day 365 |
| 090-0287 | Female | 4.20 | Wild type | AAV8.TBG.hLDLR | Day 365 | |
| 4 | 19498 | Male | 10.30 | LDLR+/− | AAV8.TBG.hLDLR | Day 365 |
| 20031 | Female | 9.60 | LDLR+/− | AAV8.TBG.hLDLR | Day 365 | |
| 5 | 02C059 | Male | N/A | Wild type | Control | N/A |
| 21737 | Male | N/A | Wild type | Control | N/A |
On study day 0, macaques were administered 1.25 × 1013 genome copies (GC)/kg of AAV8.TBG.hLDLR in a volume of 10 mL into the saphenous vein at a rate of 1 mL/min via a Harvard infusion pump. Body weight, temperature, respiratory rate, and heart rate were monitored and recorded at all blood sampling time points.
Two necropsy time points were chosen for the study: day 28 and 1 year post vector administration. The day 28 time point was an early time point chosen to allow for collection of important information regarding vector safety and toxicity early after administration. Tissue evidence of innate and adaptive immune toxicities should still be present at this time point. The 1 year time point was chosen to provide important insights into long-term issues of safety and toxicity.
Clinical findings
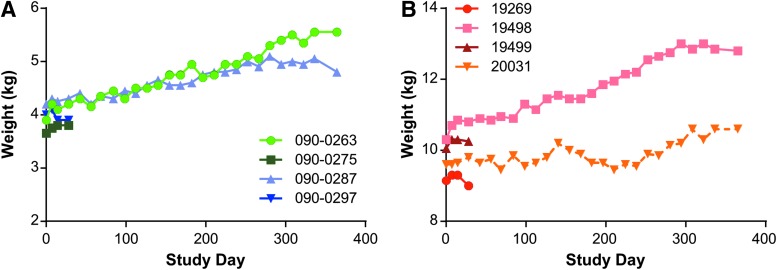
All rhesus macaques survived until their scheduled necropsy time point at day 28 or day 365. All rhesus macaques were observed daily and more thoroughly examined each time that they were anesthetized. There were no clinical abnormalities noted during the course of the study. Throughout the study, the body weight of the animals was monitored, and all animals continued to gain weight gradually over the course of the study (Fig. 1). It should be noted that at baseline, the LDLR+/− animals, which were 8 years old at the time of vector injection, were much larger than the wild-type animals, which were 3 years old at the time of vector injection (Table 2). This rate of growth is consistent with the normal development of the animals at these ages.
Figure 1.
Body weight of rhesus macaques following vector administration. (A) Wild type and (B) LDLR+/− rhesus macaques were injected intravenously (i.v.) with 1.25 × 1013 GC/kg of AAV8.TBG.hLDLR, and weights were measured throughout the study duration.
Clinical pathology
Changes in the blood chemistries, cell profiles, and coagulation parameters of the animals were analyzed throughout the study on a biweekly basis by the contract facility, Antech Diagnostics. There were occasional sporadic abnormalities that were mild in some of the parameters, but there was nothing that was considered to be related to vector treatment, except for transaminases as described below.
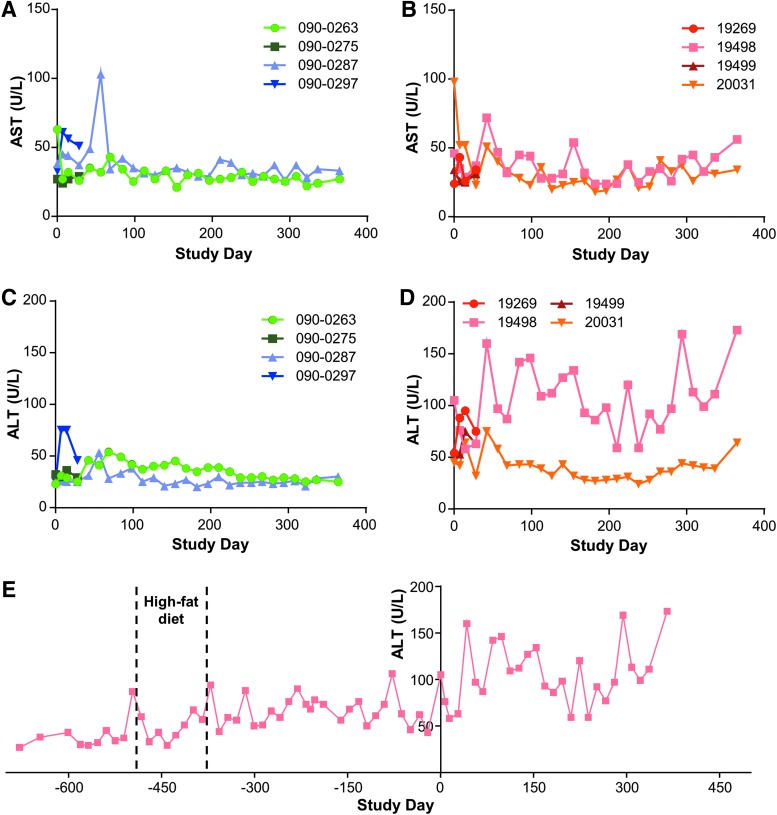
Due to the concern over toxicity in the target organ, liver function tests (LFTs) focusing on AST and ALT levels were monitored throughout the study in both the wild-type and LDLR+/− macaques. Figure 2 summarizes AST levels for wild-type (Fig. 2A) and LDLR+/− (Fig. 2B) macaques, and ALT levels for wild-type (Fig. 2C) and LDLR+/− (Fig. 2D) macaques. A more extensive presentation of the ALT levels of the LDLR+/− macaque with elevated baseline abnormalities (animal 19498) is shown in Fig. 2E. Other parameters reflective of liver pathology, such as bilirubin, were within normal limits throughout the study.
Figure 2.
Aspartate aminotransferase AST and (alanine aminotransferase) ALT levels in rhesus macaques injected with vector. Wild-type and LDLR+/− rhesus macaques were injected i.v. with 1.25 × 1013 GC/kg of AAV8.TBG.hLDLR. AST and ALT levels were measured in serum samples taken throughout the study. AST levels for (A) wild-type and (B) LDLR+/− rhesus macaques, and ALT levels for (C) wild-type and (D) LDLR+/− rhesus macaques. (E) LDLR+/− rhesus macaque 19498 was injected i.v. with 1.25 × 1013 GC/kg of AAV8.TBG.hLDLR on study day 0. ALT levels were measured in serum samples taken both during the study and prior to initiation of the study. The animal was fed normal chow, except from day −491 to day −378 pre vector administration when it was on a high-fat diet.
For wild-type animals, the normal ranges for AST and ALT levels were 37 ± 11 and 28 ± 6 IU/L, respectively, and the normal ranges for LDLR+/− macaques were 29 ± 11 and 50 ± 6 IU/L, respectively. Except for one animal with high baseline abnormalities in transaminase levels, there were no increases in AST or ALT that exceeded 103 IU/L. Additionally, these elevations were often observed early after administration of vector, and all were transient, with the exception of the animal with high pretreatment levels. A more specific summary of the transaminases for individual animals is provided below.
Wild-type animals:
• 090-0275 (day 28): the AST and ALT measurements fell within the normal ranges.
• 090-0297 (day 28): the AST was abnormal at day 0 and ALT increased to abnormal levels (i.e., 75 IU/L) immediately after vector administration, then quickly declined.
• 090-0263 (day 365): there was a slight increase in AST at baseline with other values remaining normal. ALTs were abnormal sporadically during months 2 and 3 post vector administration, but were never >54 IU/L and returned to normal levels for the duration of the study.
• 090-0287 (day 365): an acute increase in AST on day 56 to 103 IU/L was followed by a rapid decline, with other values remaining in the normal range. This corresponded to an abnormal ALT at the same time point with the rest of the values remaining normal.
LDLR+/− animals:
• 19499 (day 28): the AST and ALT measurements remained within the normal ranges.
• 19269 (day 28): the AST levels were normal, although ALT increased to abnormal levels in the first month but never exceeded 95 IU/L.
• 19498 (day 365): the majority of ALT values post vector administration were >2 SDs from the normal reference range and displayed the largest variability of any of the study animals, but they did not exhibit any time trend (Fig. 2E). In addition, the baseline of animal 19498 was greater than that of the other three LDLR+/− rhesus macaques. Further, the variability in measurements was consistent pre and post vector administration (Fig. 2E). A trend suggesting increased ALT values post vector administration was observed. However, the analysis was not sufficiently powered to confirm statistically significant differences in this elevation of ALT following vector administration.
• 20031 (day 365): the AST was elevated on day 0 to 98 IU/L and remained normal except for some elevations during the first 6 weeks, but levels never exceeded 98 IU/L. Aside from an increase in ALT to 75 IU/L on day 42, all other values remained normal.
NAbs to AAV8 capsid and peripheral T-cell responses to AAV8 capsid and hLDLR transgene
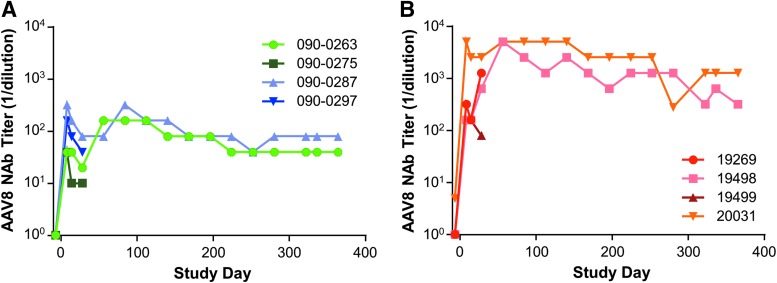
Prior to initiation of the study, all rhesus macaques were screened for NAbs to AAV8 capsid. Seven out of the eight animals selected for the study were seronegative to AAV8 (NAb titer <1:5), with macaque 20031 having a NAb titer to AAV8 of 1:5 on the 7th day prior to vector injection. Following vector administration, all animals developed an AAV8-specific NAb response. On day 7 post vector administration, AAV8 NAb titers increased from <1:5 to a range of 1:40 to 1:320 in wild-type macaques (Fig. 3A). In the LDLR+/− macaques, a greater increase in AAV8 NAb titers was seen, with titers ranging from 1:160 to 1:5,120 (Fig. 3B).
Figure 3.
Neutralizing antibody (NAb) levels in rhesus macaques injected with vector. (A) Wild-type and (B) LDLR+/− rhesus macaques were injected i.v. with 1.25 × 1013 GC/kg of AAV8.TBG.hLDLR. NAb levels to the AAV8 capsid were measured in serum samples taken throughout the study duration.
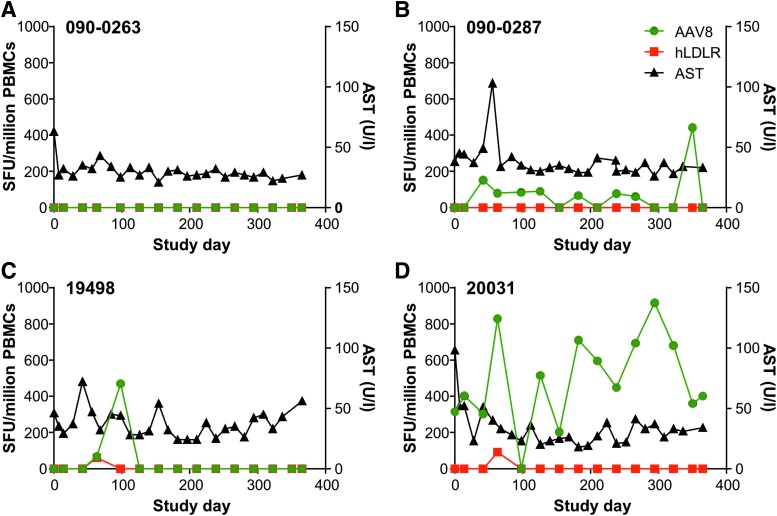
Blood was collected and PBMCs were isolated throughout the course of the study at approximately monthly intervals. T-cell responses to AAV8 and hLDLR were analyzed by IFN-γ ELISPOT and compared with serum AST. A positive response is defined when the total SFU when stimulated with antigen per 106 cells is >55 and three times greater than the medium-only negative control value (no stim). None of the four animals necropsied at day 28 post vector administration developed an AAV8 capsid-specific or LDLR-specific peripheral T-cell response following vector administration. However, three of the animals that were followed for 1 year post vector administration did develop peripheral T-cell responses to AAV8 capsid and/or the hLDLR transgene. A more detailed description of the long-term animals is presented in Fig. 4 and described below. Note that the no-stimulation control is subtracted from the antigen-stimulated result.
Figure 4.
Peripheral T-cell responses in rhesus macaques injected with vector. Peripheral T-cell responses to AAV8 capsid and the hLDLR transgene were measured by interferon-gamma (IFN-γ) enzyme-linked immunospot assay (ELISPOT) following i.v. injection with 1.25 × 1013 GC/kg of AAV8.TBG.hLDLR. Data presented show the time course of T-cell response and AST levels for macaques (A) 090-0263, (B) 090-0287, (C) 19498, and (D) 20031. The plot includes only T-cell responses that met the positive response criteria, defined when the total spot forming units (SFU) when stimulated with antigen per 106 cells is >55 and three times greater than the medium-only negative control value (no stim).
No peripheral T-cell response to AAV8 capsid or hLDLR was detected for macaque 090-0263 at any time point measured (Fig. 4A). In macaque 090-0287, an AAV8 capsid-specific response was detected at day 42 post vector administration, and remained detectable for almost the entire study (Fig. 4B). A transient peripheral T-cell response to AAV8 capsid was detected in macaque 19498 on days 63 and 98 post vector administration, which coincided with a hLDLR transgene-specific peripheral T-cell response on day 63 (Fig. 4C). Macaque 20031 had pre-existing AAV8 capsid-specific T cells and remained positive for AAV8 capsid-specific T cells throughout the course of the study (Fig. 4D). There was a transient increase in T cells to LDLR on day 63, similar to what was observed in macaque 19498.
The only correlation between T-cell response and AST elevation was found in animal 090-0287, which developed an increase in AST to 103 IU/L that corresponded with the appearance of capsid T cells (Fig. 4B). Interestingly, the AST elevation was transient, although the T-cell response persisted at low levels.
Histology and T-cell immune response in tissues
Animals were necropsied on day 28 or day 365 post vector administration, and tissues were harvested for full histopathology. Tissues were fixed in 10% neutral-buffered formalin, paraffin embedded, sectioned, and stained with H&E stain. Slides were examined microscopically by a pathologist at Antech Diagnostics. Based on the results presented in the histopathology report (presence of inflammation, cellular infiltrates, changes in morphology, etc), a scoring system was developed where the terminology used in the report was converted to a numerical system: 0, none; 1, minimal; 2, mild; 3, moderate; and 4, severe. Two additional naïve animals were included in the analysis (02C059 and 21737) as age-matched controls.
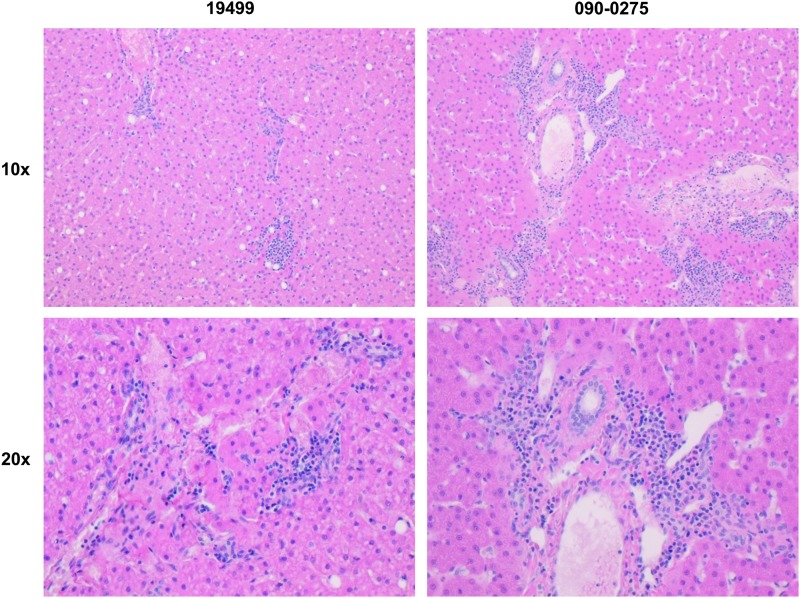
A summary of the pathologist's findings is presented in Supplementary Table S2. There were no significant findings described by the pathologist. Except for animal 090-0297, minimal to mild cholangiohepatitis was seen in liver sections from allwild-type macaques, as exemplified in Fig. 5. LDLR+/− macaques ranged from presenting no liver pathology to mild diffuse hepatocellular swelling. Similar findings were described in one of the two control animals evaluated.
Figure 5.
Liver histopathology following vector administration in rhesus macaques. Wild-type and LDLR+/– rhesus macaques were injected i.v. with 1.25 × 1013 GC/kg of AAV8.TBG.hLDLR. Following tissue harvest at necropsy, H&E-stained slides were prepared and examined microscopically. Histopathology readings from sections of the liver of animal 19499 were determined to be minimal (score = 1) and minimal to mild for 090-0275 (score = 1–2).
Prior to the current study discussed here, LDLR+/− rhesus macaques 19269, 19498, 19499, and 20031 were fed a high-fat diet for approximately 5 months, during which cholesterol levels reached a maximum and stable peak. These four macaques were then taken off the high-fat diet, and serum cholesterol of all four macaques stabilized to levels observed before initiation of the high-fat diet after approximately 1 month. Mild to moderate aortic atherosclerosis was seen in animal 19499, and minimal aortic atherosclerosis with minimal mineralization was seen in animal 20031. A review by the pathologist suggests that these lesions may have been caused by the high-fat diet administered to these animals, but may also be found as incidental lesions in adult macaques.
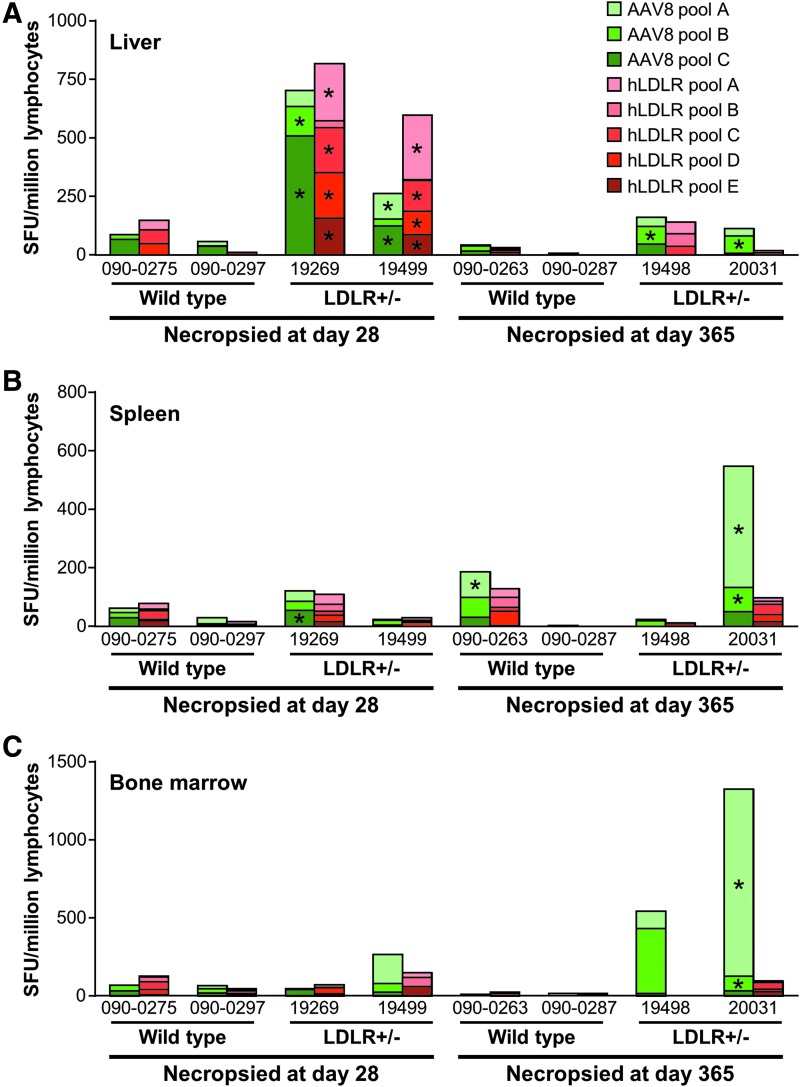
At the time of necropsy, the liver, spleen, and bone marrow were collected, and lymphocytes were isolated from each tissue. T-cell responses to AAV8 and hLDLR were analyzed by IFN-γ ELISPOT (Fig. 6A liver, B spleen, and C bone marrow). No T-cell responses were detected in the tissues tested from the two wild-type macaques (090-0275 and 090-0297) necropsied on day 28 post vector administration. Very high frequencies of AAV8 capsid-specific and hLDLR-specific T cells were detected in the liver and spleen for macaque 19269 and in the liver for macaque 19499, both of which were LDLR+/− and were necropsied on day 28. The only antigen-specific T cells detected in the day 365 wild-type animals were to capsid from spleen in macaque 090-0263. Both day 365 LDLR+/− animals developed capsid-specific T cells (the liver for animal 19498 and the liver, spleen, and bone marrow for animal 20031). No hLDLR-specific T cells were detected in any animal necropsied at 1 year post vector administration (Fig. 6).
Figure 6.
Capsid and hLDLR-specific T cells from tissues. Wild-type and LDLR+/– rhesus macaques were injected i.v. with 1.25 × 1013 GC/kg of AAV8.TBG.hLDLR. Following necropsy at 1 year post vector administration, lymphocytes were isolated from (A) the liver, (B) the spleen, and (C) bone marrow. T-cell responses to AAV8 capsid and the hLDLR transgene were measured by IFN-γ ELISPOT. An asterisk (*) denotes samples that meet positive response criteria, defined when the total SFU when stimulated with antigen per 106 cells is >55 and three times greater than the medium-only negative control value (no stim).
Vector biodistribution
Animals were necropsied on days 28 or 365 post vector administration, and tissues were harvested for evaluation of vector genome distribution. Vector GC per diploid genome are presented in Supplementary Table S3. AAV vector genomes were detectable in all tissues harvested on both day 28 and day 365. Vector was sequestered mainly in the liver, the intended target organ, and no apparent sex preference was observed. Vector GCs in the liver were, in general, more than two logs greater than those detected in other tissues at both time points, and they remained relatively stable over time, with a slight decrease from ∼60 GC to ∼20 GC/diploid genome. The decrease in liver vector genomes over time could also be due to a cytotoxic T-lymphocyte response against either the transgene or the vector capsid. However, this is less likely due to the relative lack of pathology seen in this organ. The decline in vector GC number was observed in all tissues analyzed, but was more rapid in tissues with higher cell turnover rates, such as the large intestine, thymus, and spleen. Low, but detectable, levels of AAV genome copies were also found in the brain and spinal cord harvested from both time points. Furthermore, low, but persistent, levels of vector genome copies were detected in the gonads from both sexes 365 days post vector administration.
hLDLR mRNA expression
hLDLR mRNA was extracted from liver samples taken at the time of necropsy and analyzed by TaqMan qPCR (Table 3). RNA levels were only determined for two out of the four animals necropsied on day 28 post vector administration.
Table 3.
hLDLR mRNA expression in NHP liver
| Necropsy time point | ID (genotype) | hLDLR message/μg RNA | Average vector GC/μg DNA |
|---|---|---|---|
| Day 28 | 19499 (LDLR+/−) | 5.78 × 104 | 3.21 × 106 |
| 19269 (LDLR+/−) | 3.52 × 105 | 2.37 × 107 | |
| Day 365 | 19498 (LDLR+/−) | 5.24 × 104 | 2.37 × 106 |
| 3.30 × 104 | |||
| 20031 (LDLR+/−) | 4.12 × 104 | 5.12 × 106 | |
| 3.67 × 104 | |||
| 090-0263 (wild type) | 3.62 × 104 | 3.47 × 106 | |
| 3.73 × 104 | |||
| 090-0287 (wild type) | 5.67 × 104 | 6.41 × 106 | |
| 4.99 × 104 |
Wild-type and LDLR+/− rhesus macaques were injected i.v. with 1.25 × 1013 GC/kg AAV8.TBG.hLDLR. Following tissue harvest at necropsy, RNA was extracted from the liver, and mRNA levels for the hLDLR transgene were determined.
Vector mRNA level was closely correlated with vector GC number in liver samples collected from both short- and long-term groups. Animal 19499 had the lowest vector copy number in the group, with 10-fold lower GC and mRNA levels than those of animal 19269, which was also necropsied on day 28. In general, the level of vector-derived RNA was reduced over the time course of the study. This possibly resulted from normal hepatocyte turnover throughout the 1 year study.
Discussion
The goal of this study was to evaluate toxicity of AAV8.TBG.hLDLR in wild-type and LDLR+/− macaques. The pharmacology of this product was assessed in a mouse model of HoFH and not in this study, since the LDLR+/− macaques did not recapitulate the dyslipidemia of FH patients.19
The animals tolerated the infusion of vector well, without any apparent long- or short-term clinical sequelae. Biodistribution studies confirmed the predicted high level and stable targeting of liver, with far less, but still detectable, extrahepatic distribution, which declined over time. These data suggest that the target organ for efficacy, the liver, is also the most likely source of toxicity. A detailed review of tissues harvested at necropsy performed 28 and 365 days post vector administration revealed some minimal to mild findings in liver, with some evidence of atherosclerosis in the LDLR+/− macaques. The nature of the liver pathology, and the fact that similar pathology was observed in one of the two untreated wild-type animals, suggest that these findings were unrelated to the vector.
The growing clinical experience of systemically delivered AAV8 to target the liver in patients with hemophilia B provides a context for evaluating the findings.5,6 For purposes of comparisons to the NHP data in this paper, the only published clinical data with AAV8 in the liver are summarized in Table 4. Expression of the transgene was stable in all patients, who segregated into four general categories with respect to the impact of vector on transaminases and appearance of T cells to capsid in PBMCs. Category 1 included four patients with no abnormalities in transaminases and no appearance of T cells. Category 2 included four patients with no abnormalities in transaminases but the appearance of T cells. Two patients developed both an elevation in transaminases and the appearance of T cells. However, these events occurred at different times in one patient (category 3) while occurring at the same time in the other patient (category 4). The data in the category 4 patient support the hypothesis that destructive T cells targeted transduced hepatocytes, leading to damage as evidenced by elevations in transaminases. It has been speculated that the target for the destructive T cells is input AAV capsid in what is called the “capsid T-cell hypothesis.” However, studies to date in hemophilia B do not suggest that the transient and low-level transaminitis represents dose-limiting toxicities. The concern is that the mechanism underlying these laboratory abnormalities may diminish transgene expression, which has been suggested by the clinical data but is difficult to discern because of the emerging practice of treatment with a short course of high-dose steroids.
Table 4.
Summary of human and nonhuman primate experience with AAV8 in liver
| Humans | NHPs | |||||||
|---|---|---|---|---|---|---|---|---|
| Category | Number of subjects | Expression | ALT (xULN) | T cells | Number of NHPs | Expression | AST (xULN) | T cells |
| 1 | 4 | Stable | WNL | – | 1 | Stable | WNL | – |
| 2 | 4 | Stable | WNL | + | 0 | Stable | – | – |
| 3 | 1 | Stable | 1.3 (9 weeks) | + (2 weeks) | 1 | Stable | 1.5 × (6 weeks) | + (9, 14 weeks) |
| 4 | 1 | Stable | 4 × (8 weeks) | + (9 weeks) | 1 | Stable | 2.1 × (8 weeks) | + (6 weeks onwards) |
Key aspects of vector performance (i.e., longevity of expression, elevations of transaminases, and appearance of capsid T cells in peripheral blood) in the St. Judes/UCL AAV8 hemophilia B trial are presented.5,6 Similar outcome measures of the long-term rhesus macaques presented in this study are also summarized. The transaminase ALT is presented for humans and AST for rhesus macaques, as AST appeared to be more stable in primates. When there were elevations in transaminases and/or the appearance of T cells, the time at which these events occurred are indicated. The categories of outcomes are as follows: 1, no abnormality in transaminase and no appearance of T cells; 2, no abnormality in transaminase but appearance of T cells; 3, an abnormality in transaminase and an appearance of T cells, but these events did not occur at the same time; and 4, an abnormality in transaminase and an appearance of T cells that coincided.
ULN, upper limit of normal; WLN, within normal limits.
Data from animals in this study euthanized on day 28 were not informative in terms of evaluating the capsid T-cell hypothesis, since the occurrence of transaminitis and the appearance of capsid T cells in the clinical trials occurred much later. In fact, none of these animals developed capsid T cells in PBMCs. All but one of the long-term animals, however, yielded data relevant to the findings of the clinical trials. The exception was animal 20031, which had baseline abnormalities in transaminases and pre-existing and persistent elevations of T cells that confounded the interpretation of vector-mediated effects. Each of the three informative long-term animals presented with different performance profiles that ran the spectrum of what was seen in the clinic, including no transaminase abnormalities and no T cells (category 1, 090-0263), transaminase abnormalities and T cells that occurred at different times (category 3, 19498), and transaminase abnormalities and T cells that appeared at the same time (category 4, 090-0287). In this study, the absence of a quantitative, non-invasive measure of LDLR expression made addressing the issue of expression durability more challenging than it was for coagulation Factor IX in the hemophilia B studies. Liver tissue harvested at days 28 and 365 post vector administration was analyzed for expression of the transgene by RT-PCR. While there was a decrease over time when comparing animals necropsied on days 28 and 365 post vector administration, there was no difference between animals harvested at each time point, including the animals that developed AST/ALT abnormalities with or without capsid/hLDLR-specific T cells. This strongly suggests that expression was not affected by the process that led to abnormalities in clinical pathology or the appearance of T cells.
It has been argued that no animal model has simulated the hypothesized capsid T-cell-mediated liver toxicity observed in the hemophilia B clinical trials. This study, in fact, resembles what has been observed in the clinic: low-level transient elevations of transaminases in some recipients of vector with and without the concomitant appearance of T cells, all of which did not have an impact on transgene expression. Therefore, NHPs may be useful for investigating the mechanism of this toxicity and developing strategies to prevent it from occurring.
Tissue-derived mononuclear cells harvested at necropsy were also analyzed for antigen-specific T cells, providing insight into adaptive immune responses beyond what can be measured from PBMCs. Measurement of T cells from a single harvest of tissue revealed a greater number of capsid T-cell positive animals than what was revealed by sequential measurements of peripheral blood. Furthermore, very robust LDLR-specific T cells were detected in the livers of two animals at day 28, despite the lack of detectable LDLR-specific T cells in peripheral blood. The appearance of capsid and hLDLR-specific T cells in the liver that were not present in PBMCs suggests that peripheral blood may not be an accurate reflection of the state of T-cell activation that exists in the target organ. Lymphocytes from PBMCs, as well as those isolated from secondary lymphoid organs such as the spleen and liver, differ significantly in their phenotypic and functional profile. Studies carried out in macaques using recombinant AAV vectors have shown immune responses to the transgene in liver lymphocytes that were lower or not detected in blood PBMCs.18 In addition, transgene-specific liver lymphocytes had a cytokine profile that differed considerably from the one observed in blood PBMCs. The absence of IFN-γ-expressing T cells to coagulation Factor IX in PBMCs in the clinical trials led to the hypothesis that the capsid may be the culprit. However, the data showing transgene product-specific T cells in the liver that are not found in PBMCs suggest that the capsid T-cell hypothesis should be revisited, and that destructive T cells to antigenic transgene products may be contributing to toxicity.
It is expected that some of the animals would have developed T cells to human LDLR, since it differs from endogenous macaque LDLR, but it is surprising that T-cell responses to the transgene product were found primarily in the LDLR+/− animals and not the wild-type animals. Both animal groups should demonstrate the same central tolerance to LDLR, since both have at least one normal allele. The wild-type animals were derived from a different colony than the LDLR+/− macaques, suggesting that other genetic factors may have been involved, such as MHC class I genotypes.
In conclusion, no dose-limiting toxicity was observed at a dose of 1.25 × 1013 GC/kg of AAV8.TBG.hLDLR, which means that the maximally tolerated dose is equal to or greater than the dose tested here. Vector-related elevations in transaminases were observed, which were low and transient but nevertheless present, meaning that the no-effect dose is less than the single dose evaluated in this study.
Supplementary Material
Acknowledgments
We would like to thank Alexandra L. Hanlon (University of Pennsylvania, School of Nursing) and the Human Immunology Core (University of Pennsylvania, Philadelphia, PA). We also thank Jennifer Stewart, PhD for editorial assistance with this manuscript. This work was funded by the National Institutes of Health: National Heart, Lung, and Blood Institute (P01 HL059407).
Author Disclosure
J.M.W. is an advisor to REGENXBIO, Dimension Therapeutics, and Solid Gene Therapy, and is a founder of, holds equity in, and has a sponsored research agreement with REGENXBIO and Dimension Therapeutics. In addition, he is a consultant to several biopharmaceutical companies and is an inventor on patents licensed to various biopharmaceutical companies. No competing financial interests exist for the remaining authors.
References
- 1.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986;232:34–47 [DOI] [PubMed] [Google Scholar]
- 2.Goldstein JL, Brown JC. Familial hypercholesterolemia, lipoprotein and lipid metabolism disorders. In: Scriver CR, Beaudet AL, Sly WS, et al., eds. Metabolic Basis of Inherited Disease. Volume II New York: McGraw-Hill, 1989:1215–1250 [Google Scholar]
- 3.Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. J Clin Invest 2003;111:1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman M, Raper SE, Kozarsky K, et al. Successful ex vivo gene therapy directed to liver in a patient with familial hypercholesterolaemia. Nat Genet 1994;6:335–341 [DOI] [PubMed] [Google Scholar]
- 5.Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. New Engl J Med 2014;371:1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kassim SH, Li H, Bell P, et al. Adeno-associated virus serotype 8 gene therapy leads to significant lowering of plasma cholesterol levels in humanized mouse models of homozygous and heterozygous familial hypercholesterolemia. Hum Gene Ther 2013;24:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassim SH, Li H, Vandenberghe LH, et al. Gene therapy in a humanized mouse model of familial hypercholesterolemia leads to marked regression of atherosclerosis. PLoS One 2010;5:e13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scanu A, Khalil A, Neven L, et al. Genetically determined hypercholesterolemia in a rhesus monkey family due to a deficiency of the LDL receptor. J Lipid Res 1988;29:1671–1681 [PubMed] [Google Scholar]
- 10.Hummel M, Li Z, Pfaffinger D, et al. Familial hypercholesterolemia in a rhesus monkey pedigree: Molecular basis of low density lipoprotein receptor deficiency. Proc Natl Acad Sci U S A 1990;87:3122–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuchel M, Meagher EA, du Toit Theron H, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, Phase 3 study. Lancet 2013;381:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2010;7:195–203 [DOI] [PubMed] [Google Scholar]
- 13.Lock M, Alvira M, Vandenberghe LH, et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther 2010;21:1259–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calcedo R, Vandenberghe LH, Gao G, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009;199:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Calcedo R, Wang H, et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther 2010;18:126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calcedo R, Vandenberghe LH, Roy S, et al. Host immune responses to chronic adenovirus infections in human and nonhuman primates. J Virol 2009;83:2623–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S-J, Sanmiguel J, Lock M, et al. Biodistribution of AAV8 vectors expressing human low-density lipoprotein receptor in a mouse model of homozygous familial hypercholesterolemia. Hum Gene Ther Clin Dev 2013;24:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao G, Wang Q, Calcedo R, et al. Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses. Hum Gene Ther 2009;20:930–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greig JA, Limberis MP, Bell P, et al. Nonclinical pharmacology/toxicology study of AAV8.TBG.mLDLR and AAV8.TBG.hLDLR in a mouse model of homozygous familial hypercholesterolemia. Hum Gene Ther Clin Dev 2017; 28: 10.1089/humc.2017.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.