Abstracts
Background: Arm lymphedema after breast cancer surgery affects women both from physical and psychological points of view. Lymphedema leads to adipose tissue deposition. Liposuction and controlled compression therapy (CCT) reduces the lymphedema completely.
Methods and Results: Sixty female patients with arm lymphedema were followed for a 1-year period after surgery. The 36-item short-form health survey (SF-36) was used to assess health-related quality of life (HRQoL). Patients completed the SF-36 questionnaire before liposuction, and after 1, 3, 6, and 12 months. Preoperative excess arm volume was 1365 ± 73 mL. Complete reduction was achieved after 3 months and was sustained during follow-up. The adipose tissue volume removed at surgery was 1373 ± 56 mL. One month after liposuction, better scores were found in mental health. After 3 months, an increase in physical functioning, bodily pain, and vitality was detected. After 1 year, an increase was also seen for social functioning. The physical component score was higher at 3 months and thereafter, while the mental component score was improved at 3 and 12 months. Compared with SF-36 norm data for the Swedish population, only physical functioning showed lower values than the norm at baseline. After liposuction, general health, bodily pain, vitality, mental health, and social functioning showed higher values at various time points.
Conclusions: Liposuction of arm lymphedema in combination with CCT improves patients HRQoL as measured with SF-36. The treatment seems to target and improve both the physical and mental health domains.
Keywords: : SF-36, quality of life, lymphedema, liposuction, breast cancer
Introduction
Arm lymphedema remains a significant clinical problem, with an incidence of 20% in women developing the condition following treatment for breast cancer.1,2 Despite recent sentinel node dissection in breast cancer treatment, 5.6% are still affected.1 Lymphedema with a swollen and heavy limb introduces an additional burden for the patient.3–6
Traditionally, lymphedema has been viewed as a relatively unimportant and untreatable side effect of cancer treatment.7 This perception is changing and lymphedema is increasingly recognized as a significant and complex problem representing a considerable challenge to a person's health-related quality of life (HRQoL).8
Patients with a newly diagnosed lymphedema are traditionally first treated conservatively with combined decongestive therapy (CDT), which includes manual lymph drainage (MLD), bandaging, skin care, exercises, and compression garments.9,10 Several studies have shown that MLD does not contribute to the volume reduction of the lymphedema and can therefore be omitted.10–15
Chronic lymphedema contains large amounts of adipose tissue.16,17 A slow lymph flow accelerates lipogenesis and fat deposition.18 This process is enhanced by the transformation of macrophages into adipocytes.19,20
The edema reduction itself is an advantage in terms of reduced weight of the arm, but the perceived impact of treatment, liposuction, and controlled compression therapy (CCT) on HRQoL has to be investigated to evaluate the overall outcome of treatment.21 Most studies have used generic measures without any attempt to validate them in chronic edema. However, Franks et al. examined leg lymphedema using the 36-item short-form health survey (SF-36) and concluded that it appeared to be the most appropriate tool for use in patients with lymphedema of the lower limb.22
Earlier studies have demonstrated that arm lymphedema following breast cancer surgery is associated with reduced HRQoL as analyzed with questionnaires such as SF-36.23–28 Improved outcomes of CDT using SF-36 have been shown in several studies for both arm and leg lymphedema.13,22,29,30
SF-36 has not yet been used to evaluate the outcome of HRQoL parameters in breast cancer-related arm lymphedema treated with liposuction and CCT.
The aim of the present study is to test the hypothesis that liposuction improves HRQoL.
Materials and Methods
Patients
From 1999 to 2013, a consecutive cohort of 90 patients who attended our lymphedema center agreed to complete the SF-36 before and following liposuction at different time points; that is, before surgery and at 1, 3, 6, and 12 months thereafter. When responses to the surveys were analyzed, 30 patients had failed to answer one or more of the questionnaires at the different time points. This left 60 patients in the study cohort.
The lymphedema center maintains a registry of all patients who have been diagnosed with limb lymphedema and treated with liposuction and CCT. All data of patients eligible for surgery are entered into the registry at the first consultation and then at each follow-up. Data collected include, for example, type of breast cancer surgery, lymph node removal, postoperative chemotherapy, and irradiation; previous conservative treatment, pre- and postoperative bouts of erysipelas, type of compression garments, age at cancer surgery, age at lymphedema start, and at liposuction, onset (time from cancer surgery to lymphedema debut), duration (time from lymphedema start to liposuction). In addition, after oral and written instructions, each patient is asked to complete the SF-36 before surgery, and at follow-up. There is currently no gold standard for the definition of lymphedema, but a 10% difference in limb volume is a common definition.31
Patients who met the following inclusion criteria were recruited: (1) they had been diagnosed with secondary arm lymphedema following breast cancer treatment based on medical history and physical observations, (2) there was a significant excess volume ( = the difference between the volume of the affected and nonaffected arm) where the affected arm was at least 10% larger than the unaffected one, and concomitant subjective discomfort, (3) previous conservative treatment had not been able to reduce the excess volume completely, and (4) the lymphedema showed no or minimal pitting (less than 5 mm); a sign of adipose tissue hypertrophy (Fig. 1a, b).16,17 Exclusion criteria were (1) generalized disease or local ulcers and (2) any doubts about undergoing continuous CCT after liposuction.
FIG. 1.
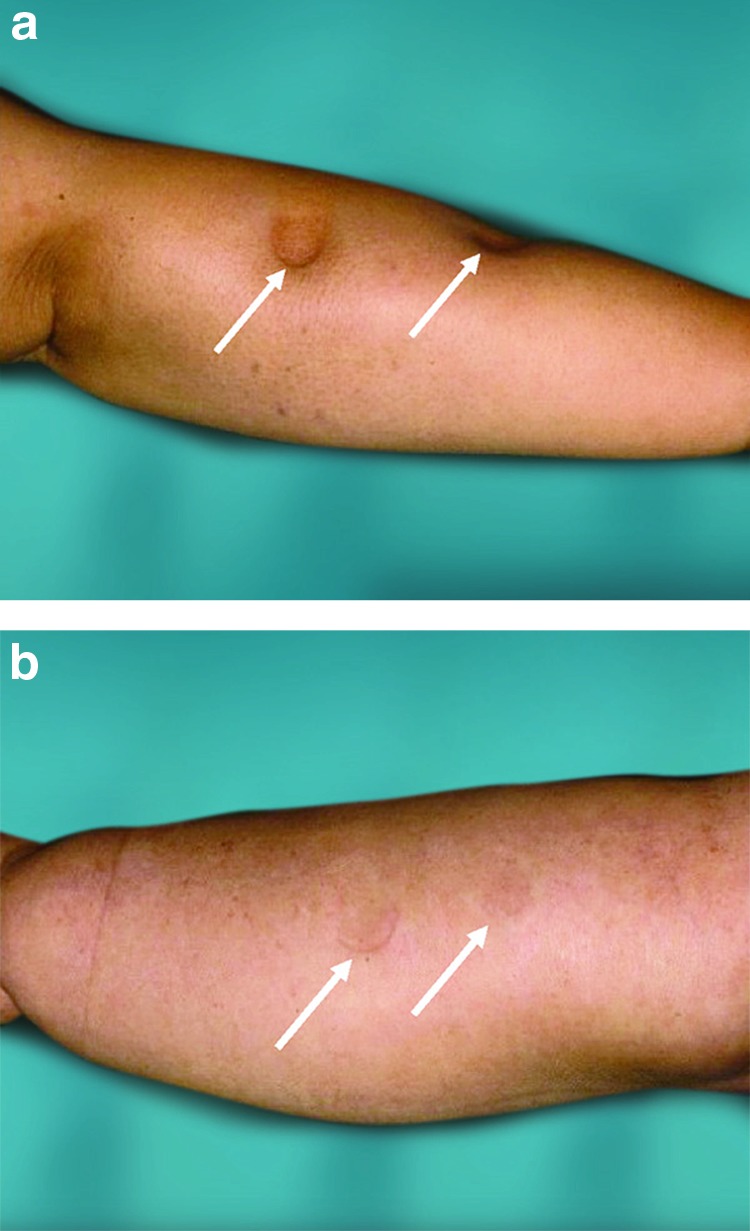
(a) Marked lymphedema of the arm after breast cancer treatment, showing pitting several centimeters (white arrows) in depth (stage I–II edema). The arm swelling is dominated by the presence of fluid, that is, the accumulation of lymph. (b) Pronounced arm lymphedema after breast cancer treatment (stage II–III edema). There is no pitting despite hard pressure by the thumb for 1 minute (white arrows). The “edema” is completely dominated by adipose tissue. The term “edema” is improper at this stage, as the swelling is dominated by hypertrophied adipose tissue and not by lymph. At this stage, the aspirate contains either no lymph or a minimal amount. Color images available online at www.liebertpub.com/lrb
The study was approved by the Ethics of Human Investigation Committee at Lund University (LU 503/2006). All participants provided their written informed consent to participate. The procedures were in accordance with the 2008 Declaration of Helsinki.
Liposuction and CCT
Our surgical technique has previously been described in detail.32,33 In brief, liposuction was performed by making around 15, 4 mm-long, incisions and the hypertrophied fat was removed by vacuum aspiration as completely as possible.
CCT was instituted with a custom-made compression sleeve-and-glove garment (JOBST–Elvarex BSN Medical, Smith & Nephew, Mölndal, Sweden) with compression in the range 32–40 mmHg (class 2). Measurements for these garments were taken 2 weeks before surgery using the healthy arm as a template. The garments were renewed three to four times during the first year to compensate for the decrease in excess volume ( = the difference between the volume of the affected and nonaffected arm). In addition, when needed, the garments were taken in at each visit using a sewing machine to compensate for loss of elasticity. The same staff, one physiotherapist and one occupational therapist, measured arm volumes before liposuction and after 1, 3, 6, and 12 months.
Arm volume measurement
Lymphedema volumes were recorded using the water displacement technique, which is considered the gold standard for volume measurements.33–35 A container with a faucet was filled with water. The whole arm was then submerged until the fingertip reached the bottom of the container. In cases of short arms, a fixed ruler was used to define the arm position. The displaced water was collected and weighed on a balance to the nearest 5 g, corresponding to 5 mL. Both arms were always measured at each visit and the difference in volume between the two was designated the edema volume.33–35 Our water displacement technique has previously been validated and showed a coefficient of variation of 0.609%.35
Volume of removed fat
Aspirate volumes were collected in graded 2000-mL canisters and the amount of removed fat was calculated with an accuracy of 10 mL.
Quality of life before and after liposuction
The SF-36 was used to assess HRQoL. This is a standardized questionnaire and is one of the most widely used measures of health related to HRQoL research. The Swedish version has been validated, and normative data for Swedish women are available.36,37 Consequently, we asked patients to complete the SF-36 questionnaires before liposuction and after 1, 3, 6, and 12 months. All questionnaires were self-administered, that is, they were completed by the patients themselves at home and then sent back to the clinic.
The SF-36 consists of 36 items constituting 8 domains: physical functioning, role limitations as a result of physical problems, bodily pain, general health perception, vitality, social functioning, role limitations due to emotional problems, and mental health. The first three domains: physical function, role physical, and bodily pain measure physical well-being; and the last three domains: social function, role emotional, and mental health relate to emotional well-being. The two remaining domains, general health and vitality, are associated with both physical and emotional dimensions. All items relate to the situation during the 4 weeks before answering the questionnaire.
These eight domains can be aggregated into two summary measures: the physical and mental component score. General health and vitality are domains shared by the physical and mental component score. In addition, the physical component score encompasses physical function, role physical, and bodily pain, whereas the mental component score includes social function, role emotional, and mental health. A high score on the subscales signifies a higher level of function and HRQoL.
Statistical analyses
To compare data from our sample and the Swedish normative data, the sample was age standardized. The normative data were based on 4582 women from the age of 35 to 75+, divided into 5-year intervals.36
Data were entered in Microsoft Excel version 14.5.3 and all statistical analyses were carried out using IBM SPSS Statistics for Mac OSX (Version 22.0; SPSS, Inc.). The Shapiro–Wilk test was used to test sample distribution, which showed that only physical functioning before surgery, general health, and vitality at 1 month after surgery showed normal distribution. In 2009, Torrance et al. stated that although scores in each of the eight domains of SF-36 rarely conform to a normal distribution SF-36 is most widely analyzed using simple parametric statistical techniques.38 We therefore used a parametric test, the Student's t-test, to analyze the outcome. Our hypothesis was that liposuction improves HRQoL over time postoperatively, and given this a priori assumption, we chose to use uncorrected p-values.
SF-36 scores and excess volume values are presented as mean and standard error of the mean (SEM). The Student's paired t-test, two tailed, was used to test changes over time. One sample t-test was used for statistical comparison of normative data. The outcome of the significance tests was considered to show exploratory results, and therefore, nominal p-values are presented without any adjustment for multiple comparisons. A p-value less than 0.05 was considered statistically significant.
Results
A total of 60 female patients with arm lymphedema participated in the study and were followed for a 1-year period. Nine missing answers on single questions were detected and were replaced with the values from the previous time point.
Characteristics of the study population
Thirty-six patients (60%) were older than 60 years. Table 1 shows age at the time of breast cancer treatment, onset (interval between breast cancer treatment and lymphedema start), duration of lymphedema (time from lymphedema start to liposuction), and duration from breast cancer treatment to liposuction.
Table 1.
Characteristics of the Study Population (Mean ± Standard Error of the Mean)
| Study patients | Nonresponding patients | |
|---|---|---|
| Number of patients | 60 | 30 |
| Age at breast cancer treatment, years | 52 ± 1.3 | 49 ± 2.0 |
| Age at liposuction, years | 64 ± 1.3 | 60 ± 2.1 |
| Onset (time from breast cancer operation to lymphedema start), years | 2 ± 0.4 | 4 ± 1.1 |
| Duration of lymphedema, years | 10 ± 1.3 | 8 ± 1.2 |
| Duration from breast cancer treatment to liposuction, years | 12 ± 1.0 | 12 ± 1.5 |
There were no significant differences between the characteristics of the study group and the group of nonresponding patients.
Volume reduction and aspirated fat
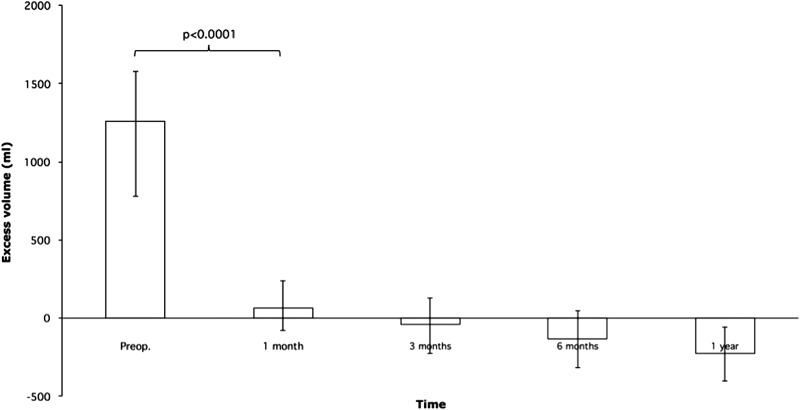
The total aspirated volume was 1361 ± 66 mL, of which the fat volume was 1373 ± 56 mL. The mean ± SEM preoperative excess volume was 1365 ± 73 mL, rapidly declining to 75 ± 35 mL at 1 month, −26 ± 40 mL at 3 months, −133 ± 40 mL at 6 months, and at 1 year −213 ± 35 mL; thus, the treated arm became somewhat smaller than the normal one (Fig. 2). Neither minor nor major complications to liposuction treatment were observed. In 49 patients (82%), the excess volume was reduced completely.
FIG. 2.
Excess volumes before and after liposuction (mean ± SEM). SEM, standard error of the mean.
Comparison between pre- and postoperative SF-36 scores within the group
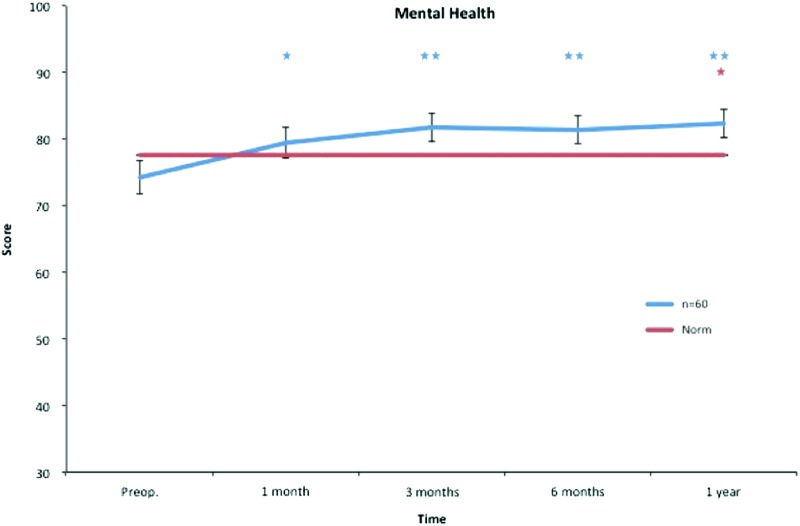
Mean and SEM for all SF-36 subscales are presented in Table 2. Already at 1 month after liposuction, significantly better values were seen in mental health that continued during the follow-up. At 3 months, physical functioning, bodily pain, and vitality showed significant improvements that continued throughout the study. At the 1-year follow-up, a significant increase was also seen for social functioning (Figs. 3–10).
Table 2.
Comparison of Pre- and Postoperative SF-36 Scores Within Each Subdomain and Its Relation to Swedish Reference Data
| Subdomain | Measurement | Preoperative | 1 month | 3 months | 6 months | 1 year |
|---|---|---|---|---|---|---|
| Physical functioning | Score | 67 (2.4) | 72 (2.4) | 76 (2.4) | 75 (2.7) | 75 (2.5) |
| p-value vs. preoperative | — | 0.06 | 0.001 | 0.001 | 0.001 | |
| Swedish reference score | 73 | 73 | 73 | 73 | 73 | |
| p-value vs. reference | 0.03 | 0.72 | 0.24 | 0.34 | 0.31 | |
| Role physical | Score | 65 (5.3) | 42 (5.5) | 61 (5.2) | 64 (5.5) | 67 (4.8) |
| p-value vs. preoperative | — | 0.001 | 0.43 | 0.79 | 0.68 | |
| Swedish reference score | 68 | 68 | 68 | 68 | 68 | |
| p-value vs. reference | 0.63 | 0.001 | 0.20 | 0.49 | 0.92 | |
| Bodily pain | Score | 65 (3.4) | 60 (2.9) | 78 (2.8) | 79 (3.2) | 79 (3.2) |
| p-value vs. preoperative | — | 0.11 | 0.001 | 0.001 | 0.001 | |
| Swedish reference score | 66 | 66 | 66 | 66 | 66 | |
| p-value vs. reference | 0.80 | 0.04 | 0.001 | 0.001 | 0.001 | |
| Social functioning | Score | 83 (3.2) | 83 (2.4) | 89 (2.5) | 87 (2.5) | 90 (2.3) |
| p-value vs. preoperative | — | 1.00 | 0.05 | 0.28 | 0.01 | |
| Swedish reference score | 84 | 84 | 84 | 84 | 84 | |
| p-value vs. reference | 0.71 | 0.63 | 0.07 | 0.40 | 0.01 | |
| Role emotional | Score | 71 (5.1) | 63 (5.5) | 75 (4.9) | 77 (4.8) | 78 (4.7) |
| p-value vs. preoperative | — | 0.24 | 0.38 | 0.25 | 0.07 | |
| Swedish reference score | 77 | 77 | 77 | 77 | 77 | |
| p-value vs. reference | 0.21 | 0.02 | 0.69 | 0.95 | 0.78 | |
| Mental health | Score | 74 (2.5) | 80 (2.3) | 82 (2.2) | 81 (2.0) | 82 (2.1) |
| p-value vs. preoperative | — | 0.02 | 0.002 | 0.01 | 0.01 | |
| Swedish reference score | 78 | 78 | 78 | 78 | 78 | |
| p-value vs. reference | 0.18 | 0.43 | 0.06 | 0.07 | 0.03 | |
| General health | Score | 68 (2.9) | 72 (2.5) | 70 (2.6) | 70 (0.35) | 69 (2.7) |
| p-value vs. preoperative | — | 0.06 | 0.37 | 0.35 | 0.80 | |
| Swedish reference score | 66 | 66 | 66 | 66 | 66 | |
| p-value vs. reference | 0.44 | 0.01 | 0.08 | 0.07 | 0.28 | |
| Vitality | Score | 66 (2.7) | 68 (2.3) | 73 (2.3) | 73 (2.3) | 72 (2.4) |
| p-value vs. preoperative | — | 0.55 | 0.02 | 0.01 | 0.03 | |
| Swedish reference score | 64 | 64 | 64 | 64 | 64 | |
| p-value vs. reference | 0.37 | 0.10 | 0.001 | 0.001 | 0.001 | |
| PCS | Score | 43 (1.3) | 41 (1.1) | 45 (1.1) | 45 (1.3) | 45 (1.2) |
| p-value vs. preoperative | — | 0.09 | 0.03 | 0.01 | 0.03 | |
| Swedish reference score | — | — | — | — | — | |
| p-value vs. reference | — | — | — | — | — | |
| MCS | Score | 49 (1.3) | 50 (1.4) | 51 (1.3) | 51 (1.1) | 52 (1.2) |
| p-value vs. preoperative | — | 0.52 | 0.04 | 0.07 | 0.01 | |
| Swedish reference score | — | — | — | — | — | |
| p-value vs. reference | — | — | — | — | — |
Swedish reference data for PCS and MCS are not available in the literature. Values are presented as mean (SEM). There are two p-values for each domain. One is for change over time within the study group for each subdomain and one is for comparison with Swedish reference data. Significant p-values (p < 0.005) are presented in bold.
MCS, mental component score; PCS, physical component score; SEM, standard error of the mean.
FIG. 3.
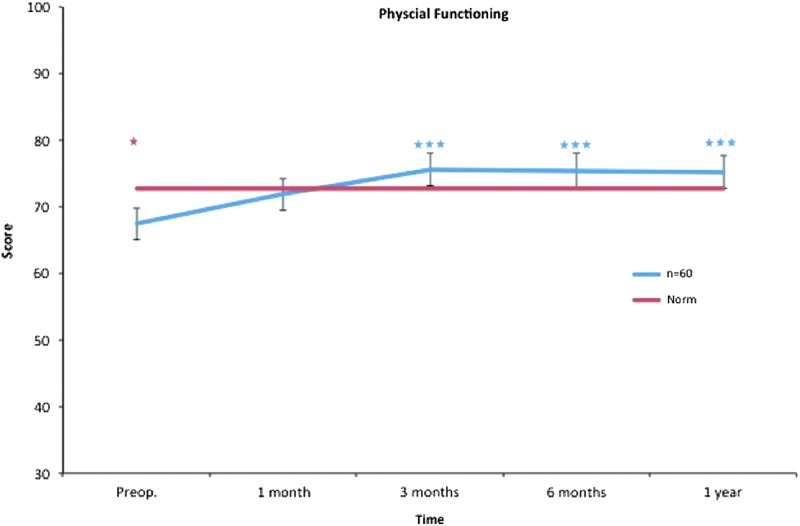
Physical functioning. All postoperative values are compared to preoperative ones. Pre- and postoperative scores within the group, and in comparison to the Swedish normative data. *p < 0.05, ***p < 0.001. Color images available online at www.liebertpub.com/lrb
FIG. 4.
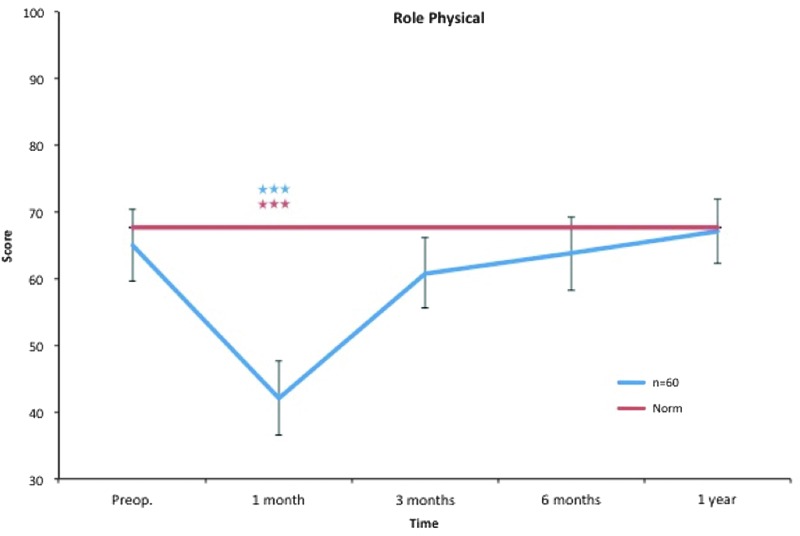
Role physical. All postoperative values are compared to preoperative ones. Pre- and postoperative scores within the group, and in comparison to the Swedish normative data. ***p < 0.001. Color images available online at www.liebertpub.com/lrb
FIG. 5.
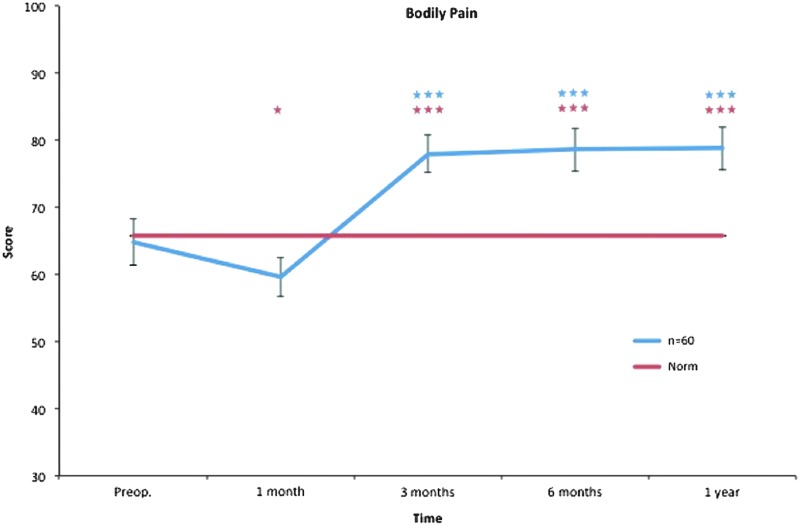
Bodily pain. All postoperative values are compared to preoperative ones. Pre- and postoperative scores within the group, and in comparison to the Swedish normative data. *p < 0.05, ***p < 0.001. Color images available online at www.liebertpub.com/lrb
FIG. 6.
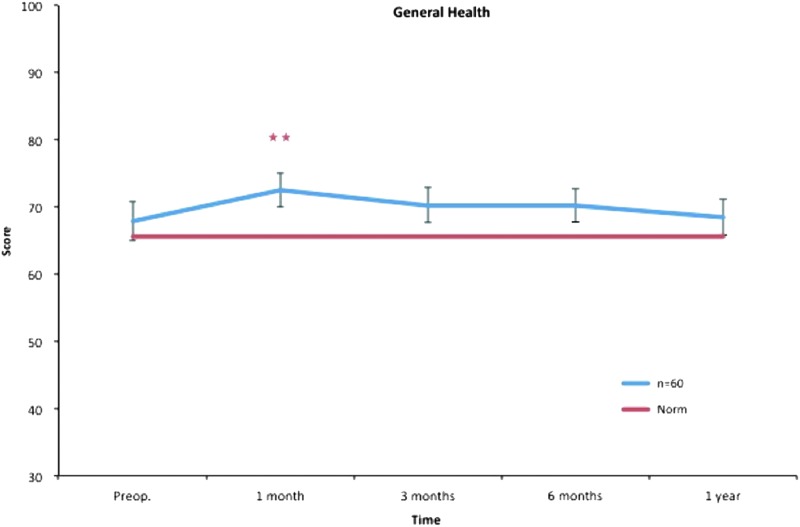
General health. All postoperative values are compared to preoperative ones. Pre- and postoperative scores within the group, and in comparison to the Swedish normative data. **p < 0.01. Color images available online at www.liebertpub.com/lrb
FIG. 7.
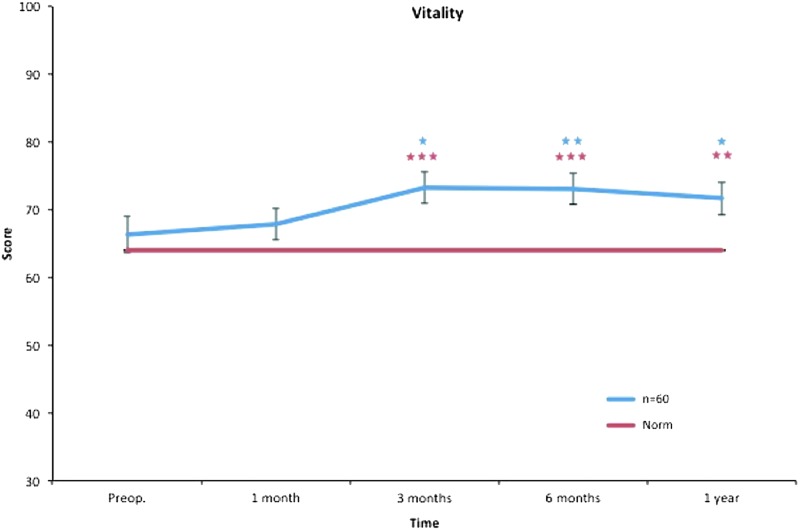
Vitality. All postoperative values are compared to preoperative ones. Pre- and postoperative scores within the group, and in comparison to the Swedish normative data. *p < 0.05, **p < 0.01, ***p < 0.001. Color images available online at www.liebertpub.com/lrb
FIG. 8.
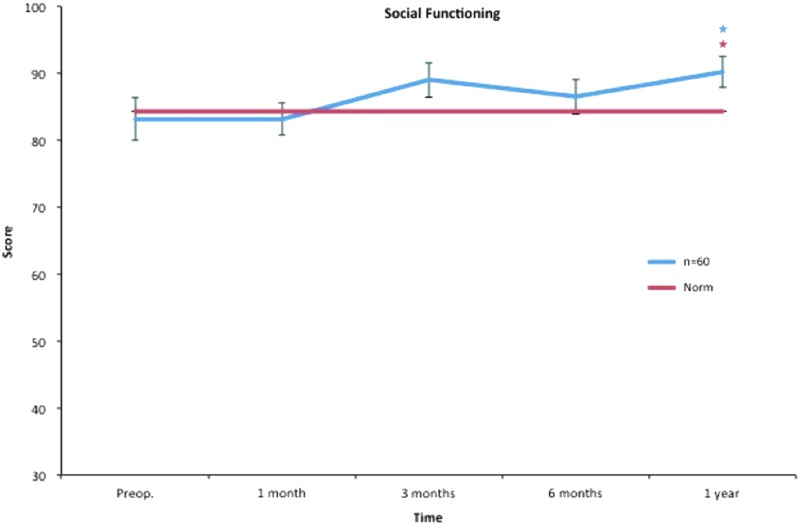
Social functioning. All postoperative values are compared to preoperative ones. Pre- and postoperative scores within the group, and in comparison to the Swedish normative data. *p < 0.05. Color images available online at www.liebertpub.com/lrb
FIG. 9.
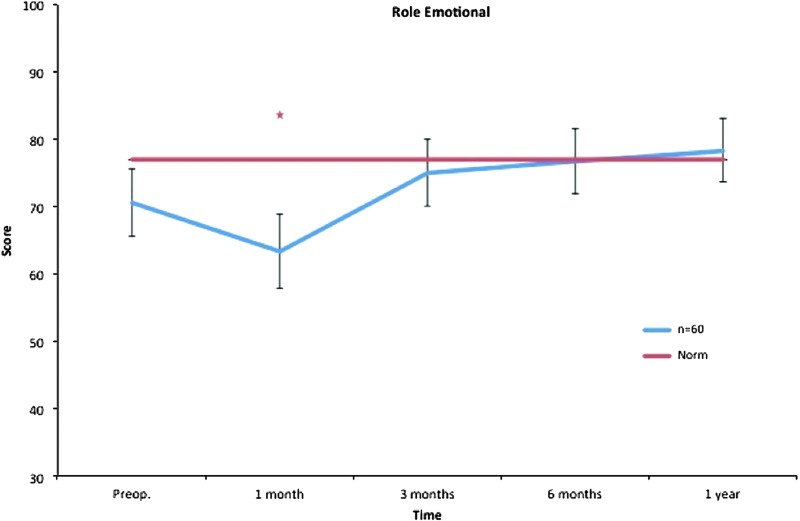
Role emotional. All postoperative values are compared to preoperative ones. Pre- and postoperative scores within the group, and in comparison to the Swedish normative data. *p < 0.05. Color images available online at www.liebertpub.com/lrb
FIG. 10.
Mental health. All postoperative values are compared to preoperative ones. Pre- and postoperative scores within the group, and in comparison to the Swedish normative data. *p < 0.05, **p < 0.01. Color images available online at www.liebertpub.com/lrb
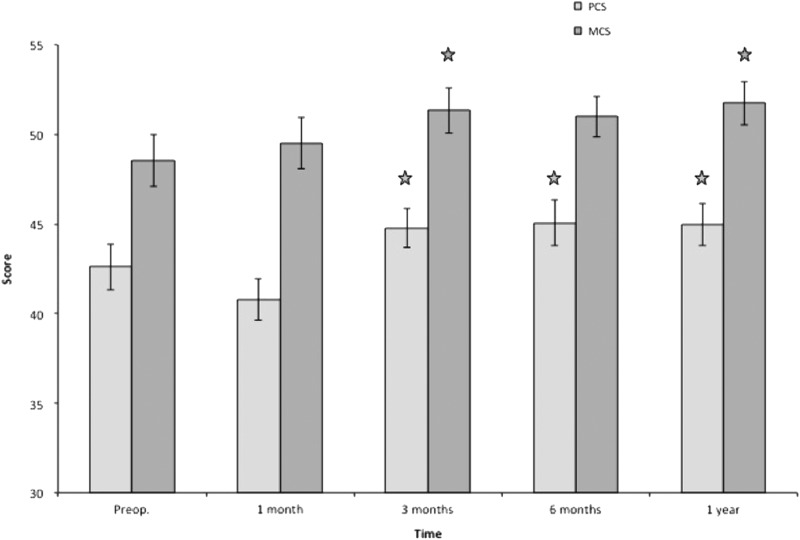
Similarly, the physical component score was significantly better at 3 months and thereafter. The mental component score was significantly improved at 3 months, and at 1 year after liposuction (Fig. 11).
FIG. 11.
All postoperative values are compared to preoperative ones. Pre- and postoperative PCS and MCS. *p < 0.05. MCS, mental component score; PCS, physical component score.
Comparison of pre- and postoperative SF-36 scores with Swedish reference population (norm)
Only physical functioning showed a significant difference at baseline, with worse values than the norm. One month after treatment, role physical, bodily pain, and role emotional showed significantly decreased scores, while general health showed significantly increased scores. Regarding bodily pain, significantly better values were seen at 3 months and thereafter, while no difference was seen in role physical, general health, and role emotional after 1 month. Vitality displayed better values at 3 months and thereafter. At the 1-year follow-up, mental health and social functioning also showed significantly better values (Figs. 3–10).
Discussion
Lymphedema is a chronic, complex, and multifaceted condition that has major physical, psychological, and social implications for the HRQoL of patients. The World Health Organization defines health as a state of complete physical, mental, and social well-being and not merely as the absence of disease or infirmity. There is no single variable that can be used to describe health. Measurement of HRQoL requires several steps and involves the evaluation of several health-related indicators. Consequently, it is essential to evaluate the reliability of health measures (i.e., how much random error is present in the measurement) and validity (i.e., whether the scores give us meaningful information about the respondent). In addition, there is no “gold standard” for HRQoL; therefore, it is a challenge to determine whether any HRQoL measure is tapping into the intended aspect of people's experience. Such generic instruments are not developed to evaluate the specific issues of importance to lymphedema patients. As an example, the SF-36 does not capture the specific symptoms such as difficulty in grasping or holding objects.39
As yet there is no standard, routinely used, and disease-specific HRQoL tool for chronic lymphedema for either research or clinical purposes.40 However, there are tools, some of which have been described and validated in the literature but not yet used in the clinical situation.40–42
In 2005, Haywood et al. conducted a structured review of 15 generic self-assessed health instruments. This review provided an extensive synthesis of evidence for 15 generic, multi-item measures of health following completion by older people (age >60). The most extensive evidence was found for the SF-36 (i.e., reliability, validity, responsiveness).43
SF-36 has been widely used in HRQoL research.44 A Swedish version has been validated and normative data for Swedish women are available.36
In the present study, we used the Swedish version of SF-36 to measure HRQoL after treatment of arm lymphedema with liposuction in combination with CCT. As in the study of Haywood et al., 60% (n = 36) of our study group (n = 60) were older than 60 years.
Characteristics of the study population
Table 1 shows the characteristics of the study group and the group of nonresponding patients. Although there is no significant difference between the groups, it shows that the onset (time from breast cancer operation to lymphedema start) in the study group and in the group of nonresponding patients is 2 and 4 years, respectively. Corresponding values for duration are 10 and 8 years, respectively. This might have had an influence on the outcomes of the study in terms of fewer symptoms in the nonresponding group compared to the studied group.
Comparison between pre- and postoperative SF-36 scores within the group
One year after liposuction, the scores were significantly improved for physical functioning, bodily pain, vitality, social functioning, and mental health (Table 2 and Figs. 3, 5, 7, 8, and 10).
The physical functioning subscale concerns problems in daily life caused by physical restraints. It includes items about everyday life activities, for example, things we normally do such as feeding ourselves, bathing, dressing, grooming, work, homemaking, and leisure. Three months after treatment, physical functioning showed improvement, suggesting that liposuction (i.e., reduction of the excess volume of the arm) has an early impact on patients' physical restraints. This improvement was noted at each time point throughout the study period (Fig. 3).
The bodily pain subscale includes items about pain levels and to what extent pain has interfered with normal activities and work. One month after surgical treatment, we found no significant improvement; instead, we found significantly decreased scores that were probably due to postoperative pain and discomfort. After 3, 6, and 12 months, bodily pain significantly improved, suggesting reduced interference of pain with normal activities and work (Fig. 5).
The vitality subscale assesses patients' fatigue, which is known to affect patients with chronic diseases. Three months after liposuction, our patients already showed a significantly higher level of alertness compared to the preoperative state, and this continued throughout the study (Fig. 7).
The social functioning and mental health subscales include items such as interference with normal social life due to physical and emotional problems and also feelings of nervousness and depression. In our study, treatment with liposuction and CCT seemed to target these psychological restraints in a positive manner. Mental health improved significantly after only 1 month and continued to do so throughout the study. Social functioning showed a statistically significant improvement after 12 months (Figs. 8 and 10).
Three scales (physical functioning, role physical, bodily pain) correlate most highly with the physical component and contribute most to the scoring of the physical component score. The mental component correlates most highly with the mental health, role emotional, and social functioning scales, which also contribute most to the scoring of the mental component score. Three of the scales (vitality, general health, and social functioning) have correlations with both components.37 In terms of validity, subscales that load highest on the physical component are most responsive to treatments that change physical morbidity, whereas scales loading highest on the mental component respond most to drugs and therapies that target mental health.
In this study, both the physical component score and mental component score improved significantly after 3 months and continued during the 12-month follow-up, with the exception of mental component score, which was not significant at 6 months (Fig. 11). Other studies have shown that patients with arm lymphedema after breast cancer treatment suffer both from a physical and psychological point of view.5,6,45–47 Therefore, it seems that liposuction followed by CCT improves these functions.
Liposuction in combination with CCT seems to have no impact on role limitations due to emotional problems. The role emotional subscale concerns problems in daily life caused by physiological restraints. It includes, for example, cutting down the amount of time spent on work or other activities. No significant changes were found for role emotional after treatment during the 12-month follow-up. This scale is work related and the outcome might be explained by the high mean age, 64 years, of the study participants, an age when many have retired.
Comparison of preoperative and postoperative HRQoL with a Swedish reference population
Only physical functioning showed impaired scores before surgery compared to the Swedish norm. This is most likely due to the heaviness of the arm. Even at 1 month after surgery, no significant difference could be detected between the study group and the Swedish norm. This finding remained during the whole follow-up.
The decrease in role physical, bodily pain, and role emotional at 1 month is probably an effect of the surgical procedure and concomitant postoperative pain, discomfort, and convalescence.
Accordingly, the study group presented better scores in vitality at 3 months than the Swedish norm, and this continued during the follow-up. At 12 months, social functioning and mental health also showed significantly higher scores than the Swedish norm (Figs. 3–10).
Arm lymphedema and treatment
Arm lymphedema is a well-known sequel of breast cancer treatment. Lymphedema eventually transforms into adipose tissue with varying elements of fibrosis,16,17,48–50 and a surgical approach to remove the excess fat is rational in a nonpitting edema.
The increased volume/weight of the affected arm leads to substantial limitations in functioning. Also, the enlarged size of the arm may prevent patients from wearing ordinary clothing. Thus, if the lymphedema is untreated or undertreated, it might interfere with the patients' HRQoL. Also, arm lymphedema after breast cancer treatment is associated with additional psychological morbidity.51
Our findings are in par with an earlier study of patients with arm lymphedema treated with liposuction, whereas the present study shows even more pronounced positive effects on psychologically oriented domains.6
There are some limitations to this study. The number of patients was relatively small (n = 60), and using a generic questionnaire such as SF-36 causes a potential bias since it has been found that disease-specific questionnaires have an advantage over more global assessments of HRQoL. Despite this, we have shown that the treatment has beneficial effects on most of the outcome parameters.
Conclusions
Liposuction of arm lymphedema combined with CCT improves patients' HRQoL when measured with SF-36. The treatment seems to target both physical and mental health domains. Compared to the Swedish reference population, the study group showed the same, or even better HRQoL when analyzing specific subscales.
Acknowledgments
We thank occupational therapist Karin Ohlin and physiotherapist Barbro Svensson at Plastic and Reconstructive Surgery, Skåne University Hospital, Malmö, Sweden, for assistance with the measurements and questionnaires. The project was supported by the Swedish Cancer Society, Stockholm, the Skåne County Council's Research and Development Foundation, and the Scientific Committee of Blekinge County Council's Research and Development Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol 2013; 14:500–515 [DOI] [PubMed] [Google Scholar]
- 2.Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: A three-year follow-up study. QJM 2005; 98:343–348 [DOI] [PubMed] [Google Scholar]
- 3.Woods M, Tobin M, Mortimer P. The psychosocial morbidity of breast cancer patients with lymphoedema. Cancer Nurs 1995; 18:467–471 [PubMed] [Google Scholar]
- 4.Carter BJ. Women's experiences of lymphedema. Oncol Nurs Forum 1997; 24:875–882 [PubMed] [Google Scholar]
- 5.Passik SD, McDonald MV. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. Cancer 1998; 83:2817–2820 [DOI] [PubMed] [Google Scholar]
- 6.Brorson H, Ohlin K, Olsson G, Langstrom G, Wiklund I, Svensson H. Quality of life following liposuction and conservative treatment of arm lymphedema. Lymphology 2006; 39:8–25 [PubMed] [Google Scholar]
- 7.Sitzia J, Harlow W. Lymphoedema 4: Research priorities in lymphoedema care. Br J Nurs 2002; 11:531–541 [DOI] [PubMed] [Google Scholar]
- 8.Farncombe M, Daniels G, Cross L. Lymphedema: The seemingly forgotten complication. J Pain Symptom Manage 1994; 9:269–276 [DOI] [PubMed] [Google Scholar]
- 9.Oremus M, Dayes I, Walker K, Raina P. Systematic review: Conservative treatments for secondary lymphedema. BMC Cancer 2012; 12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergmann A, da Costa Leite Ferreira MG, de Aguiar SS, de Almeida Dias R, de Souza Abrahao K, Paltrinieri EM, Martinez Allende RG, Andrade MF. Physiotherapy in upper limb lymphedema after breast cancer treatment: A randomized study. Lymphology 2014; 47:82–91 [PubMed] [Google Scholar]
- 11.McNeely ML, Magee DJ, Lees AW, Bagnall KM, Haykowsky M, Hanson J. The addition of manual lymph drainage to compression therapy for breast cancer related lymphedema: A randomized controlled trial. Breast Cancer Res Treat 2004; 86:95–106 [DOI] [PubMed] [Google Scholar]
- 12.Devoogdt N, Christiaens MR, Geraerts I, Truijen S, Smeets A, Leunen K, Neven P, Van Kampen M. Effect of manual lymph drainage in addition to guidelines and exercise therapy on arm lymphoedema related to breast cancer: Randomised controlled trial. BMJ 2011; 343:d5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang KH, Jeong HJ, Kim GC, Sim YJ. Clinical effectiveness of complex decongestive physiotherapy for malignant lymphedema: A pilot study. Ann Rehabil Med 2013; 37:396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dayes IS, Whelan TJ, Julian JA, Parpia S, Pritchard KI, D'Souza DP, Kligman L, Reise D, LeBlanc L, McNeely ML, Manchul L, Wiernikowski J, Levine MN. Randomized trial of decongestive lymphatic therapy for the treatment of lymphedema in women with breast cancer. J Clin Oncol 2013; 31:3758–3763 [DOI] [PubMed] [Google Scholar]
- 15.Huang TW, Tseng SH, Lin CC, Bai CH, Chen CS, Hung CS, Wu CH, Tam KW. Effects of manual lymphatic drainage on breast cancer-related lymphedema: A systematic review and meta-analysis of randomized controlled trials. World J Surg Oncol 2013; 11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brorson H, Ohlin K, Olsson G, Nilsson M. Adipose tissue dominates chronic arm lymphedema following breast cancer: An analysis using volume rendered CT images. Lymphat Res Biol 2006; 4:199–210 [DOI] [PubMed] [Google Scholar]
- 17.Brorson H, Ohlin K, Olsson G, Karlsson MK. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol 2009; 7:3–10 [DOI] [PubMed] [Google Scholar]
- 18.Larsen OA, Lassen NA, Quaade F. Blood flow through human adipose tissue determined with radioactive xenon. Acta Physiol Scand 1966; 66:337–345 [DOI] [PubMed] [Google Scholar]
- 19.Smahel J. Adipose tissue in plastic surgery. Ann Plast Surg 1986; 16:444–453 [PubMed] [Google Scholar]
- 20.Ryan TJ. Lymphatics and adipose tissue. Clin Dermatol 1995; 13:493–498 [DOI] [PubMed] [Google Scholar]
- 21.Sitzia J, Stanton AW, Badger C. A review of outcome indicators in the treatment of chronic limb oedema. Clin Rehabil 1997; 11:181–191 [DOI] [PubMed] [Google Scholar]
- 22.Franks PJ, Moffatt CJ, Doherty DC, Williams AF, Jeffs E, Mortimer PS. Assessment of health-related quality of life in patients with lymphedema of the lower limb. Wound Repair Regen 2006; 14:110–118 [DOI] [PubMed] [Google Scholar]
- 23.Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema. Am J Surg 1999; 177:184–187; discussion 188 [DOI] [PubMed] [Google Scholar]
- 24.Pereira de Godoy JM, Braile DM, de Fatima Godoy M, Longo O., Jr. Quality of life and peripheral lymphedema. Lymphology 2002; 35:72–75 [PubMed] [Google Scholar]
- 25.Oliveri JM, Day JM, Alfano CM, Herndon JE. 2nd, Katz ML, Bittoni MA, Donohue K, Paskett ED. Arm/hand swelling and perceived functioning among breast cancer survivors 12 years post-diagnosis: CALGB 79804. J Cancer Surviv 2008; 2:233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nesvold IL, Fossa SD, Holm I, Naume B, Dahl AA. Arm/shoulder problems in breast cancer survivors are associated with reduced health and poorer physical quality of life. Acta Oncol 2010; 49:347–353 [DOI] [PubMed] [Google Scholar]
- 27.Nesvold IL, Reinertsen KV, Fossa SD, Dahl AA. The relation between arm/shoulder problems and quality of life in breast cancer survivors: A cross-sectional and longitudinal study. J Cancer Surviv 2011; 5:62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sackey H, Johansson H, Sandelin K, Liljegren G, MacLean G, Frisell J, Brandberg Y. Self-perceived, but not objective lymphoedema is associated with decreased long-term health-related quality of life after breast cancer surgery. Eur J Surg Oncol 2015; 41:577–584 [DOI] [PubMed] [Google Scholar]
- 29.Oldenburg CS, Boll D, Nicolaije KA, Vos MC, Pijnenborg JM, Coebergh JW, Beijer S, van de Poll-Franse LV, Ezendam NP. The relationship of body mass index with quality of life among endometrial cancer survivors: A study from the population-based PROFILES registry. Gynecol Oncol 2013; 129:216–221 [DOI] [PubMed] [Google Scholar]
- 30.Noh S, Hwang JH, Yoon TH, Chang HJ, Chu IH, Kim JH. Limb differences in the therapeutic effects of complex decongestive therapy on edema, quality of life, and satisfaction in lymphedema patients. Ann Rehabil Med 2015; 39:347–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol 2005; 3:208–217 [DOI] [PubMed] [Google Scholar]
- 32.Brorson H, Svensson H. Complete reduction of lymphoedema of the arm by liposuction after breast cancer. Scand J Plast Reconstr Surg Hand Surg 1997; 31:137–143 [DOI] [PubMed] [Google Scholar]
- 33.Brorson H, Svensson H. Liposuction combined with controlled compression therapy reduces arm lymphedema more effectively than controlled compression therapy alone. Plast Reconstr Surg 1998; 102:1058–1067; discussion 1068 [PubMed] [Google Scholar]
- 34.Brorson H, Svensson H, Norrgren K, Thorsson O. Liposuction reduces arm lymphedema without significantly altering the already impaired lymph transport. Lymphology 1998; 31:156–172 [PubMed] [Google Scholar]
- 35.Brorson H. Liposuction normalizes—in contrast to other therapies—lymphedema-induced adipose tissue hypertrophy. Handchir Mikrochir Plast Chir 2012; 44:348–354 [DOI] [PubMed] [Google Scholar]
- 36.Sullivan M, Karlsson J, Ware JE., Jr. The Swedish SF-36 Health Survey—I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med 1995; 41:1349–1358 [DOI] [PubMed] [Google Scholar]
- 37.Gandek B, Ware JE, Jr., Aaronson NK, Alonso J, Apolone G, Bjorner J, Brazier J, Bullinger M, Fukuhara S, Kaasa S, Leplege A, Sullivan M. Tests of data quality, scaling assumptions, and reliability of the SF-36 in eleven countries: Results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998; 51:1149–1158 [DOI] [PubMed] [Google Scholar]
- 38.Torrance N, Smith BH, Lee AJ, Aucott L, Cardy A, Bennett MI. Analysing the SF-36 in population-based research. A comparison of methods of statistical approaches using chronic pain as an example. J Eval Clin Pract 2009; 15:328–334 [DOI] [PubMed] [Google Scholar]
- 39.Pusic AL, Cemal Y, Albornoz C, Klassen A, Cano S, Sulimanoff I, Hernandez M, Massey M, Cordeiro P, Morrow M, Mehrara B. Quality of life among breast cancer patients with lymphedema: A systematic review of patient-reported outcome instruments and outcomes. J Cancer Surviv 2013; 7:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keeley V. Quality of life assessment tools in chronic oedema. Br J Community Nurs 2008; 13:S22–S27 [DOI] [PubMed] [Google Scholar]
- 41.Klernas P, Johnsson A, Horstmann V, Kristjanson LJ, Johansson K. Lymphedema Quality of Life Inventory (LyQLI)-development and investigation of validity and reliability. Qual Life Res 2015; 24:427–439 [DOI] [PubMed] [Google Scholar]
- 42.Klernas P, Kristjanson LJ, Johansson K. Assessment of quality of life in lymphedema patients: Validity and reliability of the Swedish version of the Lymphedema Quality of Life Inventory (LQOLI). Lymphology 2010; 43:135–145 [PubMed] [Google Scholar]
- 43.Haywood KL, Garratt AM, Fitzpatrick R. Quality of life in older people: A structured review of generic self-assessed health instruments. Qual Life Res 2005; 14:1651–1668 [DOI] [PubMed] [Google Scholar]
- 44.Garratt A, Schmidt L, Mackintosh A, Fitzpatrick R. Quality of life measurement: Bibliographic study of patient assessed health outcome measures. BMJ 2002; 324:1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piller NB, Thelander A. Treatment of chronic postmastectomy lymphedema with low level laser therapy: A 2.5 year follow-up. Lymphology 1998; 31:74–86 [PubMed] [Google Scholar]
- 46.Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer 2005; 13:904–911 [DOI] [PubMed] [Google Scholar]
- 47.Dominick SA, Natarajan L, Pierce JP, Madanat H, Madlensky L. The psychosocial impact of lymphedema-related distress among breast cancer survivors in the WHEL Study. Psychooncology 2014; 23:1049–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borley NR, Mortensen NJ, Jewell DP, Warren BF. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn's disease: Evidence for a possible causative link. J Pathol 2000; 190:196–202 [DOI] [PubMed] [Google Scholar]
- 49.Sadler D, Mattacks CA, Pond CM. Changes in adipocytes and dendritic cells in lymph node containing adipose depots during and after many weeks of mild inflammation. J Anat 2005; 207:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pond CM. Adipose tissue and the immune system. Prostaglandins Leukot Essent Fatty Acids 2005; 73:17–30 [DOI] [PubMed] [Google Scholar]
- 51.Tobin MB, Lacey HJ, Meyer L, Mortimer PS. The psychological morbidity of breast cancer-related arm swelling. Psychological morbidity of lymphoedema. Cancer 1993; 72:3248–3252 [DOI] [PubMed] [Google Scholar]