Abstract
Identification and complete resection of colorectal polyps provide a significant mortality benefit from colorectal cancer. With improvements in colonoscopic techniques and advanced endoscopic imaging techniques, polyp detection has taken on greater complexity since the establishment of bowel cancer screening programmes internationally. All endoscopists operating within symptomatic and screening populations should be aware of endoscopic features associated with advanced neoplasia. Chromoendoscopy and advanced imaging techniques, such as narrow spectrum technologies (narrow band imaging, flexible spectral imaging colour enhancement (FICE) and i-Scan digital contrast (iSCAN)), have specific classification systems to support accurate lesion characterisation. This review summarises the evidence in relation to polyp detection, recognition and characterisation as well as the identification of features of invasion. Future areas of interest include optimal management of large polyps, incorporation of a ‘detect, resect and discard’ strategy for small and diminutive polyps, expected wider use of computer decision support tools (artificial intelligence and deep learning) and the use of fluorescently labelled molecular probes to improve detection and assessment of neoplasia.
Keywords: ADENOMA, COLORECTAL CANCER, COLONOSCOPY, POLYP
Background
The detection of colorectal polyps has become an area of intense interest since the original description of adenoma–carcinoma sequence and further strengthened by robust large-scale trial data supporting the role for colonoscopy and resection of adenomatous polyps as being associated with reduced colorectal cancer-related mortality.1 As endoscopic technology advances, our understanding of appropriate colonoscopic surveillance and identification and management of specific polyp subtypes that may place individuals at higher risk of developing cancer has become clearer. In the era of national bowel cancer screening, we are detecting increasing numbers of polyps and those polyps that we are detecting are increasingly complex, requiring specialised methods for their characterisation and management.
Polyp detection
There are now clear epidemiological data from multiple large studies linking the adenoma detection rate (ADR; the percentage of colonoscopies where at least one adenoma is detected) with outcomes such as interval colorectal cancer incidence and interval cancer mortality.2 Adenoma detection is influenced by many factors, including patient factors such as age, gender and bowel preparation and endoscopist factors such as experience, withdrawal time, time of day, use of antispasmodics, adequate luminal distension and rectal retroflexion. Specific technologies such as high-definition endoscopes and chromoendoscopy and devices such as cap attachments can also influence ADR.
Adequate bowel preparation is a prerequisite for acceptable polyp detection, particularly for right-sided, smaller and flat polyps. The British Society of Gastroenterology (BSG) guidance on bowel cleansing considers sodium phosphate preparations and polyethylene glycol (PEG)-based preparations to be equivalent, however they do recognise that most studies show a superior cleansing effect in the proximal colon with PEG-based preparations.3 The importance of ‘split dose’ preparation in achieving high-quality bowel preparation is emphasised in the 2014 US Multi-society Task Force on Colorectal Cancer guidelines and is now the standard of care, demonstrating improved adenoma detection in randomised trials.4
Increased lesion recognition may be achieved with the use an antispasmodic such as hyoscine butylbromide (Buscopan; Boehringer Ingelheim, Bracknell, UK) prior to scope withdrawal, dynamic position changes (luminal distension), and adherence to key performance indicators such as a 6 min withdrawal time and retroflexion in the rectum. Combining these four factors in an ‘evidence bundle’ and supporting endoscopists and units to make these practice changes improve adenoma detection, particularly for the lowest detectors.5 Within specific higher risk groups such as inflammatory bowel disease (IBD) and Lynch syndrome, chromoendoscopy has been shown to result in improved lesion recognition.6 7
Polyp characterisation
Polyps have been classically defined as pedunculated or sessile, with ‘flat’ or non-polypoid lesions only recently widely appreciated by Western endoscopists. In research studies, characterisation is usually done with the Paris classification for gastrointestinal polyps, and the National Health Service bowel cancer screening programme has recently adopted this for clinical use.8 However, there are doubts about its reproducibility, especially for diminutive polyps where interobserver kappa values were 0.27 (fair) even among experts.9 Invasive potential can be estimated based on morphology. Polypoid lesions (Paris 0-I), sessile or pedunculated polyps (>10 mm), have a low invasive potential (7%), whereas as those with a depressed component (either Paris IIc or IIa+c) have a 31% risk of submucosal invasion.10 Type III (excavated) lesions are invasive and should be biopsied and referred for multidisciplinary team (MDT) discussion with surgical management unless comorbidity precludes this approach.
The Kudo pit pattern with narrow band imaging (NBI) has high sensitivity and specificity for distinguishing adenomas (type III/IV) from hyperplastic polyps (type I/II), particularly when used by experts. Even polyps of <10 mm have a malignant potential and this can be largely determined by a careful endoscopic evaluation. The Kudo type V pit pattern is associated with a 56% rate of submucosal invasion, compared with 5% for type III/IV and 0% for type I/II.11
The Sano classification uses blood vessel irregularity and capillary patterns to predict invasive potential and considers meshed (type I) or branched–around-crypts (type II) vessels to indicate hyperplastic and adenomatous histopathology, respectively, while irregular, blind-ending, branched or absent vessel pattern (type III) indicates submucosal invasion (table 1).
Table 1.
Pit pattern, microvessel pattern and surface pattern classifications
| Classification | Hyperplastic | SSP | Dysplasia | Early invasion (SM1) | Deep invasion (SM2/3) |
|---|---|---|---|---|---|
| Kudo | Asteroid or star shaped-pit (type II) | Asteroid or star shaped-pit (type II) or type II-O (open shape) | Tubular or round pit either smaller (IIIs) or larger (IIIL) than regular pits. Gyrus/branched type pits (IV) | Irregular aggregation of type IIIs, IIIL and IV pits (Vi) | Loss of pit pattern, amorphous (Vn) non-structural |
| Sano | Meshed capillary vessels invisible with NBI (type I) | – | Broader meshed capillary vessels surround mucosal glands (type II) | Broad irregular vessels, unevenly sized and branching (type IIIa) | Avascular appearance due to desmoplastic change in stroma (type IIIb) |
| NICE NBI | Type I | Type II | Type III | ||
| Colour (vs background) | Same or lighter | – | Brown | – | Brown to dark brown |
| Vessels | None or isolated lacy | – | Brown vessels surround (white) pits | – | Disrupted vessels |
| Surface | Dark or white uniform spots | – | Tubular or branched | – | Amorphous or absent pattern |
WASP
|
<2 features present | ≥2 features present | <2 features present | – | – |
| JNET | Type I | Type II | Type IIa | Type IIb | Type III |
| Vessel pattern | Invisible | Invisible | Regular calibre Meshed pattern |
Variable calibre Irregular distribution |
Loose vessel areas Interruption of thick vessels |
| Surface pattern | Regular white or dark spots, similar to surrounding mucosa | Regular white or dark spots, similar to surrounding mucosa | Tubular, branched or papillary | Irregular or obscure | Amorphous areas |
JNET, Japan NBI Expert Team; NBI, narrow band imaging; NICE, narrow band imaging international colorectal endoscopic; SM, submucosal invasive cancer; SSP, sessile serrated polyp; WASP, workgroup serrated polyps and polyposis.
Narrow band imaging international colorectal endoscopic (NICE) classification12 is a consensus-derived, validated classification system based on colour change, vessel thickness and surface pattern and divides polyps into type I (hyperplastic), type II (adenoma), and was extended to include type III (deep submucosal invasion or cancer). Further to this, the Japan NBI Expert Team (JNET) classification subdivides into type IIa (adenoma) or type IIb (high-grade shallow submucosal invasive cancers, SM1). This latest classification system, which attempts to unify aspects of all of the others (and includes Hiroshima, Showa and Jikei classifications, not mentioned in this article), has been recognised by the World Endoscopy Organization but is limited by its requirement for optical zoom magnification and lack of validation outside of expert Japanese endoscopists to date (table 1).13
For serrated polyps, a modification of the Kudo pit pattern classification has been proposed with type II-O (open) pits proposed to indicate sessile serrated polyps (SSP).14 The Dutch ‘Workgroup serrAted polypS and Polyposis’ (WASP) classification15 combines the NICE classification (types I and II) and four typical sessile serrated features, that is, clouded surface, indistinct border, irregular shape and dark spots inside the crypts. This classification appears to have good accuracy (in diminutive and small polyps) both for differentiating hyperplastic and serrated lesions from adenomas and for identifying neoplasia. Further predictors of neoplasia identified in a recent retrospective series of serrated polyps included (semi) pedunculated morphology, double elevation, central depression and reddishness, along with a pit pattern analysis by the Kudo classification.16 The features of these classifications and how they interact are summarised in table 1.
Specific descriptive features for inflammatory or postinflammatory polyps (PIPs) in patients with IBD have not been well studied and the application of advanced digital imaging technology (NBI) has not been shown to be helpful, although chromoendoscopy is suggested to improve accuracy. The characteristic features of PIPs include the presence of a fibrinous cap, surface friability, ulceration, an appendage-like or filiform appearance and a halo sign on application of dye.
The classification systems mentioned above that use pit pattern or microvessel patterns to determine risk can be complementary to one another and it is suggested that they be used in combination, depending on the information required (table 1). To determine the invasive potential of a polyp before embarking upon attempted resection, a combination of the Kudo and Sano classifications (in conjunction with other factors) has been suggested (box 1),10 while to differentiate adenomas, hyperplastic polyps and serrated polyps from one another, a combination of the NICE and WASP classifications has been suggested.12
Box 1. Polyp features that indicate higher risk of malignant invasion (adapted from Rutter et al10).
Kudo type V pit pattern (irregular or loss of pit pattern)
Paris 0-IIc or 0-IIa+c morphology (depressed component)
Non-granular-type laterally spreading polyp (LST-NG ‘flat or smooth’)
Granular-type LST (G-LST) with a dominant nodule (≥10 mm in size)
Distorted surface pattern, colour and vessels (narrow band imaging international colorectal endoscopic classification type III)
Thick and irregular microvessels (Sano capillary pattern type III)
Classification systems for FICE and iSCAN exist and show good accuracy for optical diagnosis of polyps, but these are not necessarily interchangeable with those derived for use with NBI, such as NICE and JNET.
For pedunculated polyps (Paris, 0-Ip), careful assessment of the polyps by one or more of the classification systems mentioned above is recommended. Invasive characteristics should favour a lower resection point on the stalk, that is, closer to the mucosal surface and further from the polyp head, to maximise the chances of a clear margin and to facilitate the pathologist in being able to make an assessment using the Haggitt classification.17
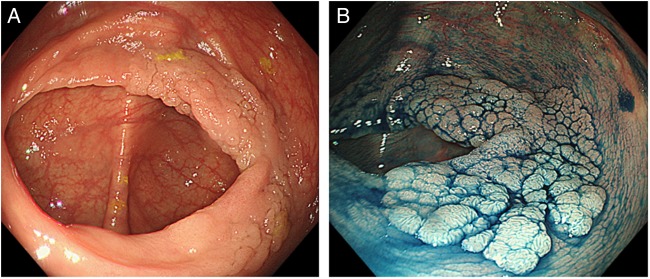
Laterally spreading tumours (LSTs, figure 1) are those with a horizontal growth pattern around the circumference of the bowel wall, and are greater than 10 mm and can be divided into granular (G) and non-granular (NG) subtypes. Further distinction should be made to identify granular-type LST (LST-G) where a dominant nodule is present. LST-NG and LST-G with a dominant nodule >10 mm have a high risk of invasion (30%) compared with a LST-G without a nodule (<5%). Depressed (Paris 0-IIc or 0-IIa+c) lesions can carry as high as 60%–75% risk of deep submucosal invasion.10 18 Key points in the identification and initial endoscopic management of these larger lesions are: a photograph or video on discovery (after washing), estimation of size (ideally against an open snare), characterisation by the Paris and Kudo/NICE NBI classifications and biopsy only where there is concern for invasion (targeted biopsies from the most suspicious area) (box 1).
Figure 1.
Forty-millimetre granular-type laterally spreading tumour in the caecum from a patient referred after diagnostic colonoscopy at another centre. (A) High-definition (CF-H290DL, Olympus, Tokyo, Japan) white-light image. (B) After dye spray with indigo carmine chromoendoscopy. This lesion was resected with piecemeal endoscopic mucosal resection (EMR) and histology showed a tubulovillous adenoma with low-grade dysplasia. Images were provided by Dr Malcolm Tan, Translational Gastroenterology Unit, Oxford/Changi General Hospital, Singapore.
A broad approach to assess the difficulty of colorectal polyp resection has been recommended by the consensus guidelines.10 Lesion assessment should include four components, that is, size, morphology, site and access, and polyps are thus assigned points for each component and assigned a level of difficulty (I–IV) based on a cumulative points total. Level I and II polyps should be within the capability of all fully trained independent colonoscopists, while those participating in bowel cancer screening should be comfortable with level III polyps. A specialist referral or surgery should be considered for level IV polyps. A size >4 cm, right-sided/caecal location and endoscopist inexperience are factors that are suggested to be associated with higher risk for adverse outcomes. Location including involving the appendix, ileocaecal valve or adjacent to the dentate line, as well as polyps within a segment of previous colitis or previous attempt at resection, are all factors associated with higher risk for an incomplete resection. A specialist referral should be considered in these circumstances, along with a careful discussion at complex polyp MDT meetings and informed consent of the patient including alternative management and subsequent requirements for follow-up. Polyps that have not been biopsied or partly resected (scarred) with a non-lifting sign after an accurate submucosal injection19 should be considered to have a high risk of deep submucosal invasion and these lesions should be assessed by an experienced colonoscopist to assess endoscopic resectability, combining other factors that may indicate invasive risk. The challenges in and importance of correct decision making for suspicious polyps and early colorectal cancer have recently been highlighted in a national series of ongoing development lectures and case presentations that aim to promote multidisciplinary approaches to make sure patients receive optimal care, with regional or even supraregional referral if necessary (http://www.pelicancancer.org/specc). Current variations in surgical referral rates for large polyps, which are sevenfold in the bowel cancer screening programme in the North of England, are not acceptable.
Recent developments
A device-assisted colonoscopy (cap-assisted colonoscopy, Endocuff, EndoRings) may confer a benefit in terms of increased adenoma detection but early studies have shown mixed results and the benefit in terms of adenoma detection remains unclear.
New-generation endoscopes with bright illumination, high definition, surface/edge enhancement, optical and digital zoom magnification, and wider field of visualisation (170° or more) endoscopes and endoscopy systems, may further improve white-light detection rates. The European Society for Gastrointestinal Endoscopy (ESGE) guidelines suggest the use of high-definition endoscopes for the detection of colorectal neoplasia in average-risk populations.12
An expert group of gastroenterologists, interventional endoscopists, pathologists and colorectal surgeons recently published guidance on the management of large non-pedunculated colorectal polyps (LNPCPs).10 In this guideline, a structured approach to polyp recognition and further management is suggested, along with evidence-based recommendations for the identification of technically challenging and high-risk lesions both in terms of identification of markers of deep or submucosal invasion (box 1) and polyps with high risk or procedure-related complications, best managed by expert colonoscopists. The approach described above is largely in concordance with these guidelines.
Future potential
Evidence suggests that there is good correlation between optical diagnosis (using the NICE criteria) for small and diminutive polyps and pathology, especially where the optical diagnosis is made with high confidence. This is true for colonoscopy carried out by experts in academic settings but more recent evidence suggests that the correlation is significantly less strong when applied in the general hospital or non-expert setting, even after a defined period of training.12 The National Institute for Health and Care Excellence is considering the clinical efficacy and cost effectiveness of virtual chromoendoscopy for this application and is expected to deliver a final report in May 2017 (https://www.nice.org.uk/guidance/indevelopment/gid-dg10004).
Computer-aided diagnosis systems have developed rapidly in the past few years, especially since the advent of ‘deep learning’ methods.12 20 Such a technology is already in use within radiology to support clinical decision making and it is envisaged that similar applications within gastrointestinal endoscopy will be available shortly. A key criterion not yet fully overcome will be the ability of such systems to detect and characterise lesions, where current models are unable to define the borders of a specific lesion of interest, though this seems to be less problematic with deep learning-based approaches (https://vimeo.com/185052677). Currently described accuracy levels are approaching those which are considered to be required to support community-based endoscopists to adopt a ‘resect and discard’ strategy for diminutive colorectal polyps, perhaps as a ‘second reader’.
Molecular imaging using fluorescently labelled molecular probes such as lectins has shown promise in the detection of Barrett's dysplasia in the oesophagus. Lectins and other molecular probes, for example, c-Met, may have utility in the identification and characterisation of colonic neoplasia with detection using fluorescence-enabled endoscopes for wide-field ‘red flag’ detection.21
These technologies may be of particular benefit in situations where detection and characterisation are challenging, for example, IBD-associated dysplasia.
The ESGE guidelines on advanced endoscopic imaging techniques and the BSG-Association of Coloproctology of Great Britain and Ireland (ACPGBI) guidelines on LNPCPs identify areas where evidence is lacking in relation to lesion recognition and management. Both guidelines suggest several areas where we can improve our knowledge as a community, including training in lesion recognition. By use of virtual and standard chromoendoscopy in selected settings and by validation of competency measures/key performance indicators for polyp recognition and resection, we can achieve improved preresection identification of malignant features and enhance polyp management in general endoscopy. A correct diagnosis of invasiveness risk by the endoscopist first encountering a large polyp is the key step to making correct management decisions.
Footnotes
Contributors: CL designed and drafted the original manuscript and reviewed and updated subsequent and final drafts for important intellectual content. JEE designed and reviewed the original manuscript and was responsible for updating subsequent drafts and for important intellectual content of the manuscript.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33. doi:10.1016/S0140-6736(10)60551-X [DOI] [PubMed] [Google Scholar]
- 2.Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:2541 doi:10.1056/NEJMc1405329 [DOI] [PubMed] [Google Scholar]
- 3.Connor A, Tolan D, Hughes S, et al. Consensus guidelines for the safe prescription and administration of oral bowel-cleansing agents. Gut 2012;61:1525–32. doi:10.1136/gutjnl-2011-300861 [DOI] [PubMed] [Google Scholar]
- 4.Radaelli F, Paggi S, Hassan C, et al. Split-dose preparation for colonoscopy increases adenoma detection rate: a randomised controlled trial in an organised screening programme. Gut 2015;66:270–7. doi:10.1136/gutjnl-2015-310685 [DOI] [PubMed] [Google Scholar]
- 5.Rajasekhar PT, Rees CJ, Bramble MG, et al. A multicenter pragmatic study of an evidence-based intervention to improve adenoma detection: the Quality Improvement in Colonoscopy (QIC) study. Endoscopy 2015;47:217–24. doi:10.1055/s-0034-1391563 [DOI] [PubMed] [Google Scholar]
- 6.Kamiński MF, Hassan C, Bisschops R, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2014;46:435–49. doi:10.1055/s-0034-1365348 [DOI] [PubMed] [Google Scholar]
- 7.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc 2015;81:489–501.e26. doi:10.1016/j.gie.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 8.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3–43. [DOI] [PubMed] [Google Scholar]
- 9.van Doorn SC, Hazewinkel Y, East JE, et al. Polyp morphology: an interobserver evaluation for the Paris classification among international experts. Am J Gastroenterol 2015;110:180–7. doi:10.1038/ajg.2014.326 [DOI] [PubMed] [Google Scholar]
- 10.Rutter MD, Chattree A, Barbour JA, et al. British Society of Gastroenterology/Association of Coloproctologists of Great Britain and Ireland guidelines for the management of large non-pedunculated colorectal polyps. Gut 2015;64:1847–73. doi:10.1136/gutjnl-2015-309576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 2011;140:1909–18. doi:10.1053/j.gastro.2011.02.062 [DOI] [PubMed] [Google Scholar]
- 12.East JE, Vleugels JL, Roelandt P, et al. Advanced endoscopic imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review. Endoscopy 2016;48(11):1029–45. doi:10.1055/s-0042-118087 [DOI] [PubMed] [Google Scholar]
- 13.Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc 2016;28:526–33. doi:10.1111/den.12644 [DOI] [PubMed] [Google Scholar]
- 14.Kimura T, Yamamoto E, Yamano HO, et al. A novel pit pattern identifies the precursor of colorectal cancer derived from sessile serrated adenoma. Am J Gastroenterol. 2012;107:460–9. doi:10.1038/ajg.2011.457 [DOI] [PubMed] [Google Scholar]
- 15.IJspeert JE, Bastiaansen BA, van Leerdam ME, et al. Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Gut 2016;65:963–70. doi:10.1136/gutjnl-2014-308411 [DOI] [PubMed] [Google Scholar]
- 16.Murakami T, Sakamoto N, Ritsuno H, et al. Distinct endoscopic characteristics of sessile serrated adenoma/polyp with and without dysplasia/carcinoma. Gastrointest Endosc 2016. doi: 10.1016/j.gie.2016.09.018. [Epub ahead of print 20 Sep 2016]doi:10.1016/j.gie.2016.09.018 [DOI] [PubMed] [Google Scholar]
- 17.Haggitt RC, Glotzbach RE, Soffer EE, et al. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology 1985;89:328–36. doi:10.1016/0016-5085(85)90333-6 [DOI] [PubMed] [Google Scholar]
- 18.Uraoka T, Saito Y, Matsuda T, et al. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut 2006;55:1592–7. doi:10.1136/gut.2005.087452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uno Y, Munakata A. The non-lifting sign of invasive colon cancer. Gastrointest Endosc 1994;40:485–9. doi:10.1016/S0016-5107(94)70216-0 [DOI] [PubMed] [Google Scholar]
- 20.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature 2015;521:436–44. doi:10.1038/nature14539 [DOI] [PubMed] [Google Scholar]
- 21.Ket SN, Bird-Lieberman E, East JE. Electronic imaging to enhance lesion detection at colonoscopy. Gastrointest Endosc Clin N Am 2015;25:227–42. doi:10.1016/j.giec.2014.11.011 [DOI] [PubMed] [Google Scholar]