Abstract
We report the case of a 76-year-old man who presented with moderate active Crohn's colitis that was refractory to high-dose corticosteroids, mesalazine and 6-mercaptopurine. He subsequently received a trial of infliximab with poor response and was diagnosed with cytomegalovirus (CMV) colitis, improving on antiviral therapy. Three weeks into treatment he developed acute respiratory distress with hypoxaemia and diffuse pulmonary interstitial infiltrates. This was confirmed as Pneumocystis jirovecii on bronchoalveolar lavage. He responded well to treatment with trimethoprim-sulfamethoxazole (TMP-SMX) and was subsequently discharged home. Despite the favourable outcome, our case raises the question of whether chemoprophylaxis against opportunistic infections in immunosuppressed patients with inflammatory bowel disease (IBD) is appropriate. There are currently no recommendations on providing chemoprophylaxis against CMV colitis and so we focus on pneumocystis pneumonia (PCP) where wide debate surrounds the use of prophylactic TMP-SMX in HIV-negative patients. Contrasting approaches to chemoprophylaxis against PCP in IBD likely relates to a lack of clear parameters for defining risk of PCP among patient groups. This must be addressed in order to develop universal guidelines that take into account patient-dependent risk factors. Awareness of the severity of PCP among HIV-negative individuals and the current consensus on PCP prophylaxis in IBD must be raised in order to minimise the risk of PCP and drive research in this controversial area.
Keywords: 6-MERCAPTOPURINE, 5-AMINOSALICYLIC ACID (5-ASA), INFLAMMATORY BOWEL DISEASE, CROHN'S DISEASE, IMMUNODEFICIENCY
A 76-year-old man presented with a 2-week history of profuse bloody diarrhoea, poor appetite, vomiting and three stone weight loss since the onset of symptoms. There was no history of recent travel, use of antibiotics or non-steroidal anti-inflammatory drugs. On examination, he was underweight and clinically dehydrated with tachycardia and hypotension. His abdomen was soft, non-tender with active bowel sounds. Digital rectal examination was unremarkable. Blood tests on admission showed an acute inflammatory response and acute kidney injury (WCC 19.4, C reactive protein (CRP) 176, Cr 107—baseline 65, Ur 10.7). Stool culture and Clostridium difficile toxin were negative. He underwent a CT abdomen/pelvis and flexible sigmoidoscopy with colonic biopsies. These confirmed moderately active colitis with patchy inflammation, fissuring ulcers and submucosal fibrosis suggestive of Crohn's disease. The patient was treated for moderate Crohn's colitis with 7 days of intravenous hydrocortisone, oral and rectal mesalazine leading to improved stool frequency and improvement in CRP (WCC 22.9, CRP 6).
Following conversion to oral prednisolone and 6-mercaptopurine, our patient suffered symptom relapse and was restarted on intravenous hydrocortisone. There was no clinical response after 3 days of intravenous steroids with persistent bloody diarrhoea and raised inflammatory markers (CRP 80). He received a trial of infliximab but failed to respond despite initial improvement in stool consistency.
Prior to consideration for colectomy, a repeat flexible sigmoidoscopy was performed to rule out infective colitis (figure 1). The repeat colonic biopsies demonstrated cytomegalovirus (CMV) on immunohistochemistry, present on a background of Crohn's colitis (figure 2). The patient was started on intravenous ganciclovir and responded well with improvement in stool frequency and consistency.
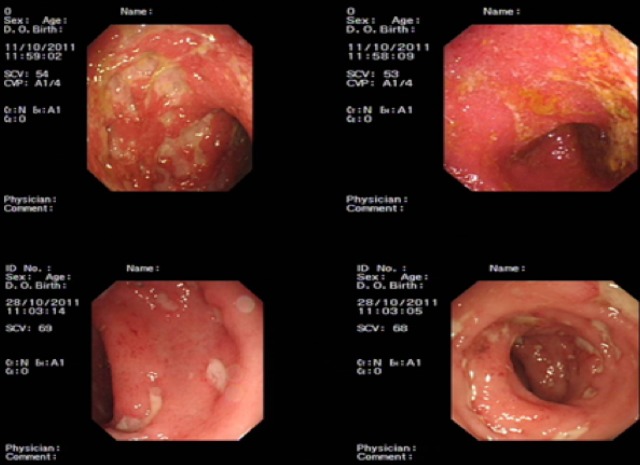
Figure 1.
The top two images are from flexible sigmoidoscopy performed during initial diagnosis of Crohn's disease on admission, while the bottom two images are from flexible sigmoidoscopy performed before the initiation of ganciclovir.
Figure 2.
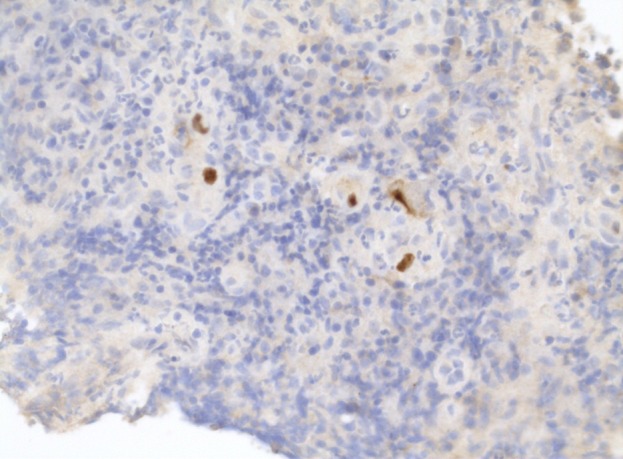
Cytomegalovirus inclusion bodies seen within stromal cells in repeat colonic biopsies.
His condition remained stable and he was subsequently stepped down to oral valganciclovir. Three weeks into the course of treatment for CMV colitis, our patient developed acute dyspnoea and a non-productive cough. On examination he was tachypnoeic, saturating 84% on air with fine crackles in the upper zones bilaterally. Arterial blood gas showed type 1 respiratory failure with pO2 8 kPa on 2 L of oxygen. Chest X-ray taken demonstrated bilateral interstitial shadowing (figure 3). Bronchoscopy with bronchoalveolar lavage was performed and Pneumocystis jirovecii was subsequently confirmed. He was started on a course of intravenous trimethoprim-sulfamethoxazole (TMP-SMX). Immunological testing demonstrated low titres of CD4 and CD8 lymphocytes. HIV test was negative. Our patient's symptoms improved gradually with a course of TMP-SMX and he was discharged home.
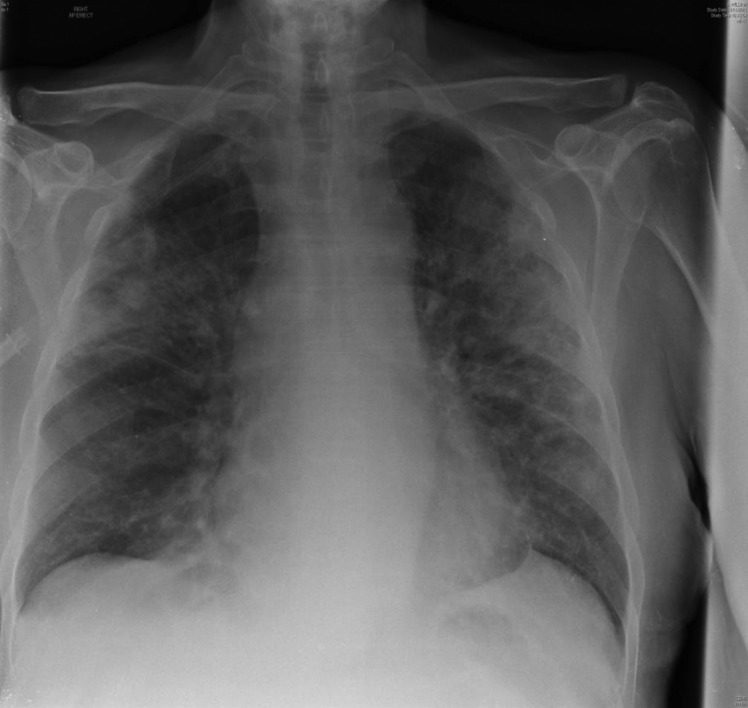
Figure 3.
Radiographic changes of Pneumocystic jirovecii pneumonia.
History of pneumocystis pneumonia
P. jirovecii is a potentially life-threatening fungal infection first described as the cause of interstitial plasma cell pneumonitis among premature infants in 1952.1 2 Its prevalence had greatly increased following the emergence of HIV in the 1980s, and soon became the leading AIDS-defining diagnosis in HIV-infected patients; with pneumocystis pneumonia (PCP) rates as high as 20 per 100 person-years for those with CD4 counts <200 cells/μL.3 The introduction of PCP prophylaxis using TMP-SMX in 1989, together with highly active antiretroviral therapy has led to the first substantial reduction in rates of PCP among HIV-infected individuals.4 Despite this decline, we have witnessed a rise in the number of cases of PCP in HIV-negative immunocompromised individuals owing to the greater role of immunosuppressive therapies in managing haematological malignancies, inflammatory conditions and solid organ transplantations.5
The increasing use of monoclonal antibodies in the management of rheumatoid arthritis and inflammatory bowel disease (IBD) has led to dramatic improvements in disease control and relapse rates.6 7 Unfortunately, this has been associated with an increase in the number of opportunistic infections including tuberculosis, histoplasmosis, aspergillosis, pneumocystosis and CMV.8 Review of the US Food and Drug Administration adverse events data between 1998 and 2003 identified 84 cases of PCP following infliximab therapy. The report showed concomitant use of immunosuppressive medications including methotrexate, prednisolone, azathioprine, 6-mercaptopurine and ciclosporin.
Twenty-three of the 84 (27%) patients died, highlighting the significant morbidity and mortality associated.9 Another study looking at case notes of 78 non-HIV-infected patients with PCP showed similar findings with 72 (92%) and 55 (71%) patients on corticosteroids and cytotoxic drugs, respectively. Interestingly, there was higher mortality from PCP associated with corticosteroid use.10
This is important given many of our patients require high-dose corticosteroids to induce remission as in the case of our patient.
Transmission of P. jirovecii
There is widespread debate surrounding the transmission of P. jirovecii. The question still remains whether PCP occurs following reactivation of latent disease or by de novo infection. A cross-sectional and longitudinal study of normal infants demonstrated that by 4 years of age, two-thirds of children possessed antibodies against P. jirovecii favouring the existence of subclinical PCP among immunocompetent hosts.11 A further study looking at pneumocystis colonisation of autopsied lungs in the general population found that 50 of 77 individuals were carriers of P. jirovecii despite immunocompetence.12 Wakefield et al13 demonstrated transient colonisation of hosts with P. jirovecii up to 9.5 months after treatment of an acute episode of PCP. Of interest, the genotype of P. jirovecii found at follow-up was different from that identified during the acute infection. This provides strong evidence supporting the model of reinfection rather than latency, and a potential for those demonstrating carriage of P jirovecii to act as a reservoir for infection.
Clinical course of PCP
Patients with PCP often develop progressive dyspnoea, non-productive cough, fever, hypoxaemia and radiological evidence of diffuse pulmonary infiltrates.14 PCP among HIV-infected patients tends to have a more insidious onset compared with non-HIV-infected individuals where PCP manifests as an acute illness often leading to severe respiratory distress within several days of presentation. As a consequence, the severity of pneumonia, rates of ITU admission and mortality are higher in non-HIV-infected patients.15 16 A further observation from Li et al16 was the significant delay in initiating PCP-specific antimicrobial therapy in non-HIV-infected patients compared with HIV-infected counterparts (10 vs 1 days). This is likely due to a delay in diagnosis given the non-specific symptoms at presentation and a lack of clinical vigilance for PCP among non-HIV-infected patients. The treatment of choice for the management of PCP is TMP-SMX, with patients showing clinical and radiological response within four days of treatment.17 Unfortunately, adverse events are common, particularly gastrointestinal and cutaneous symptoms, which are attributed to the sulfonamide portion of the drug. These reactions are generally mild and do not lead to discontinuation of therapy.18 In patients with PCP who are hypoxic (PaO2 <70 mm Hg on air or alveolar-arterial gradient >35), oral corticosteroid therapy in addition to TMP-SMXhas been shown to be beneficial.19
Chemoprophylaxis against P. jirovecii
Primary prophylaxis against PCP in HIV-infected patients is recommended when the CD4 count is <200 cells/μL.20 Such clear guidelines for PCP prophylaxis among non-HIV-infected patients have not yet been established. A meta-analysis looking at PCP prophylaxis in HIV-negative patients showed TMP-SMX significantly reduced PCP occurrence.21 When balanced against the risk of severe adverse events, the study recommended PCP prophylaxis in patients with an expected risk of PCP of 3.5% or more which are reported in solid organ or allogeneic bone allografts throughout the period of immunosuppression. The incidence of PCP in patients on corticosteroid treatment has not been reported, but PCP prophylaxis may be warranted in those who are young where adverse events from prophylaxis are rare.21
Focusing specifically on patients taking immunosuppressive therapy for IBD, there is no consistency in the approach to PCP prophylaxis. Several risk factors have been identified for developing PCP following treatment with tumour necrosis factor (TNF)-α inhibitors including old age, coexisting pulmonary disease and high-dose corticosteroid use.22 23 A further study demonstrated that the risk of PCP correlates with the use of high-dose corticosteroid, and the authors recommend PCP prophylaxis be offered in these cases.24
The Italian Society of Gastroenterology has since recommended the consideration of PCP prophylaxis in patients treated with anti-TNF-α agents who are also receiving other immunosuppressive medications, particularly high doses of corticosteroids.25 A contrasting message is conveyed by the European evidence-based consensus which only recommends PCP prophylaxis for patients on triple immunomodulator therapy with one of those being a calcineurin inhibitor or anti-TNF α agent. For those on double immunomodulators, with concomitant calcineurin inhibitor or anti-TNF α use, a consensus could not be reached.26
Discussion
There must be extra vigilance among clinicians for the non-specific presentation of PCP that is often diagnosed late in the HIV-negative immunosuppressed individuals not on chemoprophylaxis. There is potential for de novo infection with P. jirovecii and likely transmission from person to person, with some patients demonstrating transient latent carriage of PCP. One study described an outbreak of PCP among immunosuppressed patients with rheumatoid arthritis in an outpatient clinic.27 The authors suggested that in the event of a new case of PCP, one may be able to justify short-term chemoprophylaxis for exposed patients in order to prevent further latent carriage of PCP and reduce the risk of a PCP outbreak.
There is a clear need to identify immune parameters for defining the risk of PCP among patient groups which will allow the initiation of chemoprophylaxis based on universal guidelines that take into account patient-dependent risk factors. General measures to prevent infection may be equally as important as PCP chemoprophylaxis. Nutritional state of the patient, dose, duration and combination of immunomodulator therapy can all contribute to risk of developing opportunistic infections. There have been several cases of PCP pneumonia reported following initiation of infliximab therapy in patients with Crohn's disease.28–30 Some of these patients were on a thiopurine in addition to infliximab.28–30
Our case describes an unusual scenario where an elderly man developed two opportunistic infections secondary to immunosuppressive therapy to induce remission of Crohn's colitis. Our patient was appropriately screened for CMV colitis when he developed colitis refractory to immunomodulatory therapy.26 There are currently no recommendations on providing chemoprophylaxis against CMV colitis. Immunosuppressive therapy was only initiated on admission to hospital and there was no previous history of immunosuppressive therapy raising the question of whether duration of therapy is important in developing PCP. There was no delay in diagnosis and the patient was promptly started on TMP-SMX to good effect. Awareness of such cases and the current consensus on PCP prophylaxis in IBD must be raised in order to minimise cases of PCP and drive research in this widely debated subject.
Recommendations
There are a few patient-dependant risk factors which can predict the risk of opportunistic infection in the immunosuppressed cohort. These include the elderly (over 75 years of age), undernourished and those with underlying chronic diseases such as chronic obstructive airway disease, chronic liver diseases and diabetes which can contribute to poor nutritional status and impaired immune system. Individuals on two or more immunosuppressive agents with the above mentioned risk factors should strongly be considered for PCP chemoprophylaxis.
Our patient illustrates the importance of recognising these risk factors. He was an elderly patient with diabetes who was undernourished and had received high-dose steroids, thiopurine and anti-TNF α agent. This puts him at a higher risk of developing opportunistic infections than the general population and should be started on PCP chemoprophylaxis based on our recommendations above.
These risk factors should be taken into account when discussing treatment options so that an informed clinical decision can be made. This is of particular importance now given the widespread use of anti-TNF α agents and other immunomodulators in treating patients with a complicated course of their IBD.
Footnotes
Correction notice: Last author's name was published with mistake. It has been corrected since published Online First.
Contributors: OO co-wrote the case report and wrote the background, discussion and conclusion of the paper as well as putting together the final draft of the paper. PC reviewed and photographed the immunohistochemistry slides used. SFN co-wrote the case report, and reviewed and edited the penultimate draft of the paper. SFN is also responsible for the final revised version of the accepted manuscipt. GVS reviewed and edited the final draft of the paper prior to submission.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vanek J, Jirovec O. Parasitic pneumonia. Interstitial plasma cell pneumonia of premature, caused by pneumocystis Carinii. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg 1952;158:120–7. [PubMed] [Google Scholar]
- 2.Gajducek DC. Pneumocystis carinii; etiologic agent of interstitial plasma cell pneumonia of premature and young infants. Pediatrics 1957;19:543–65. [PubMed] [Google Scholar]
- 3.Phair J, Munoz A, Detels R, et al. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. N Engl J Med 1990;322:161–5. [DOI] [PubMed] [Google Scholar]
- 4.Fischl MA, Dickinson GM, La Voie L. Safety and efficacy of sulfamethoxazole and trimethoprim chemoprophylaxis for Pneumocystis carinii pneumonia in AIDS. JAMA 1988;259:1185–9. [DOI] [PubMed] [Google Scholar]
- 5.Reid AB, Chen SCA, Worth LJ. Pneumocystis jirovecii pneumonia in non-HIV-infected patients: new risks and diagnostic tools. CurrOpin Infect Dis 2011;24:534–44. [DOI] [PubMed] [Google Scholar]
- 6.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762–84. [DOI] [PubMed] [Google Scholar]
- 7.Hanauer S, Feagan B, Lichtenstein G, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 8.Salvana EMT, Salata RA. Infectious complications associated with monoclonal antibodies and related small molecules. Clin Microbiol Rev 2009;22:274–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur N, Mahl T. Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy: a review of 84 cases. Dig Dis Sci 2007;52:1481–4. [DOI] [PubMed] [Google Scholar]
- 10.Arend SM, Kroon FP, van'tWout JW. Pneumocystis carinii pneumonia in patients without AIDS, 1980 through 1993. An analysis of 78 cases. Arch Intern Med 1995;155:2436–41. [PubMed] [Google Scholar]
- 11.Pifer LL, Hughes WT, Stagno S, et al. Pneumocystis carinii infection: evidence for high prevalence in normal and immunosuppressed children. Paediatrics 1978;61:35–41. [PubMed] [Google Scholar]
- 12.Ponce CA, Gallo M, Bustamante R, et al. Pneumocystis colonization is highly prevalent in autopsied lungs of the general population. Clin Infect Dis 2010;50:347–53. [DOI] [PubMed] [Google Scholar]
- 13.Wakefield AE, Lindley AR, Ambrose HE, et al. Limited asymptomatic carriage of Pneumocystis jiroveci in human immunodeficiency virus-infected patients. J Infect Dis 2003;187:901–8. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs JA, Hiemenz JW, Macher AM, et al. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med 1984;100:663–71. [DOI] [PubMed] [Google Scholar]
- 15.Mansharamani NG, Garland R, Delaney D, et al. Management and outcome patterns for adult Pneumocystis carinii Pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest 2000;118:704–11. [DOI] [PubMed] [Google Scholar]
- 16.Li MC, Lee NY, Lee CC, et al. Pneumocystis jiroveci pneumonia in immunocompromised patients: Delayed diagnosis and poor outcomes in non-HIV-infected individuals. J MicrobiolImmunol Infect 2014;47:42–7. [DOI] [PubMed] [Google Scholar]
- 17.Young LS. Trimethoprim-sulfamethoxazole in the treatment of adults with pneumonia due to Pneumocystis carinii. Clin Infect Dis 1982;4:608–13. [DOI] [PubMed] [Google Scholar]
- 18.Masters PA, O'Bryan TA, Zurlo J, et al. Trimethoprim-sulfamethoxazole revisited. Arch Intern Med 2003;163:402–10. [DOI] [PubMed] [Google Scholar]
- 19.Masur H, Meier P, McCutchan JA, et al. Consensus statement on the use of corticosteroids as adjunctive therapy for pneumocystis pneumonia in the acquired immunodeficiency syndrome. N Engl J Med 1990;323:1500–4. [DOI] [PubMed] [Google Scholar]
- 20.Masur H, Kaplan JE, Holmes KK. Guidelines for preventing opportunistic infections among HIV-infected persons—2002: recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. Ann Intern Med 2002;137:435–78. [DOI] [PubMed] [Google Scholar]
- 21.Green H, Paul M, Vidal L, et al. Prophylaxis of pneumocystis pneumonia in immunocompromised non–HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc 2007;82:1052–9. [DOI] [PubMed] [Google Scholar]
- 22.Poppers DM, Scherl EJ. Prophylaxis against Pneumocystis pneumonia in patients with inflammatory bowel disease: toward a standard of care. Inflamm Bowel Dis 2008;14:106–13. [DOI] [PubMed] [Google Scholar]
- 23.Harigai M, Koike R, Miyasaka N. Pneumocystis pneumonia associated with infliximab in Japan. N Engl J Med 2007;357:1874–6. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez M, Fishman JA. Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clin Microbiol Rev 2004;17:770–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orlando A, Armuzzi A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis 2011;43:1–20. [DOI] [PubMed] [Google Scholar]
- 26.Rahier J, Ben-Horin S, Chowers Y, et al. European evidence-based Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2009;3:47–91. [DOI] [PubMed] [Google Scholar]
- 27.Mori S, Sugimoto M. Pneumocystis jirovecii infection: an emerging threat to patients with rheumatoid arthritis. Rheumatology 2012;51:2120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velayos FS, Sandborn WJ. Pneumocystis carinii pneumonia during maintenance anti-tumor necrosis factor-α therapy with infliximab for Crohn's disease. Inflamm Bowel Dis 2004;10:657–60. [DOI] [PubMed] [Google Scholar]
- 29.Seddik M, Meliez H, Seguy D, et al. Pneumocystis jirovecii (carinii) pneumonia following initiation of infliximab and azathioprine therapy in a patient with Crohn's disease. Inflamm Bowel Dis 2004;10:436–7. [DOI] [PubMed] [Google Scholar]
- 30.Kaur N, Mahl TC. Pneumocystis carinii pneumonia with oral candidiasis after infliximab therapy for Crohn's disease. Dig Dis Sci 2004;49:1458–60. [DOI] [PubMed] [Google Scholar]