Abstract
Primary central nervous system tumors are the most common solid neoplasm of childhood and the leading cause of cancer-related death in pediatric patients. Survival rates for children with malignant supratentorial brain tumors are poor despite aggressive treatment with combinations of surgery, radiation, and chemotherapy, and survivors often suffer from damaging lifelong sequelae from current therapies. Novel innovative treatments are greatly needed. One promising new approach is the use of a genetically engineered, conditionally replicating herpes simplex virus (HSV) that has shown tumor-specific tropism and potential efficacy in the treatment of malignant brain tumors. G207 is a genetically engineered HSV-1 lacking genes essential for replication in normal brain cells. Safety has been established in preclinical investigations involving intracranial inoculation in the highly HSV-sensitive owl monkey (Aotus nancymai), and in three adult phase 1 trials in recurrent/progressive high-grade gliomas. No dose-limiting toxicities were seen in the adult studies and a maximum tolerated dose was not reached. Approximately half of the 35 treated adults had radiographic or neuropathologic evidence of response at a minimum of one time point. Preclinical studies in pediatric brain tumor models indicate that a variety of pediatric tumor types are highly sensitive to killing by G207. This clinical protocol outlines a first in human children study of intratumoral inoculation of an oncolytic virus via catheters placed directly into recurrent or progressive supratentorial malignant tumors.
Keywords: : HSV, oncolytic, virotherapy, pediatric, brain tumors, G207
Introduction
Malignant childhood brain tumors
Central nervous system tumors are the most common solid neoplasm of childhood accounting for approximately 25% of all childhood malignancies and the leading cause of cancer-related morbidity and mortality.1,2 While outcomes for children with low-grade, localized tumors are excellent, there remains a significant subset of patients with high-grade malignancies that have very poor outcomes despite conventional therapies including surgery, chemotherapy and radiation. For patients with high-grade recurrent tumors, traditional treatment usually only provides a brief interval of disease control.3,4 Furthermore, patients who survive their disease after traditional therapy often have damaging lifelong sequelae such as hormone dysfunction, neurosensory impairment, and neurocognitive changes that are attributed to the treatment.5–7 Novel innovative treatments that improve outcomes and lessen harmful side effects are urgently needed.
Neoplastic therapy using genetically engineered herpes simplex virus
Brain tumors are suitable targets for intervention using conditionally replicating viruses that replicate and kill tumor cells while sparing the postmitotic, nondividing cell population of the brain. Attenuated herpes simplex virus (HSV) has a number of potential advantages as a neoplastic therapy for pediatric brain tumors. HSV-1 has been extensively studied; it is a large, enveloped, double-stranded DNA virus with a genome of approximately 152 kb, encoding at least 80 genes.8 The genome is divided into long and short unique regions, each flanked by characteristic repeated sequences. Essential and nonessential genes for HSV-1 replication in the brain and in brain tumors have been identified, making it possible to genetically engineer a virus capable of selective replication in pediatric brain tumor cells.9–12 HSV is a neurotropic virus, making nervous system tumors ideal targets for the virus. The attenuated virus remains immunogenic and promotes danger signals that can reverse tumor immune evasion, increase cross-presentation of tumor antigens, and promote an antitumor immune response.13 This antitumor immune response may occur even in the absence of virus permissivity.14 Lastly, because these viral constructs retain the thymidine kinase gene, they are highly susceptible to readily available, effective antiviral agents in the unlikely event that a mutant virus produces toxicity in normal brain tissue.
Engineered HSV-1 G207 (Fig. 1) contains deletions in both loci of the γ134.5 gene and a disabling lacZ insertion in UL39, that encodes the large subunit of viral ribonucleotide reductase.12 The γ134.5 “neurovirulence” gene encodes a critical multifunctional protein (ICP34.5) that enables the virus to replicate in normal cells by blocking host cell shutoff of protein synthesis, inhibiting the induction of antiviral host cell genes, and preventing autophagy that can limit virus replication.15,16 In the absence of this protein, the virus fails to induce HSV encephalitis, even when injected in high amounts directly into the central nervous system (CNS) of susceptible animal models.17,18 Additionally, a portion of the latency associated transcripts is encoded on the DNA strand opposite the γ134.5 genes. Thus, the capacity to express genes necessary for the virus to establish and escape latency is lost when the γ134.5 gene is altered.19 The lacZ insertion provides added protection for normal cells because ribonucleotide reductase is important for nucleotide synthesis needed for viral replication.20,21 In the postmitotic brain, dividing tumor cells provide cellular ribonucleotide reductase, which is utilized by the mutant virus to facilitate replication in cancer cells.
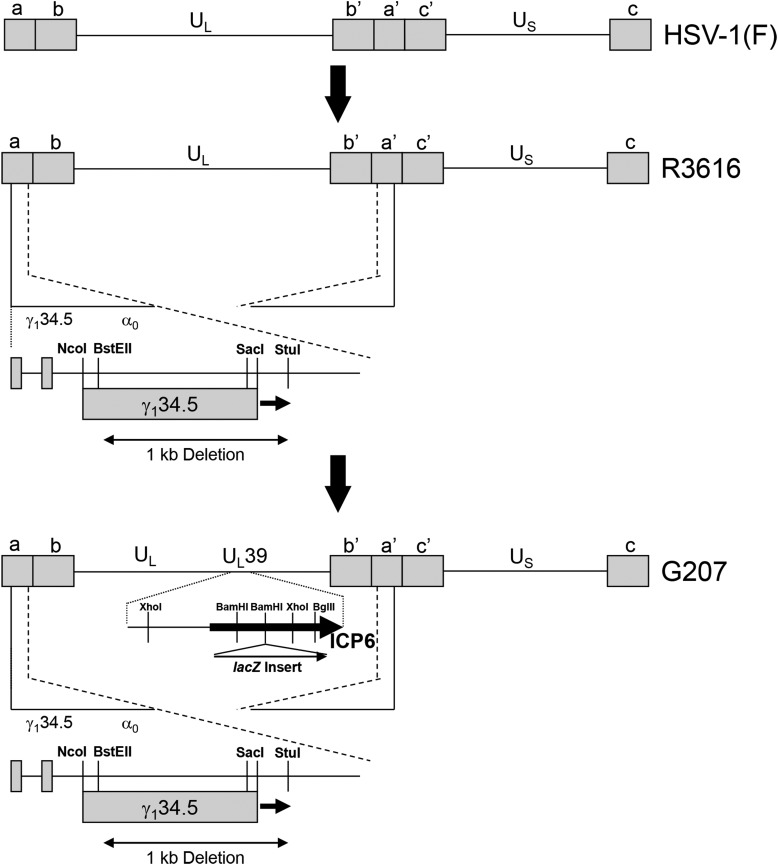
Figure 1.
Schematic representation of the derivation of G207 HSV. Chou et al. deleted both copies of the γ134.5 gene from herpes simplex virus strain F (HSV-1(F)), a temperature-sensitive clinical isolate, to create recombinant virus R3616.9 Only one of the two deletions is shown for simplicity. This deletion ablated the ability of the virus to overcome interferon resistance in normal cells and made the virus aneurovirulent. Mineta et al. modified R3616 by inserting the Escherichia coli β-galactosidase gene (lacZ) to produce a functional deletion of HSV UL39, which encodes the heavy chain or ribonucleotide reductase (infected cell protein 6 [ICP6]), as described by Goldstein and Weller.12, 20 This second disabling deletion was performed to ensure added safety for intracerebral human trials. (Copyright [2009] The American Society of Gene Therapy. Reproduced with permission from Molecular Therapy [Friedman et al.38]).
Preclinical and clinical data to support the safety and efficacy of the proposed study
G207 has been inoculated intracerebrally without deleterious side effects in BALB/c mice and in Aotus nancymai owl monkeys at single doses up to 109 plaque-forming units (pfu).17,18 Owl monkeys are exquisitely sensitive to wild-type HSV-1, similar to human neonates, with 103 pfu of wild-type HSV-1(F) causing death.18 Magnetic resonance imaging (MRI) analysis of G207-treated nonhuman primates showed no CNS abnormalities, and neuropathologic evaluation demonstrated local inflammation at the site of injection, but no other brain abnormalities. Histopathological evaluation of multiple organs revealed no evidence of altered cellular architecture or HSV immunoreactive cells.
A phase 1 clinical trial of G207 in adults with high-grade glioma was conducted at the University of Alabama at Birmingham (UAB) and Georgetown University, followed by two additional phase 1 studies conducted at UAB.22–24 Twenty-one adult patients were treated in seven cohorts of three each by stereotactic intratumoral injection of HSV G207 in a standard dose escalation fashion from 1 × 106 pfu as a single injection in 0.1–0.3 mL up to 3 × 109 pfu given at 0.2 mL each in five sites.22 While some patients developed complications frequently seen with malignant glioma, no serious adverse events were ascribed to G207, and therefore a maximum tolerated dose was not reached. Eight of twenty MRI-evaluable patients had a decrease in their enhancement volume at 1 month postinoculation. Two patients had an extended disease-free survival of >5.5 years, and another patient who died of an unrelated stroke 9 months after G207 treatment had no evidence of recurrent disease at autopsy.22,25
In a phase 1b study, six patients with recurrent glioblastoma multiforme received two doses of G207 totaling 1.15 × 109 pfu, with 13% of this total dose injected prior to tumor resection via a catheter placed stereotactically into enhancing portion of the tumor.23 Two to five days later, the tumor was resected en bloc with the catheter in place, and the remainder of G207 dose was injected into the brain surrounding the resection cavity. Radiographic and neuropathologic evidence suggestive of viral replication and antitumor activity was seen in three patients. Furthermore, increases in tumor-infiltrating T lymphocytes and macrophages were seen in three of six and five of six patients respectively. This study suggested that multiple administrations of G207 was safe and that injection into brain bordering the tumor bed was safe.
A third phase 1 study examined the safety of stereotactic intratumoral administration of G207 at 1 × 109 pfu followed within 24 h with a single 5 Gy radiation dose in nine adults with recurrent high-grade gliomas.24 Preclinical studies demonstrated in vivo synergism of engineered HSV-1 and radiation through several potential mechanisms.26–30 Ionizing radiation increases transcription of cellular genes that complement viral gene deletions such as ribonucleotide reductase and potentially activates late HSV-1 promoters through activation of the p38 mitogen–activated protein kinase pathway thereby enhancing virus replication.28,29 Also, increased cellular activity due to the DNA damage response after radiation may improve viral replication. Lastly, radiation may synergize with oncolytic HSV-1 by facilitating enhancement of antitumor innate and adaptive immune responses. In the phase 1 trial, treatment was well tolerated and no dose limiting toxicities occurred. Six of nine patients had a stable or partial response for at least one time point, and three had marked radiographic responses to treatment (see Fig. 2 for example of response). Two patients had an initial response, then demonstrated recurrences and were able to be retreated under patient-specific protocols; both patients exhibited antitumor effects in response to the second dose of virus as well. Taken together, approximately half of the 35 adults treated in the phase 1 adult high-grade glioma G207 trials had radiographic or neuropathologic evidence of response at a minimum of one time point.
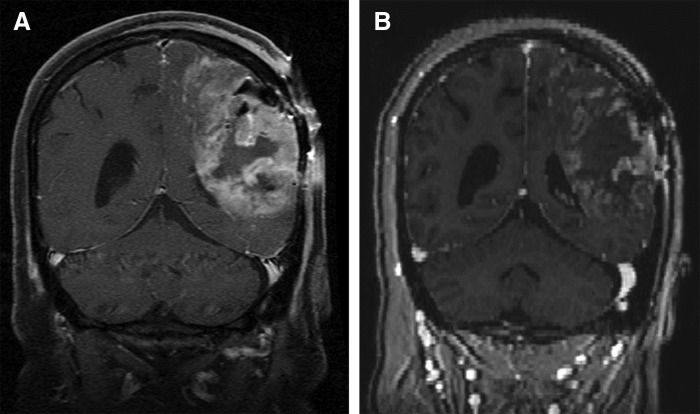
Figure 2.
Response to G207 in an adult with recurrent glioblastoma multiforme. T1-weighted gadolinium-enhanced coronal magnetic resonance imaging images. (A) Pre-inoculation of G207. (B) One month post-G207 inoculation demonstrating clear response to G207.
Preclinical evidence supports advancing G207 to clinical testing in children with malignant brain tumors; preclinical studies demonstrated that a variety of pediatric tumor types, including neural and glial tumors, express the primary HSV entry receptor nectin-1 (CD111), an adhesion molecule, and are highly sensitive to engineered HSV both in vitro and in vivo.31–34 Comparing the sensitivity of eight pediatric patient–derived high-grade brain tumor xenografts (4 medulloblastoma, 2 glioblastoma, 1 supratentorial primitive neuroectodermal tumor [PNET], 1 anaplastic ependymoma) with six adult glioblastoma xenografts to G207, Ring et al. determined that on average, pediatric tumor cells expressed CD111 in significantly greater amounts and were 22-fold more sensitive than the adult tumors in vitro, requiring a substantially lower dose to kill 50% of the cells in 72 h.35 These data suggest that malignant pediatric brain tumors may be more sensitive to G207 than adult glioblastoma, and pediatric patients may be the ideal candidates for this therapy. Importantly, CD133+ glioma and medulloblastoma cancer stem cells—which play a significant role in tumor aggressiveness, chemotherapy resistance, radioresistance, recurrence, and metastasis—were highly sensitive to G207 and showed no inherent resistance to oncolytic HSV-1 compared with CD133- tumor cells.32,33,36 Furthermore, three pediatric patient-derived xenograft models of molecular group 3 medulloblastoma, which is the most difficult to treat subgroup of medulloblastoma and portends a poor prognosis, were very sensitive to killing by G207 in vitro, and G207 significantly prolonged survival in athymic nude mice bearing intracranial tumors compared with saline.33
The safety and efficacy established in the three phase 1 adult studies and the promising preclinical data in pediatric patient-derived xenograft models form the bases to extend HSV G207 alone or combined with a single dose of radiation to the pediatric population. Certainly, pediatric patients have the potential to respond differently than adults to G207. They are more likely to be seronegative for HSV at the time of injection and therefore will require close monitoring for seroconversion and/or any evidence of HSV-related sequelae.
Study Objectives
The primary objective of this study is to assess the safety and tolerability of G207 administered intratumorally via stereotactic infusion alone or followed by a single dose of radiation within 24 h of G207 administration in children three years of age or older with recurrent or progressive malignant supratentorial brain tumors. Safety and tolerability will be assessed by adverse events and laboratory tests.
The secondary objectives are to obtain preliminary information concerning the potential efficacy of and biological response to G207 alone or combined with a single dose of radiation in pediatric patients with recurrent or progressive malignant brain tumors. This will be assessed by radiographic response, patient performance scale, progression-free and overall survival, measuring antiviral immune response and presence of G207 in blood, saliva, and conjunctiva. Additionally, the impact of this new therapy on patients' quality of life and caregiver stress will also be assessed.
Study Design
This study is a phase 1, open-label clinical trial of HSV G207 to assess its safety and tolerability in pediatric patients (3–18 years old) with recurrent or progressive supratentorial malignant brain tumors who have failed standard of care therapy: surgery, radiotherapy, and who may or may not have had chemotherapy or another form of treatment. A traditional 3 + 3 design is used (Table 1). The initial dose is 1 × 107 pfu of G207 in a volume of 2.4 mL for the first three patients (dose level 1). If two or more patients have toxicity at the starting dose level, a cohort will be added at 1 × 106 pfu (dose level −1). If two or more patients have toxicity at this dose, the study will be terminated. Three additional patients may be added at any dose level if toxicity in one patient is observed. If toxicity in a second patient is observed at any dose level, no further patients will receive that dose level, and the dose immediately below will be declared the maximum tolerated dose. There will be a minimum 28-day observation period between the first and second patient of each cohort, a 7-day observation period between patient 2 and 3 enrolled in a cohort, and a 28-day observation period from one cohort to the next to allow for evaluation of potential toxicity.
Table 1.
Dose escalation
| Dose Level | Patients | Dose (pfu) | Volume | No. of Loci |
|---|---|---|---|---|
| −1 | 3 | 1 × 106 | 2.4 mL | 1–4 |
| 1 | 3 (+3) | 1 × 107 | 2.4 mL | 1–4 |
| 2 | 3 (+3) | 1 × 108 | 2.4 mL | 1–4 |
| 3 | 3 (+3) | 1 × 107 + 5 Gy radiation | 2.4 mL | 1–4 |
| 4 | 3 (+3) | 1 × 108 + 5 Gy radiation | 2.4 mL | 1–4 |
Dose level −1 will be utilized only if needed due to toxicity at dose level 1.
pfu, plaque-forming units.
Any grade 3 or 4 nonhematologic toxicity, as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v4.0, that is possibly, probably, or likely related to G207 will be considered a dose-limiting toxicity. Based on previous clinical trial experience with G207, worsening neurologic deficits will not be considered dose-limiting if it resolves back to the patient's baseline within one week of completing treatment. For example, increased weakness or aphasia that occurs during the infusion will be treated with increasing steroid doses and/or slowing of infusion rates, but not treated as a dose-limiting toxicity if symptoms improve to baseline within 7 days of completing treatment. Patients will be followed closely for potential HSV toxicity. Medical monitors with expertise in diagnosis and treatment of herpes encephalitis will review all patient data and will be available for immediate consultation should any suspicion or concerns for infection arise.
HSV antibody titers will be measured preoperatively and at 1, 3, 5, 7, 9, and 12 months (see Table 2 for study schedule). PCR and cultures will also be performed at the same time points to check for HSV in saliva, conjunctival secretions, and blood. MRI will be performed on day 3 or 4 after treatment and at months 1, 3, 5, 7, 9, and 12. Because the virus is immunogenic and may stimulate an immune cell–related inflammatory response in the tumor and cause pseudoprogression, which has been seen in engineered HSV-1 studies in adults, the Immunotherapy Response Assessment in Neuro-Oncology (iRANO) criteria will be used for assessing tumor response.37 The appearance of enlarging or new lesions 6 months or less from the initiation of an immunotherapy alone does not define progressive disease. Confirmation of disease progression on follow-up imaging after initial radiographic progression will be determined if there is no new or substantially worsened neurological deficits that are not due to comorbid events or concurrent medication, and it is 6 months or less from receiving G207. If follow-up imaging confirms disease progression, the date of actual progression will be back-dated to the date of initial radiographic progression. Patients whose tumor shows radiographic and clinical evidence of progression will be declared a treatment failure and may be considered as candidates for any other available therapy. Nonetheless, patients who have received G207 will continue long-term follow-up even after receiving other cancer therapies.
Table 2.
Patient study schedule
| Pre | Day 0 | Day 1 | Day 2 | Day 3 or 4 | Day 7 | Day 14 | Day 28 | Month 3 | Month 5 | Month 7 | Month 9 | Month 12‡ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| History and physical | X | X | X | X | X | X | X | X | |||||
| Complete metabolic panel, magnesium and phosphorous | X | X | X | ||||||||||
| Complete blood count with differential and platelets including lymphocyte markers | X | X | X | X | X | ||||||||
| Cystatin C | X | ||||||||||||
| HIV serology | X | ||||||||||||
| HSV antibody titer | X | X | X | X | X | X | X | ||||||
| HSV detection (Saliva, conjunctival secretions, and blood)** | X | X | X | X | X | X | X | X | X | X | X | ||
| Serum pregnancy | X | ||||||||||||
| Head CT scan | X | X# | |||||||||||
| G207 administration | X | ||||||||||||
| MRI | X* | X | X | X | X | X | X | X† | |||||
| UA | X | ||||||||||||
| PT/PTT | X | ||||||||||||
| Neurological status on exam | X | X§ | X | X | X | X | X | X | X | X | X | X | X |
| Performance score | X | X | X | X | X | X | X | X | X | X | X | X | |
| Quality of life evaluation*** | X | X | X | X | X | ||||||||
| Vital signs | X | X§ | X | X | X | X | X | X | X | X | X | X | X |
Must be within 14 days of scheduled treatment
Additional samples for shedding analysis should be taken when clinical signs warrant (i.e. if patients show signs of herpes virus infection due to reactivation)
Optional consent
Only for patients who receive radiation therapy
Frequency post-operatively defined by institutional standards and medical need
Will also be done at 18 and 24 months, and yearly thereafter, as patient's health permits
All subjects will continue to receive yearly follow-up by clinic visits (or telephone call for those who cannot be seen in person) for assessment of adverse events / serious adverse events for up to 15 years after treatment with G207. Particular attention will be paid to any symptoms (neurological or otherwise) consistent with HSV infection
CT, computed tomography; HSV, herpes simplex virus; MRI, magnetic resonance imaging; PT/PTT, Prothrombin Time/Partial Thromboplastin Time; UA, urinalysis.
Dosing will start at an initial dose level of 1 × 107 pfu of G207 and will then escalate by a one log increment to a dose of 1 × 108 pfu. The rationale to start at 1 × 107 pfu, which is higher than the initial starting dose of G207 in adults but lower than the highest safe dose used (3 × 109), was based on our primary concern for safety. Children may respond differently to the virus than adults, both in terms of the oncolytic effect, based on our preclinical data suggesting that pediatric tumors may be more sensitive than adult tumors, and the immune response generated against the virus and/or tumor.36 Furthermore, responses to G207 in adults were not dose dependent, with responses seen at this starting dose and below this dose (1 × 106). If there are no unacceptable (dose-limiting) toxicities in the initial cohorts, the dose levels will be repeated in combination with a single 5 Gy dose of radiation. The decision to repeat the same two doses of G207 with a single dose of radiation was based on the previously discussed preclinical and clinical rationale to combine radiation with G207 and to monitor closely for any additional toxicity caused by the combination therapy. Subjects will be enrolled sequentially. Treatment will be escalated as outlined in Table 1.
Subject Selection and Withdrawal
Inclusion criteria
All study subjects must meet the following inclusion criteria to be included in the study.
1. Age ≥36 months and <19 years
2. Pathologically proven malignant supratentorial brain tumor (including glioblastoma multiforme, giant cell glioblastoma, anaplastic astrocytoma, PNET, atypical teratoid/rhabdoid tumor, germ cell tumor, or other high-grade malignant tumor) which is progressive or recurrent despite standard of care treatment including surgery, radiotherapy, and/or chemotherapy and have a life expectancy of ≥8 weeks
3. Lesion must be ≥1.0 cm in diameter and surgically accessible as determined by MRI
4. Patients must have fully recovered from acute treatment related toxicities of all prior chemotherapy, immunotherapy, or radiotherapy prior to entering this study
5. Myelosuppressive chemotherapy: patients must have received their last dose at least 3 weeks prior (or at least 6 weeks if nitrosurea)
6. Investigational/biologic agents: patients must have recovered from any acute toxicities potentially related to the agent and received last dose ≥7 days prior to entering this study (this period must be extended beyond the time during which adverse events are known to occur for agents with known adverse events ≥7 days)
7. Monoclonal antibodies: At least three half-lives must have elapsed prior to study entry
8. Radiation: Patients must have received their last fraction of craniospinal radiation (>24 Gy) or total body irradiation ≥3 months prior to study entry. Patients must have received focal radiation to symptomatic metastatic sites or local palliative radiation >4 weeks prior to study entry
9. Autologous bone marrow transplant: Patients must be ≥3 months posttransplant prior to study entry
10. Normal hematological, renal, and liver function (absolute neutrophil count ≥1000/mm3, platelets ≥100,000/mm3, PT Prothrombin Time or PTT (Partial Thromboplastin Time) ≤1.3 × control, creatinine ≥70 mL/min/1.73 m2, total bilirubin ≤1.5 mg/dL, transaminases ≤3 times above the upper limits of the institutional norm)
11. Patients <10 years, modified Lansky score ≥60; patients ≥10 years, Karnofsky score ≥60
12. Written informed consent in accordance with institutional and U.S. Food and Drug Administration (FDA) guidelines must be obtained from patient or legal guardian
Exclusion criteria
All patients with the following conditions will be excluded from participation in the study:
1. Any treatment outside the allowable guidelines outlined in inclusion criteria
2. Acute infection, granulocytopenia, or medical condition precluding surgery
3. Pregnant or lactating females
4. Prior history of encephalitis, multiple sclerosis, or other CNS infections
5. Tumor involvement which would require ventricular, cerebellar, or brainstem inoculation
6. Prior participant in experimental viral therapy (e.g., adenovirus, retrovirus, or herpes virus protocol)
7. Required steroid increase within 1 week prior to injection
8. Known HIV seropositivity
9. Concurrent therapy with any drug active against HSV (acyclovir, valaciclovir, penciclovir, famciclovir, ganciclovir, foscarnet, cidofovir) or any immunosuppressive drug therapy (except dexamethasone or prednisone)
Subject recruitment and screening
Up to 24 pediatric patients, ages 3 to 18 years, with recurrent/progressive malignant supratentorial brain tumors will be enrolled in this study.
Informed consent
The general principles underlying the therapeutic strategy of this trial will be explained in nonmedical, lay language to the patient and/or the patient' caretakers. Potential risks and benefits will be discussed, as well as the time involvement necessary for appropriate follow-up care. Alternative therapies for pediatric patients with recurrent/progressive malignant brain tumors will be discussed, including standard (re-resection, repeat fractionated radiotherapy, and/or chemotherapy) and experimental interventions (biological agents, gene therapy, and novel combinations and administration routes of existing agents). The patient's caretaker(s) will be provided with a summary of this information as contained in the informed consent form (see Supplementary Data; Supplementary Data are available online at www.liebertpub.com/humc) and will be given as much time as needed to make a deliberate decision concerning the involvement of their child in this trial. Additionally, patients will be provided an assent form (see Supplementary Data) asked if they assent to the therapy except in cases where assent is waived due to the child's age, maturity, or psychological state. Those patients and families who do not decide to enroll in this trial will be provided with the best standard of care available.
Study Drug
G207 is a genetically engineered altered herpes simplex virus that has been demonstrated to be aneurovirulent secondary to deletions of both copies of the γ134.5 gene and a disabling LacZ insertion at UL39. G207 is supplied by Aettis, Inc., in sterile, labeled, 1.0 mL cryovials containing 0.12 mL of G207 suspended in the storage buffer Dulbecco's phosphate-buffered saline and 10% glycerol. The vials should remain frozen at −60°C or below until use. G207 will be stored in the pediatric hematology/oncology pharmacy at Children's of Alabama prior to being injected.
Study Procedures
After informed consent has been obtained and all screening procedures completed according to the protocol (Table 2), treatment may commence. Utilizing the frameless stereotactic system, children under general anesthesia will undergo a stereotactic guided biopsy. First, a frozen section biopsy for histopathological confirmation of neoplastic cells will be taken. If tumor is not present on frozen section, patients will not undergo G207 inoculation. Following frozen section demonstration of recurrent tumor, up to four silastic catheters (PIC-030 Neuro-Infusion Catheter (Sophysa; 30 cm × 2.0 mm outer diameter, 1.00 mm inner diameter), equipped with a stainless steel stylet and a 6 French compression hub) will be passed to stereotactically predefined coordinates of enhancing tumor. The catheters will be exteriorized, primed with sterile vehicle (Dulbecco's phosphate-buffered saline +10% glycerol; Alanza, Inc.) for injection, the scalp wound(s) closed and the patient allowed to recover in the Pediatric Intensive Care Unit. The following morning, a postoperative head computed tomography (CT) scan will be obtained to confirm the location of each catheter within the tumor. If needed, and it is possible to do so at the bedside, the surgeon will adjust the position of the catheter tip by slightly withdrawing it to a more desirable location. Otherwise, malplaced catheters will not be utilized for the infusion of virus.
The total amount of G207, as defined by each patient's dose level, will be delivered in a total volume of 2.4 mL administered in up to four catheters, each attached to a separate syringe mounted in a microprocessor-controlled infusion pump for a total infusion rate of 0.4 mL/h (Table 3). The study drug syringe will be connected to the infusion tubing that has been flushed with sterile vehicle. The infusion tubing (PIT-400; Sophysa) will then be primed with 3.4 mL of the diluted G207 virus preparation in the syringe and connected to the catheter. The infusion period will begin with a 35 min flush of the sterile vehicle solution from the catheter at a rate of 0.4 mL/h/catheter. After completion of the flush the pump will be reprogramed to the appropriate rate for the study drug infusion, and the infusion will occur over 6 h. This interval was chosen based on previous stability testing to ensure no detectable loss of virus activity. If delivery at the desired rate is not possible through an individual catheter, the rate of delivery through that catheter may be slowed and the rate of delivery through the remaining catheters increased as possible to attempt to maintain total delivery rate at 0.4 mL/h. Infusion may extend up to 8 h, if needed due to catheter malfunction. Vitals signs (temperature, blood pressure, pulse, and respiratory rate) and neurologic function (Glascow Coma Scale and limited neurological exam) will be monitored every hour during the infusion. Approximately 1 h after the completion of the infusion, the catheters will be removed at the bedside. Stable patients may be transferred to be a general inpatient room at that time and will have vital signs and neurologic function monitored every 2 h for the first 24 h. Subsequently, the frequency of monitoring will be determined by the physician(s) based on the medical condition of the patient.
Table 3.
Plan for loading rate and infusion rate for HSV G207
| Number of Catheters | Initial Loading Rate per Catheter | Final Infusion Rate per Catheter |
|---|---|---|
| 1 | 0.4 cc/hour | 0.4 cc/hour |
| 2 | 0.4 cc/hour | 0.2 cc/hour |
| 3 | 0.4 cc/hour | 0.13 cc/hour |
| 4 | 0.4 cc/hour | 0.1 cc/hour |
For patients receiving radiation, a 100 SAD linear accelerator with at least 6 MV beam energy will be utilized for radiation treatment. On day 1 after receiving G207, all patients will undergo simulation and radiation treatment. A custom aquaplast immobilization device will be fabricated, and axial CT images will be collected at a slice thickness of no less than 3 mm. The preoperative MRI will be utilized for treatment planning. This study will be registered/fused to the simulation CT scan. The target volumes and prescription doses for radiation planning are shown in the Table 4 and follow The International Commission on Radiation Units and Measurements (ICRU)-62 conventions. The goal of treatment planning is coverage of the planning target volume 1 (PTV1) and 2 (PTV2) by the prescription 100% isodose line with none of the PTV1 to receive greater than 120% (6 Gy). The dose to brainstem and optic apparatus (nerves and chiasms) will be limited to 3 Gy or less unless coverage of the gross tumor volume would be compromised. The 2 mm margin for setup and registration error may be dosimetrically compromised in cases where gross tumor is very close to critical structures such as the brainstem or optic apparatus. In no case will gross disease receive less than the prescription dose. Generally, it is expected that multiple axial or nonaxial beams (5–9) will be required to generate an acceptable plan.
Table 4.
Target volume for radiation planning
| Volume | Definition | Dose |
|---|---|---|
| Gross tumor volume | Enhancing residual plus resection cavity | – |
| Clinical target volume | T2 abnormality | – |
| Planning target volume 1 | Gross tumor volume plus 2 mm | 5 Gy |
| Planning target volume 2 | Clinical target volume plus 2 mm | 3 Gy |
Statistical Plan
This study is a phase 1 standard dose-escalation study of safety and toxicity. It is anticipated that patients with several different histologic tumor types will be enrolled in this study, since the primary endpoint is safety and not efficacy. Data from the study will be presented in a descriptive manner with careful analysis of any effects that G207 may have on survival, cognitive function, or development of herpes infection. In general, descriptive statistics will be used to summarize the safety and efficacy variables collected and the baseline demographic data. Continuous variables will be summarized using mean, standard deviation, minimum, medium, and maximum. Categorical variables will be summarized using frequency counts and percentages. Additional exploratory analyses of the data on some of the secondary endpoints will be conducted as deemed appropriate.
Safety and Adverse Events Analyzed
Any serious, life-threatening, suspected or unexpected adverse event that occurs during the conduct of this study, regardless of relationship to test article or procedures, will be reported within 24 h and a written report filed within 7 days after the event to the medical monitors, the investigator's Institutional Review Board, the UAB Clinical Trials Monitoring Committee, and the FDA. Also, grade 2–3 unexpected events will be filed as soon as possible, but in no case later than 15 calendar days after determination that the event qualifies for reporting with the Institutional Review Board and FDA.
Risk–Benefit Assessment
The primary objective of the study is to provide safety and tolerability data on intratumoral injection of G207 in small cohorts of pediatric patients with malignant supratentorial brain tumors. The children's risk level for this study is 2, because the research involves greater than minimal risk but presents the prospect of direct benefit to the individual child (45 CFR 46.405). G207 has been tested on very few brain tumor patients (<40), and therefore, little is known about side effects in humans. Side effects that have been seen in patients shortly after experimental treatment with G207 include headache, nausea, drowsiness, weakness, anemia, and leukopenia. Although not reported, side effects could include more severe reactions such as viral infections with flu-like symptoms and allergic reactions to G207 ranging from itching and hives to difficulty breathing or the development of brain irritation or edema including swelling due to HSV encephalitis and/or hepatitis. If there is concern for encephalitis, a CT scan or MRI scan will be performed and the antivirus drug acyclovir started. Surgical biopsy or lumbar puncture may be necessary. There may also be unpredicted side effects, and participation in this study may not be of any direct medical benefit to the patient. If the patient responds to this therapy as seen in some adult patients, the tumor may shrink and the symptoms caused by the tumor may improve. We expect that there may be an improvement in the patient's quality of life and decrease in caregiver stress from baseline to subsequent administrations if there is a response to the treatment. Furthermore, there may be indirect benefits; information learned from this study may help patients with brain tumors in the future.
Supplementary Material
Acknowledgments
Supported in part by grants from the Food and Drug Administration Office of Orphan Products Development (R01FD005379), St. Baldrick's Foundation, the Rally Foundation for Childhood Cancer Research, Hyundai Hope on Wheels, Kaul Pediatric Research Institute, and the Department of Defense (W81XWH-15-1-0108) to G.K.F., and from the National Institutes of Health (P20CA151129 to G.Y.G. and P01CA071933 to J.M.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. FDA, the National Institutes of Health, or the Department of Defense.
Author Disclosure
Drs. Markert and Gillespie are founders of and own stock and stock options (<8% interest) in Aettis, Inc., a biotech company that has licensed M032 HSV from The Board of Trustees of the University of Alabama for the University of Alabama at Birmingham and is developing other oncolytic HSVs that are not the subject of this current investigation. Dr. Gillespie currently serves as one of five unpaid members of the Board of Directors for Aettis, Inc. Dr. Gillespie is a founder of and owns stock and stock options (<10%) in Maji Therapeutics, which is developing other HSVs that are not the subject of the current investigation. Drs. Markert and Gillespie were also founders of and owned stock and stock options (<8%) in Catherex Inc., a biotechnology company that had licensed additional intellectual property related to oncolytic HSV. Catherex, Inc., was sold to Amgen, Inc., on December 18, 2015, and they no longer participate in any decision making or have any control of any aspect of Catherex or Amgen, although they did receive proceeds from the sale of the company. Dr. Gillespie has served as a paid advisor to the Program Project at the Ohio State University that seeks to find improved methods for application of distinct oncolytic HSVs to treat localized and metastatic cancers. This is generally, but not specifically, related to the subject matter of this investigation.
References
- 1.Gatta G, Capocaccia R, Coleman MP, et al. . Childhood cancer survival in Europe and the United States. Cancer 2002;95:1767–1772 [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, de Blank PM, Kruchko C, et al. . Alex's Lemonade Stand Foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 2015;16 Suppl 10:x1–x36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen KJ, Pollack IF, Zhou T, et al. . Temozolomide in the treatment of high-grade gliomas in children: a report from the Children's Oncology Group. Neuro Oncol 2011;13:317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajjar A, Pizer B. Role of high-dose chemotherapy for recurrent medulloblastoma and other CNS primitive neuroectodermal tumors. Pediatr Blood Cancer 2010;54:649–651 [DOI] [PubMed] [Google Scholar]
- 5.Diller L, Chow EJ, Gurney JG, et al. . Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol 2009;27:2339–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribi K, Relly C, Landolt MA, et al. . Outcome of medulloblastoma in children: long-term complications and quality of life. Neuropediatrics 2005;36:357–365 [DOI] [PubMed] [Google Scholar]
- 7.Packer RJ, Sutton LN, Atkins TE, et al. . A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg 1989;70:707–713 [DOI] [PubMed] [Google Scholar]
- 8.Roizman B, Sears AE. Herpes simplex viruses and their replication. In: Fields BN, Knipe DM, Howley PM, eds., Fundamental Virology. Philadelphia: Lippincott-Raven, 1996:1043–1107 [Google Scholar]
- 9.Chou J, Kern ER, Whitley RJ, et al. . Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 1990;250:1262–1266 [DOI] [PubMed] [Google Scholar]
- 10.Chou J, Roizman B. Herpes simplex virus 1 gamma(1)34.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc Natl Acad Sci U S A 1994;91:5247–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martuza RL, Malick A, Markert JM, et al. . Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 1991;252:854–856 [DOI] [PubMed] [Google Scholar]
- 12.Mineta T, Rabkin S, Yazaki T, et al. . Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med 1995;1:938–943 [DOI] [PubMed] [Google Scholar]
- 13.Benencia F, Courreges MC, Fraser NW, et al. . Herpes virus oncolytic therapy reverses tumor immune dysfunction and facilitates tumor antigen presentation. Cancer Biol Ther 2008;7:1194–1205 [DOI] [PubMed] [Google Scholar]
- 14.Leddon J, Chen C, Currier M, et al. . Oncolytic HSV virotherapy in murine sarcomas differentially triggers an antitumor T cell response in the absence of virus permissivity. Mol Ther Oncolytics 2015;1:14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A 1997;94:843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox DR, Longnecker R. The Herpes simplex virus neurovirulence factor gamma 34.5: revealing virus-host interactions. PLoS Pathog 2016;12:e1005449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundaresan P, Hunter WD, Martuza RL, et al. . Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation in mice. J Virol 2000;74:3832–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter WD, Martuza RL, Feigenbaum F, et al. . Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J Virol 1999;73:6319–6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitley RJ, Kern ER, Chatterjee S, et al. . Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin Invest 1993;91:2837–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein DJ, Weller SK. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol 1988;62:196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coen DM, Goldstein DJ, Weller SK. Herpes simplex virus ribonucleotide reductase mutants are hypersensitive to acyclovir. Antimicrob Agents Chemother 1989;33:1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markert J, Medlock M, Rabkin S, et al. . Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther 2000;7:867–874 [DOI] [PubMed] [Google Scholar]
- 23.Markert JM, Liechty PG, Wang W, et al. . Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther 2009;17:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markert JM, Razdan SN, Kuo HC, et al. . A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther 2014;22:1048–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whisenhunt TR, Jr., Rajneesh KF, Hackney JR, et al. . Extended disease-free interval of 6 years in a recurrent glioblastoma multiforme patient treated with G207 oncolytic viral therapy. Oncolytic Virother 2015;4:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Advani SJ, Sibley GS, Song PY, et al. . Enhancement of replication of genetically engineered herpes simplex viruses by ionizing radiation: a new paradigm for destruction of therapeutically intractable tumors. Gene Ther 1998;5:160–165 [DOI] [PubMed] [Google Scholar]
- 27.Bradley JD, Kataoka Y, Advani S, et al. . Ionizing radiation improves survival in mice bearing intracranial high-grade gliomas injected with genetically modified herpes simplex virus. Clin Cancer Res 1999;5:1517–1522 [PubMed] [Google Scholar]
- 28.Stanziale SF, Petrowsky H, Joe JK, et al. . Ionizing radiation potentiates the antitumor efficacy of oncolytic herpes simplex virus G207 by upregulating ribonucleotide reductase. Surgery 2002;132:353–359 [DOI] [PubMed] [Google Scholar]
- 29.Mezhir JJ, Advani SJ, Smith KD, et al. . Ionizing radiation activates late herpes simplex virus 1 promoters via the p38 pathway in tumors treated with oncolytic viruses. Cancer Res 2005;65:9479–9484 [DOI] [PubMed] [Google Scholar]
- 30.Advani SJ, Markert JM, Sood RF, et al. . Increased oncolytic efficacy for high-grade gliomas by optimal integration of ionizing radiation into the replicative cycle of HSV-1. Gene Ther 2011;18:1098–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillory LA, Megison ML, Stewart JE, et al. . Preclinical evaluation of engineered oncolytic herpes simplex virus for the treatment of neuroblastoma. PLoS One 2013;8:e77753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman GK, Langford CP, Coleman JM, et al. . Engineered herpes simplex viruses efficiently infect and kill CD133+human glioma xenograft cells that express CD111. J Neuro Oncol 2009;95:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman GK, Moore BP, Nan L, et al. . Pediatric medulloblastoma xenografts including molecular subgroup 3 and CD133+ and CD15+ cells are sensitive to killing by oncolytic herpes simplex viruses. Neuro Oncol 2015;18:227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman GK, Beierle EA, Gillespie GY, et al. . Pediatric cancer gone viral. Part II: potential clinical application of oncolytic herpes simplex virus-1 in children. Mol Ther Oncolytics 2015;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ring E, Moore B, Nan L, Etminan T, Markert J, Gillespie GY, Friedman G. Comparison of the sensitivities of pediatric high-grade brain tumor versus adult glioblastoma xenografts to engineered oncolytic herpes simplex virotherapy. Abstracts from the 17th International Symposium on Pediatric Neuro-Oncology (ISPNO), June 12–15, 2016, Liverpool, United Kingdom. Neuro Oncol 2016;18:iii141 [Google Scholar]
- 36.Friedman GK, Gillespie GY. Cancer stem cells and pediatric solid tumors. Cancers (Basel) 2011;3:298–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okada H, Weller M, Huang R, et al. . Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 2015;16:e534–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman GK, Pressey JG, Reddy AT, et al. . Herpes simplex virus oncolytic therapy for pediatric malignancies. Mol Ther 2009;17:1125–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.