Abstract
Anti-tumour necrosis factor (TNF) therapies, such as infliximab, adalimumab, certolizumab pegol and golimumab, have been proven to be effective for the treatment of patients with Crohn's disease and ulcerative colitis. However, 10%–30% of patients with inflammatory bowel disease (IBD) show no initial clinical benefit to anti-TNF therapy (primary non-response), and over 50% after an initial favourable outcome will lose response over time (secondary loss of response (SLR)). Numerous recent studies in IBD have revealed an exposure–response relationship suggesting a positive correlation between high serum anti-TNF concentrations and favourable therapeutic outcomes including clinical, biomarker and endoscopic remission, whereas antidrug antibodies have been associated with SLR and infusion reactions. Currently, therapeutic drug monitoring (TDM) is typically performed when treatment failure occurs either for SLR, drug intolerance (potential immune-mediated reaction) or infusion reaction (reactive TDM). Nevertheless, recent data demonstrate that proactive TDM and a treat-to-target (trough) therapeutic approach may more effectively optimise anti-TNF therapy efficacy, safety and cost. However, implementing TDM in real-life clinical practice is currently limited by the diversity in study design, therapeutic outcomes and assays used, which have hindered the identification of robust clinically relevant concentration thresholds. This review will focus mainly on the pharmacodynamic properties of anti-TNF therapy and the role of TDM in guiding therapeutic decisions in IBD.
Keywords: INFLAMMATORY BOWEL DISEASE, INFLIXIMAB, ULCERATIVE COLITIS, CROHN'S DISEASE
Introduction
Anti-tumour necrosis factor (TNF) therapies, infliximab, adalimumab, certolizumab pegol and golimumab have revolutionised the care of the inflammatory bowel diseases (IBD), Crohn's disease (CD) and ulcerative colitis (UC).1 Nevertheless, 10%–30% of patients with IBD are primary non-responsers and another 20%–50% of patients have a secondary loss of response (SLR) within 1 year of treatment and need to dose-intensify or discontinue therapy.2 Mechanisms underlining both primary non-response (PNR) and SLR include pharmacodynamic (PD) and pharmacokinetic (PK) issues, characterised by inadequate drug concentrations due to increased non-immune clearance or a non-TNF-driven inflammatory process.3 4 Subtherapeutic or undetectable drug concentrations due to high immune clearance have been attributed to immunogenicity, or the development of antidrug antibodies (ADA). Immunogenicity may also lead to drug intolerance and consequently treatment failure due to infusion reactions.4 5
Recent studies in IBD suggest a positive correlation between high serum drug concentrations and favourable therapeutic outcomes including clinical (physician global assessment, Harvey–Bradshaw Index and the Crohn's Disease Activity Index for CD or the (partial) Mayo score for UC), biomarker (normalisation of C reactive protein (CRP) or faecal calprotectin (FC)) endoscopic (mucosal healing) or composite remission (tables 1 and 2).6–40
Table 1.
Anti-TNF therapy exposure–response relationship in IBD studies regarding clinical efficacy
| Drug | IBD type | Study design | No | Time point | TC (μg/mL) | Therapeutic outcome | SN | SP | PPV | NPV | Assay | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFX | UC | RCT* | 82 | Induction (w2) | >21.3 | Clinical remission (w14) | 61 | 69 | 72 | 58 | ELISA | 6 |
| IFX | UC | RCT* (ACT-1 and 2) | 446 | Induction (w6) | >22 | Clinical response (w8) | 60 | 62 | 78 | 41 | ELISA | 7 |
| IFX | UC | RCT* (ACT-1 and 2) | 377 | Postinduction (w14) | >5.1 | Clinical response (w30) | 66 | 63 | 74 | 54 | ELISA | 7 |
| IFX | UC | Retrospective | 112 | Postinduction (w14) | >2.5 | Absence of clinical relapse† | 81 | 75 | ND | ND | ELISA | 8 |
| IFX | CD | RCT* (ACCENT-1) | 284 | Postinduction (w14) | >3.5 | Sustained clinical response (w54) | 64 | 78 | 56 | 83 | ELISA | 9 |
| IFX | UC | RCT* (ACT-1 and 2) | 158 | Postinduction (w14) | >3.5 | Clinical response (w54) | 82 | 50 | 63 | 72 | ELISA | 7 |
| IFX | CD | Prospective | 84 | Postinduction (w14 or w22) | >3 | Sustained clinical response | 70 | 62 | 41 | 84 | ELISA | 10 |
| IFX | CD/UC | Prospective | 93 (CD: 59) | Maintenance (w22) | ≤5.5 | SLR | 25 | 84 | ND | ND | ELISA | 12 |
| IFX | UC | RCT* (ACT-1 and 2) | 377 | Maintenance (w30) | >3.7 | Clinical response (w30) | 65 | 71 | 82 | 51 | ELISA | 7 |
| IFX | UC | RCT* (ACT-1 and 2) | 158 | Maintenance (w30) | >2.4 | Clinical response (w54) | 86 | 62 | 76 | 77 | ELISA | 7 |
| IFX | CD | RCT* (SONIC) | 203 | Maintenance (w30) | ≥3 | CS-free remission (w50) | 50 | 65 | 69 | 46 | ELISA | 13 |
| IFX | UC | RCT* (ACT-1 and 2) | 158 | Maintenance (w54) | >1.7 | Clinical response (w54) | 89 | 64 | 83 | 74 | ELISA | 7 |
| IFX | CD | Retrospective | 69 | Maintenance | <0.5 | SLR | 86 | 85 | ND | ND | RIA | 14 |
| IFX | UC | Retrospective | 13 | Maintenance | <0.8 | SLR | 75 | 100 | ND | ND | RIA | 14 |
| IFX | CD/UC | Retrospective | 213 (CD: 131) | Maintenance | >2.1 | Clinical remission | 78 | 76 | ND | ND | ELISA | 19 |
| IFX | CD | Prospective | 105 | Maintenance | >1.4 | Clinical remission | ND | ND | ND | ND | ELISA | 20 |
| IFX | UC | Prospective | 115 | Maintenance | >1.4 | Clinical remission | ND | ND | ND | ND | ELISA | 17 |
| IFX | UC | Prospective | 46 | Maintenance | >6.26 | Clinical remission | 50 | 88 | NA | NA | ELISA | 21 |
| IFX | CD | Prospective | 61 | Maintenance | >2.18 | Clinical remission | 67 | 79 | NA | NA | ELISA | 21 |
| ADM | UC | Retrospective | 73 | Postinduction (w4) | >4.58 | Clinical response (w12) | 80 | 56 | 85 | 47 | ELISA | 24 |
| ADM | UC | Retrospective | 73 | Postinduction (w4) | >7 | Clinical response (w52) | 80 | 69 | 43 | 92 | ELISA | 24 |
| ADM | CD | Retrospective | 148 | Postinduction (w4) | <5 | Drug discontinuation | ND | ND | ND | ND | HMSA | 25 |
| ADM | CD/UC | Cross-sectional | 40 (CD: 22) | Maintenance | >4.85 | Clinical remission | 81 | 67 | 84 | 57 | ELISA | 27 |
| ADM | CD/UC | Retrospective | 57 (CD: 42) | Maintenance | <6.85 | SLR | 69 | 69 | 58 | 78 | RIA | 28 |
| ADM | CD | Cross-section | 71 | Maintenance | >5.85 | Clinical remission | 68 | 71 | ND | ND | ELISA | 29 |
*Post-hoc analysis.
†Within 6 months of baseline.
ACT, Active Ulcerative Colitis Trial; ADM, adalimumab; CD, Crohn's disease; CS, corticosteroids; HMSA, homogeneous mobility shift assay; IBD, inflammatory bowel disease; IFX, infliximab; NA, not applicable; ND, not defined; No, number of patients; NPV, negative predictive value; PPV, positive predictive value; RCT, randomised clinical trial; RIA, Radioimmunoassay; SLR, secondary loss of response; SONIC, study of biologic and immunomodulator naive patients in Crohn's disease; SN, sensitivity; SP, specificity; TC, trough concentration; TNF, tumour necrosis factor; UC, ulcerative colitis; w, week.
Table 2.
Anti-TNF therapy exposure–response relationship in IBD studies regarding endoscopic and biomarker outcomes
| Drug | IBD type | Study design | No | Time point | TC (μg/mL) | Therapeutic outcome | SN | SP | PPV | NPV | Assay | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endoscopic outcomes | ||||||||||||
| IFX | UC | Retrospective | 101 | Induction (w6) | ≥15 | Mucosal healing (w10–14) | 60 | 74 | 73 | 62 | ELISA | 31 |
| IFX | UC | Prospective | 19 | Induction (w6) | >6.6 | Endoscopic response* (w8) | 88 | 73 | ND | ND | RIA | 32 |
| IFX | UC | Retrospective | 101 | Postinduction (w14) | ≥2.1 | Mucosal healing (w10–14) | 84 | 62 | 78 | 71 | ELISA | 31 |
| IFX | CD | RCT† (SONIC) | 123 | Maintenance (w30) | ≥3 | Mucosal healing (w26) | 59 | 72 | 73 | 57 | ELISA | 13 |
| IFX | CD | Prospective | 105 | Maintenance | >1.4 | Endoscopic improvement | ND | ND | ND | ND | ELISA | 20 |
| IFX | UC | Prospective | 115 | Maintenance | >1.4 | Endoscopic improvement | ND | ND | ND | ND | ELISA | 17 |
| IFX | CD/UC | Prospective | 52 (CD: 34) | Maintenance | >0.5‡ | Mucosal healing | 89 | 80 | 83 | 87 | ELISA | 33 |
| IFX | CD/UC | Cross-sectional | 72 (CD: 49) | Maintenance | >8.3 | Mucosal healing | 71 | 73 | ND | ND | HMSA | 34 |
| IFX | CD/UC | Cross-sectional | 78 (CD: 53) | Maintenance | >5 | Mucosal healing | 39 | 85 | 70 | 62 | ELISA | 15 |
| IFX | CD | Retrospective | 45 | Maintenance | >4 | Mucosal healing | 71 | 70 | ND | ND | ELISA | 18 |
| ADM | CD/UC | Cross-sectional | 67 (CD: 58) | Maintenance | >7.1 | Mucosal healing | 32 | 85 | 51 | 72 | ELISA | 15 |
| ADM | CD/UC | Cross-sectional | 40 (CD: 22) | Maintenance | >4.9 | Mucosal healing | 66 | 85 | 88 | 51 | ELISA | 27 |
| CZP | CD | RCT† (MUSIC) | 89 | Postinduction (w8) | >23.3 | Endoscopic response and remission (w10) | ND | ND | ND | ND | ELISA | 30 |
| Biomarker outcomes | ||||||||||||
| IFX | CD/UC | Retrospective | 213 (CD: 131) | Maintenance | >3.9 | Normal FC (<250 µg/g) | 74 | 80 | ND | ND | ELISA | 19 |
| IFX | CD/UC | Retrospective | 213 (CD: 131) | Maintenance | >2.9 | CRP normalisation | 69 | 66 | ND | ND | ELISA | 19 |
| IFX | CD | Prospective | 105 | Maintenance | >1.4 | Lower CRP | ND | ND | ND | ND | ELISA | 20 |
| IFX | CD | Observational§ | 483 | Maintenance | >2.79 | Normal CRP | 77 | 52 | ND | ND | HMSA | 35 |
| IFX | CD | Prospective | 327 | Maintenance | <3 | Elevated CRP | ND | ND | ND | ND | HMSA | 22 |
| IFX | CD/UC | Cross-sectional | 78 (CD: 53) | Maintenance | >6.8 | CRP normalisation | 22 | 85 | 59 | 53 | ELISA | 15 |
| IFX | CD | Retrospective | 45 | Maintenance | >0.6 | Normal CRP (≤0.3 mg/dL) | 73 | 62 | ND | ND | ELISA | 18 |
| IFX | CD | Retrospective | 45 | Maintenance | >1.1 | Normal FC (≤300 µg/g) | 72 | 56 | ND | ND | ELISA | 18 |
| ADM | CD/UC | Cross-sectional | 67 (CD: 58) | Maintenance | >6.6 | CRP normalisation | 28 | 85 | 71 | 48 | ELISA | 15 |
| ADM | CD | Prospective | 40 | Maintenance | >5.9 | Normal CRP (≤0.3 mg/dL) | 67 | 92 | ND | ND | ELISA | 36 |
| ADM | CD | Cross-sectional | 71 | Maintenance | >5.85 | CRP normalisation | 68 | 71 | ND | ND | ELISA | 29 |
*≥1-point reduction in the endoscopic Mayo score.
†Post-hoc analysis.
‡Delta after dose intensification for SLR.
ADM, adalimumab; CD, Crohn's disease; CRP, C reactive protein; CZP, certolizumab pegol; FC, faecal calprotectin; HMSA, homogeneous mobility shift assay; IBD, inflammatory bowel disease; IFX, infliximab; ND, not defined; No, number of patients; NPV, negative predictive value; PPV, positive predictive value; RCT, randomised clinical trial; RIA, radioimmunoassay; SLR, secondary loss of response; SN, sensitivity; SP, specificity; TC, trough concentration; TNF, tumour necrosis factor; UC, ulcerative colitis; w, week.
This is of great clinical importance as these objective outcomes are emerging as new goals in IBD treatment.39 Currently, therapeutic drug monitoring (TDM) is typically implemented in the reactive IBD setting, when active inflammation and/or drug intolerance are present. Nevertheless, recent data demonstrate that proactive TDM and a treat-to-target therapeutic approach may arise as a novel strategy to optimise anti-TNF therapy efficacy, safety and cost.16 41–43 However, well-defined therapeutic windows and robust, clinically relevant thresholds are limited by the diversity in study design, disease outcomes and assays used in the current TDM studies (tables 1 and 2).
This review will focus on the PD properties of anti-TNF therapies, including the relationship between serum drug concentrations and immunogenicity and clinical outcomes as well as the role of TDM in guiding therapeutic decisions in IBD.
TDM and anti-TNF therapy in IBD
Drug concentration–effect relationship
The drug concentration–effect relationship for anti-TNF therapy in IBD recently has been the research objective of numerous clinical studies. These analyses have mainly focused on the association of infliximab and adalimumab concentrations with several outcomes including clinical, biomarker and/or endoscopic remission (tables 1 and 2).6–36 These studies show that higher drug concentrations during both induction and maintenance therapy are associated with favourable therapeutic outcomes, such as clinical response or remission, normalisation of CRP and FC and/or mucosal healing, whereas lower or undetectable drug concentrations are associated with treatment failure and drug discontinuation. More recently, the strategy of dosing to a therapeutic concentration, rather than using a fixed dosing schedule, has gained traction optimising care. Though this method for optimising efficacy, safety and costs of anti-TNF therapy in IBD seems to be a rational approach, before any clinical recommendations can be made, the therapeutic window for each anti-TNF agent should be more clearly defined, as current data mainly derive from cohort studies and post-hoc analysis of randomised control trials (RCTs) (tables 1 and 2).
The lower limit of the therapeutic window represents the optimal concentration threshold that best discriminates clinical, biomarker, endoscopic or composite remission (identified by receiver operating characteristic (ROC) curve analysis), while the upper limit may represent either the drug's maximal efficacy or toxicity. Nevertheless, defining precise clinically relevant cut-offs for each drug may be a difficult and rather simplistic approach taking into account our current knowledge and the variability of drug thresholds depending on the therapeutic outcome and their relatively modest sensitivity, specificity, positive and negative predictive values (tables 1 and 2). Additionally, when mucosal inflammation is present, serum anti-TNF levels may not be representative of the tissue drug concentrations, as patients with increased intestinal inflammation may have lower serum concentrations due to higher drug clearance secondary to increased tissue TNF burden and faecal loss.12 18 27 34 44 Finally, it is still unclear whether trough, currently used in treatment algorithms mainly for practical reasons, rather than peak or intermediate concentrations, are the most clinically relevant measurements for optimising anti-TNF therapy in IBD or whether other PK parameters, such as area under the curve, would be better used instead.45–47
Currently, concentration–effect studies in patients with IBD suggest that the optimal therapeutic trough concentration during maintenance therapy is 3–7 μg/mL for infliximab and 5–10 μg/mL for adalimumab, although some (including us) would argue that higher concentrations (5–10 µg/mL for infliximab and >10–12 µg/mL for adalimumab) are more preferable in order to achieve more objective outcomes, such as mucosal healing and avoid future undetectable drug concentrations and ADA development as variations in a patient's clinical course may influence the PK properties of anti-TNF drugs.15 16 Regarding induction dosing, the optimal therapeutic window has not been clearly defined yet, as there are only limited data available and mostly for patients with UC treated with infliximab (tables 1 and 2).6 7 31 32 44
Immunogenicity
Anti-TNF agents, as foreign antigens, can induce immunogenicity. Development of ADA may be affected by multiple patient, disease and treatment factors48–55 (table 3). ADA have been found in 0.4%–17% of patients with IBD in RCTs (see online supplementary table S1).
Table 3.
Factors related to immunogenicity of anti-TNF therapy in IBD
| Factor | ADA | Ref. |
|---|---|---|
| Patient-related | ||
| Genetic predisposition: HLA-DRB1*03 | ↑ | 48 |
| Gender: male† | ↑ | 7 31 45 49 |
| Ethnicity: Jewish Ashkenazi | ↓ | 50 |
| BMI: high or low† | ↑ | 45 47 49 |
| CRP: elevated† | ↑ | 7 11 31 32 49 |
| Albumin: low† | ↑ | 7 47 49 |
| Disease-related | ||
| IBD type: (acute severe) UC† | ↑ | 7 17 20 31 |
| TNF load: higher† | ↑ | 51 |
| Endoscopic severity: higher Mayo score in UC† | ↑ | 7 31 |
| Treatment-related | ||
| Concomitant medication: IMM‡ | ↓ | 7 8 10 15 20 23 49 52–54 |
| Dose and frequency: high§ | ↓ | 55 |
| Type of therapy: episodic | ↑ | 15 20 55 |
| Previous medication: prior anti-TNF therapy | ↑ | 28 36 51 |
†Probably related, via undetectable or low drug concentrations due to faster non-immune clearance as a result of higher disease inflammatory burden.
‡Thiopurines, methotrexate.
§In patients not receiving IMM, there was approximately twofold difference in ADA prevalence between 5 and 10 mg/kg doses, suggesting that lower doses were more immunogenic in the absence of IMM.
ADA, antidrug antibodies; BMI, body mass index; CRP, C reactive protein; HLA, human leucocyte antigen; IBD, inflammatory bowel disease; IMM, immunomodulators; TNF, tumour necrosis factor; UC, ulcerative colitis.
Immunogenicity of maintenance anti-TNF therapy in IBD RCTs.
flgastro-2016-100685supp_table.pdf (194.2KB, pdf)
Immunogenicity is associated with negative therapeutic outcomes, such as lack of endoscopic remission, treatment failure (PNR, SLR) and drug discontinuation, related to either subtherapeutic or undetectable drug concentrations or immune-mediated reactions including infusion reactions11 12 14 15 17 18 21–26 28 29 31 32 35 56–63 (table 4).
Table 4.
Anti-TNF therapy immunogenicity–therapeutic outcomes relationship in IBD
| Drug | IBD type | Study type | No | Time point | ADA | Therapeutic outcome | Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| Clinical efficacy | ||||||||
| IFX | CD/UC | Retrospective | 90 (CD: 64) | Induction and maintenance* | Detectable | Treatment failure and discontinuation | HMSA | 11 |
| IFX | CD/UC | Retrospective | 90 (CD: 64) | Induction and maintenance* | >9.1 U/mL | Failure of dose intensification after SLR | HMSA | 11 |
| IFX | CD/UC | Retrospective | 128 (CD: 105) | Induction after drug holiday | Undetectable | Short-term clinical response | HMSA | 63 |
| IFX | CD | Prospective | 125 | Maintenance | >8 μg/mL-eq | Shorter clinical response | ELISA | 23 |
| IFX | CD/UC | Cross-sectional (paediatric) | 134 (CD: 114) | Maintenance | >12 U/mL | Surgery | HMSA | 56 |
| ADM | CD | Retrospective | 130 | Maintenance | Detectable | Treatment discontinuation | ELISA | 26 |
| ADM | CD | Retrospective | 30 | Maintenance | Detectable† | No clinical response | RIA | 57 |
| ADM | CD | Cross-sectional | 118 | Maintenance | Detectable‡ | Active disease | ELISA | 29 |
| Endoscopic and biomarker outcomes | ||||||||
| IFX | UC | Prospective | 19 | Induction | Detectable | No endoscopic response | HMSA | 32 |
| IFX | UC | Retrospective | 101 | Induction (w6) | Detectable | No mucosal healing | ELISA§ | 31 |
| IFX | CD | Retrospective | 45 | Maintenance | Detectable | No mucosal healing | ELISA | 18 |
| IFX | CD/UC | Cross-sectional | 78 (CD: 53) | Maintenance | Undetectable | Mucosal healing | ELISA | 15 |
| IFX | CD | Prospective | 327 | Maintenance | Detectable | CRP >5 mg/L | HMSA | 22 |
| IFX | CD | Observational¶ | 483 | Maintenance | Detectable | CRP >5 mg/L | HMSA | 35 |
| ADM | CD | Prospective | 40 | Maintenance | Detectable | Higher CRP and ESR | ELISA | 36 |
| Loss of response | ||||||||
| IFX | CD/UC | Prospective | 125 (CD: 98) | Induction and maintenance | Detectable | SLR | ELISA | 52 |
| IFX | CD/UC | Case–control | 62 (CD: 51) | Maintenance | Detectable | SLR | ELISA | 58 |
| IFX | CD | Prospective | 53 | Maintenance | Detectable** | SLR | ELISA | 53 |
| IFX | CD/UC | Prospective | 93 (CD: 59) | Maintenance (w22) | Detectable stable ATI†† | SLR | ELISA | 12 |
| IFX | CD | Retrospective | 33 | Maintenance | Detectable | SLR | RIA | 59 |
| IFX | CD | Retrospective (paediatric) | 28 | Maintenance | Detectable | SLR | ELISA | 60 |
| IFX | CD | Retrospective | 106 | Maintenance | Detectable‡‡ | SLR | RIA | 14 |
| ADM | CD | Retrospective | 148 | Maintenance | Detectable | SLR§§ | HMSA | 25 |
| ADM | CD/UC | Retrospective | 72 (CD: 53) | Maintenance | Detectable | SLR§§ | RIA | 61 |
| ADM | CD/UC | Retrospective | 57 (CD: 42) | Maintenance | Detectable | SLR | RIA | 28 |
*Ever had a positive ATI.
†>12 U⁄mL.
‡≥3 μg/mL-eq.
§Recently developed drug-tolerant assay.
**IFX level ≤1.4.
††Two consecutive ATI >20 ng/mL.
‡‡>10 U/mL.
§§Drug discontinuation.
ADA, antidrug antibody; ADM, adalimumab; ATI, antibodies to infliximab; CD, Crohn's disease; CRP, C reactive protein; eq, equivalent; ESR, erythrocyte sedimentation rate; HMSA, homogeneous mobility shift assay; IBD, inflammatory bowel disease; IFX, infliximab; No, number of patients; RIA, Radio-immunoassay; SLR, secondary loss of response; U, units; UC, ulcerative colitis.
A meta-analysis including 494 patients with CD showed that patients who develop antibodies to infliximab (ATI) have a risk ratio of 3.2 for SLR compared with those without ATI.62 Infusion reactions have been directly related to the presence of ADA.14 20 23 53–59 A recent systematic review and meta-analysis showed that patients with IBD who develop ATI have a twofold risk of acute infusion reactions and a sixfold risk of serious acute infusion reactions.64 However, as the humoral immune response to anti-TNF therapy is a dynamic process, ADA can be transient or sustained, high or low titres, neutralising or non-neutralising, with each of these variables having a potentially different effect on clinical outcomes.11 23 52 These issues have yet to be clearly defined. Another issue that remains to be elucidated is the ‘double positive’ state, when both serum drug concentration and ADA are detectable. Recent studies using a drug-tolerant assay show that ‘double positive’ patients are prone to SLR, elevated CRP or lack of mucosal healing.15 31 35 65 Finally, whether low anti-TNF concentrations is the reason or the consequence of ADA development is still debated, although the use of drug-tolerant assays show that low drug concentrations, during or early after the induction therapy, were linked to a higher risk of ADA.11 25
Using TDM in clinical practice
Maintenance therapy
Reactive TDM
Numerous studies have demonstrated the utility of combining anti-TNF concentrations and ADA in the clinical management of patients with SLR.11 42 43 54 66 67 They showed that patients with subtherapeutic drug levels, but no ADA, will benefit more from dose escalation compared with switching to another anti-TNF, while those with adequate drug levels are likely to respond better to changing to a biological agent with a different mechanism of action, as inflammation in these patients may not be driven by TNF anymore. Moreover, they demonstrated that patients who lose response to anti-TNF therapy due to ADA responded well when switched to another anti-TNF agent, although those with transient and/or low titre ADA may still benefit from optimisation of original anti-TNF therapy. Though the most efficient method of dose optimisation (shortening interval, increasing dose, adding immunomodulators (IMM)) is still unclear, recent PK data suggest that shortening of the intervals may result to overall higher drug concentrations compared with dose increasing.47 Additionally, several studies have shown that elevated drug concentrations after dose escalation for SLR were associated with recapturing clinical response and improved clinical outcomes.11 26 68 In the setting of an SLR, a personalised therapeutic approach based on TDM when compared with an empiric dose escalation was found to be more cost-effective.42 43 69 Although this approach was not superior in terms of clinical and biological remission, it does more appropriately direct care.
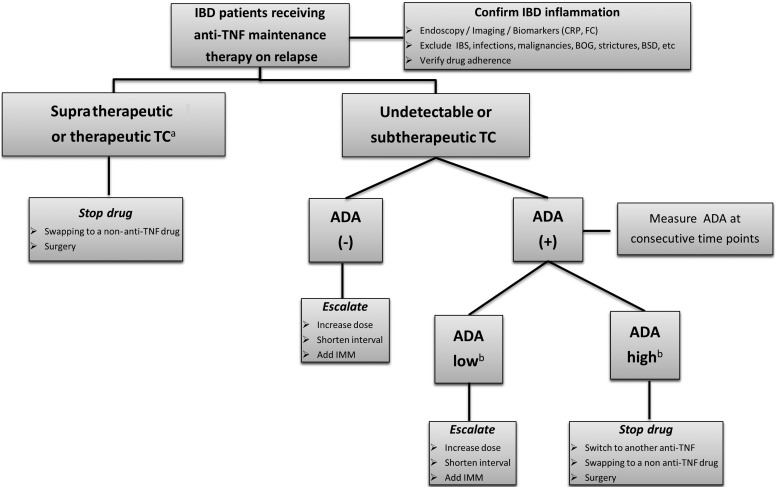
A treatment algorithm for using reactive testing to infliximab is shown in figure 1. It is important to note that when assessing for SLR, it is imperative that one proves that there is objective evidence for active IBD.
Figure 1.
Reactive TDM algorithm of patients with IBD on anti-TNF therapy. aFor relative values refer to text and tables 1 and 2. bTitre depends on the assay used, >8 μg/mL-eq for ELISA23 and >9.1 U/mL for HMSA.11 ADA, antidrug antibody; BOG, bacterial overgrowth; BSD, bile salt diarrhoea; CRP, C reactive protein; eq, equivalent; FC, faecal calprotectin; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; IMM, immunomodulators; TC, trough concentrations; TDM, therapeutic drug monitoring; TNF, tumour necrosis factor.
Proactive TDM
Proactive, in contrast to reactive, TDM has received little attention, although recent data suggest that routine monitoring of drug concentrations with dosing to a therapeutic window may become the standard of care in the very near future.16 40 46
The landmark Trough level Adapted infliXImab Treatment trial, an RCT of 251 patients with infliximab-treated IBD in stable response or remission who were first dose optimised to a therapeutic infliximab concentration window of 3–7 μg/mL and then randomised to either drug concentration or clinically based dosing, showed that among patients who underwent dose escalation for a low trough a significantly higher proportion of patients with CD achieved clinical remission.40 Additionally, dose reduction in those with a high trough concentration did not have any effect on remission rates for either CD or UC, although it significantly reduced the treatment cost.40 Following the optimisation phase, although the primary endpoint of clinical remission at 1 year was similar between the two groups, many secondary outcomes favoured continued dosing to drug concentration. Concentration-dosed group needed rescue therapy less frequently than clinically dosed group (7% vs 17.3%; p=0.004), more patients in concentration-dosed group maintained the goal trough concentration between 3 and 7 μg/mL (74% vs 57%; p<0.001) and fewer patients in the concentration-dosed group had undetectable trough concentrations (OR: 3.7; p<0.001). It is also important to note there was a similar cost between the two treatment strategies.40
The effectiveness of a dose-escalation strategy based on TDM in patients on remission with supratherapeutic drug concentrations was further confirmed by a prospective study of 20 adult patients with IBD on deep remission for at least 4 months after receiving infliximab 10 mg/kg every 8 weeks for SLR and with trough levels >8 μg/mL, whose infliximab dose was decreased 1 mg/kg until they achieved a trough level between 3 and 7 μg/mL. After a median follow-up of 8 months after de-escalation, 18/20 (90%) of patients remained in deep remission and only 2 experienced a clinical relapse.70
The first study published to demonstrate the efficacy of proactively dose optimising based on trough concentrations was an observational study of 126 patients with IBD. In this study, patients in clinical remission who underwent proactive TDM and infliximab dose optimisation based on a therapeutic window of 5–10 μg/mL had markedly improved persistence on infliximab when compared with a similar control group of patients with IBD receiving standard of care (ie, reactive testing or empiric dose escalation if needed).16 The main reasons for infliximab cessation in the control group were acute infusion reactions and SLR. Furthermore, the cumulative probability of infliximab persistence was higher in patients achieving a trough concentration >5 μg/mL compared with those who did not (HR: 0.03; 95% CI 0.01 to 0.1; p<0.0001)).16 Among these patients, there was no difference in the probability of remaining on infliximab between those on monotherapy or combination therapy with IMM, suggesting the potential for ‘optimised monotherapy’ defined as proactive therapeutic infliximab trough concentration monitoring and titration to concentration level of 5–10 μg/mL (but at least 3 μg/mL) and continued monitoring at regular interval. Several of these patients were on ‘optimised monotherapy’ throughout the course of the study, whereas others had the IMM stopped during the follow-up. No patient on ‘optimised monotherapy’ stopped infliximab at end of data collection with a median follow-up time of 3.4 years. This suggests that in patients in clinical remission on combination therapy and adequate trough levels the IMM may be safely withdrawn. This concept is in agreement with another study which showed that in patients receiving dual therapy, those with infliximab trough levels >5 μg/mL at the time the IMM discontinuation have a decreased risk for infliximab dose escalation, IBD-related surgery and infliximab cessation due to SLR compared with those without.37 Furthermore, it was previously shown that although patients who continued to receive combination therapy with IMM had higher median trough levels of infliximab and lower CRP concentrations than those who discontinued IMM, no clear clinical benefit of combo-therapy was observed beyond 6 months.71
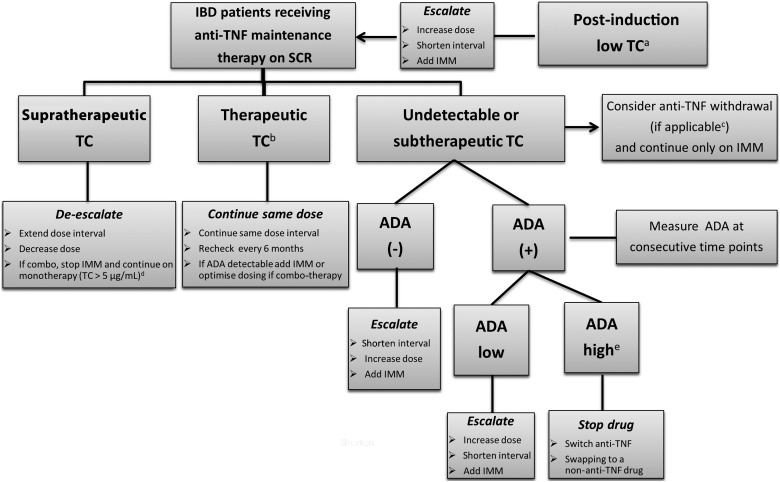
A treatment algorithm for using proactive testing for infliximab is shown in figure 2.
Figure 2.
Proactive TDM algorithm of patients with IBD on anti-TNF therapy. aFor relative values of infliximab and adalimumab concentrations at week 14 and week 4, respectively, associated with different therapeutic outcomes, refer to tables 1 and 2. bFor relative values, refer to text and tables 1 and 2. cLow risk for relapse after anti-TNF withdrawal.72 dBased on data from Vaughn et al16 and Drobne et al.37 eTitre depends on the assay used, >8 μg/mL-eq for ELISA23 and >9.1 U/mL for HMSA.11 ADA, antidrug antibody; eq, equivalent; HMSA, homogeneous mobility shift assay; IBD, inflammatory bowel disease; IMM, immunomodulators; SCR, sustained clinical remission; TC, trough concentration; TDM, therapeutic drug monitoring; TNF, tumour necrosis factor.
The same concepts should also hold for adalimumab, though data are lacking. Finally, besides the association of immunogenicity and infusion reactions, it is still unknown if TDM could improve the safety profile of anti-TNF therapy in IBD, as there are only limited data regarding the potential association of supratherapeutic drug concentrations with increased toxicity or adverse events.
Induction therapy
There are only limited data regarding the role of TDM during anti-TNF induction therapy in IBD. Nevertheless, higher infliximab concentrations during and early after the induction phase are associated with favourable short-term and long-term therapeutic outcomes, whereas low or undetectable drug concentrations and ADA are associated with PNR, SLR and treatment discontinuation, suggesting that target concentration adjusted dosing should be implement early, even during the induction therapy (tables 1 and 2).7 9 27 31–33 44 An observational study assessing the long-term clinical benefit of adalimumab in patients with CD who failed to respond to infliximab showed that patients who discontinued adalimumab by week 2 (6.5 vs 10.4 μg/mL, p=0.02) and week 4 (2.5 vs 5.9 μg/mL, p=0.012) had lower trough serum concentration compared with those who continued throughout maintenance treatment.26 In a retrospective, single-centre study regarding 285 consecutive patients with refractory UC treated with infliximab postinduction (week 14), median infliximab serum concentrations were significantly higher in patients with UC with short-term complete clinical response (5.96 vs 2.20 μg/mL, p<0.001), short-term CRP normalisation (6.27 vs 2.02 μg/mL, p<0.001) and in patients with short-term mucosal healing (5.96 vs 1.74 μg/mL, p<0.001).8 Moreover, preliminary data from post-hoc analyses of RCTs in UC show that higher infliximab concentration at week 2 (>21.3 μg/mL) and week 6 (>22 μg/mL) are associated with short-term clinical remission and response, respectively.6 7 In a recent retrospective study, a ROC analysis identified infliximab concentration thresholds of 28.3 μg/mL at week 2 (area under the ROC curve (AUROC: 0.638)) and 15 μg/mL at week 6 (AUROC: 0.688) associated with short-term mucosal healing, while infliximab concentration ≥15 at week 6 (p=0.025; OR: 4.6; 95% CI 1.2 to 17.1) was independently associated with short-term mucosal healing.31 On the other hand, there is compelling evidence that PNR in (acute) severe UC may be attributable to accelerated drug clearance due to higher baseline inflammatory burden and/or the development of ADA, characterised by high levels of faecal and low levels of serum infliximab.31 32 44
These preliminary data point out that drug concentrations during the induction phase probably need to be higher compared with maintenance therapy, as this is when there is higher inflammation and requirement for more drug, although large, prospective clinical trials are certainly warranted. As a proof of concept, an ‘accelerated’ infliximab induction regimen (3 doses within a median period of 24 days) for the treatment of (acute) severe UC was more beneficial, at least in the short-term, compared with the classic induction regimen of 0, 2 and 6 weeks.73 However, the role of TDM in guiding therapeutic decisions after PNR to anti-TNF therapy, such as early optimisation of treatment by either increasing the dose and/or shortening the intervals, switching to another anti-TNF or swapping to a non-anti-TNF agent, is still awaited. Nevertheless, if PNR to anti-TNF therapy with adequate drug concentrations occurs, it makes sense to change to a biologic drug with a different mechanism of action.3
Laboratory assays and TDM
Although there have been recent advances in the methods used for TDM of anti-TNF therapy, comparability, standardisation, clinical validation and quality control between the assays remains an open issue that limits extrapolation of values among tests and clinical interpretation of the results.65 Common assays to measure anti-TNF concentrations and ADA include the ELISA,74 the radio-immunoassay (RIA)75 and the homogeneous mobility shift assay (HMSA).76 These assays have a different setup and therefore different advantages and drawbacks relating to their sensitivity and implementation in everyday clinical practice.31 36 65 For example, in contrast to HMSA, the bridging ELISA is a drug-sensitive assay, thus ADA are only measurable when serum drug concentration is undetectable. On the other hand, ELISA is an inexpensive, clinically validated and easy-to-use assay compared with the costly and cumbersome HMSA and RIA, respectively. However, most of the comparison studies show that despite variable analytical properties, common assays result in similar classifications and interventions in patients with IBD with infliximab treatment failure, and with comparable clinical outcomes.11 65 74 77–79
Conclusions
Numerous studies in IBD have demonstrated a positive correlation between adequate serum anti-TNF concentrations and favourable therapeutic outcomes including clinical and endoscopic remission, whereas ADA have been associated with SLR and infusion reactions. Currently, most TDM is done in the reactive setting. This reactive TDM has been shown to be more cost-effective than empiric dose escalation of anti-TNF therapy and better directs care in IBD. Moreover, there is increasing evidence for the benefits of proactive TDM and dose optimisation, which is likely to be the standard of care in the near future. Nevertheless, before personalised medicine and TDM-based algorithms can be fully implemented in real-life clinical practice, an optimal concentration therapeutic window, the most clinically relevant time of measuring drug concentrations (peak, intermediate, trough) and the most suitable assay used should first be clearly determined.80 Future perspectives include the development of rapid assays that allow anti-TNF (including biosimilars) concentration measurement at the site of point of care in the hospital or even at home and software decisions support tools that will incorporate a predictive PK model based on patient and disease characteristics.81–83
Footnotes
Contributors: KP: drafting and final approval of the manuscript, ASC: critical revision and final approval of the manuscript.
Funding: KP received a fellowship grant from the Hellenic Group for the study of IBD.
Competing interests: ASC received a consultancy fee from AbbVie, Janssen, UCB, Takeda, Prometheus and Pfizer.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1.Billiet T, Rutgeerts P, Ferrante M, et al. Targeting TNF-α for the treatment of inflammatory bowel disease. Expert Opin Biol Ther 2014;14:75–101. doi:10.1517/14712598.2014.858695 [DOI] [PubMed] [Google Scholar]
- 2.Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn's disease. Aliment Pharmacol Ther 2011;33:987–95. doi:10.1111/j.1365-2036.2011.04612.x [DOI] [PubMed] [Google Scholar]
- 3.Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97. doi:10.1097/MIB.0000000000000202 [DOI] [PubMed] [Google Scholar]
- 4.Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev 2014;13:24–30. doi:10.1016/j.autrev.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 5.Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol 2003;98:1315–24. doi:10.1111/j.1572-0241.2003.07457.x [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi T, Suzuki Y, Motoya S, et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis . J Gastroenterol Published Online First:11 Jul 2015. doi:10.1007/s00535-015-1102-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014;147:1296–307.e5. doi:10.1053/j.gastro.2014.08.035 [DOI] [PubMed] [Google Scholar]
- 8.Arias MT, Vande Casteele N, Vermeire S, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:531–8. doi:10.1016/j.cgh.2014.07.055 [DOI] [PubMed] [Google Scholar]
- 9.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014;63:1721–7. doi:10.1136/gutjnl-2012-304094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn's disease. J Crohns Colitis 2013;7:736–43. doi:10.1016/j.crohns.2012.10.019 [DOI] [PubMed] [Google Scholar]
- 11.Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013;108:962–71. doi:10.1038/ajg.2013.12 [DOI] [PubMed] [Google Scholar]
- 12.Roblin X, Marotte H, Leclerc M, et al. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohns Colitis 2015;9:525–31. doi:10.1093/ecco-jcc/jjv061 [DOI] [PubMed] [Google Scholar]
- 13.Reinisch W, Colombel JF, Sandborn WJ, et al. Factors associated with short- and long-term outcomes of therapy for Crohn's disease. Clin Gastroenterol Hepatol 2015;13:539–47.e2. doi:10.1016/j.cgh.2014.09.031 [DOI] [PubMed] [Google Scholar]
- 14.Steenholdt C, Bendtzen K, Brynskov J, et al. Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn's disease. Scand J Gastroenterol 2011;46:310–18. doi:10.3109/00365521.2010.536254 [DOI] [PubMed] [Google Scholar]
- 15.Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNFα therapy: Serum levels of infliximab and adalimumab associate with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol Published Online First: 29 Oct 2015. doi:10.1016/j.cgh.2015.10.025 doi:10.1016/j.cgh.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 16.Vaughn BP, Martinez-Vazquez M, Patwardhan VR, et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis 2014;20:1996–2003. doi:10.1097/MIB.0000000000000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010;59:49–54. doi:10.1136/gut.2009.183095 [DOI] [PubMed] [Google Scholar]
- 18.Imaeda H, Bamba S, Takahashi K, et al. Relationship between serum infliximab trough levels and endoscopic activities in patients with Crohn's disease under scheduled maintenance treatment. J Gastroenterol 2014;49:674–82. doi:10.1007/s00535-013-0829-7 [DOI] [PubMed] [Google Scholar]
- 19.Roblin X, Rinaudo M, Jarlot C, et al. Infliximab trough level thresholds vary depending on the efficacy criterion chosen in IBD patients. J Crohn's Colitis 2015;9(Suppl 1):S3. [Google Scholar]
- 20.Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol 2006;4:1248–54. doi:10.1016/j.cgh.2006.06.025 [DOI] [PubMed] [Google Scholar]
- 21.Warman A, Straathof JW, Derijks LJ. Therapeutic drug monitoring of infliximab in inflammatory bowel disease patients in a teaching hospital setting: results of a prospective cohort study. Eur J Gastroenterol Hepatol 2015;27:242–8. doi:10.1097/MEG.0000000000000279 [DOI] [PubMed] [Google Scholar]
- 22.Levesque BG, Greenberg GR, Zou G, et al. A prospective cohort study to determine the relationship between serum infliximab concentration and efficacy in patients with luminal Crohn's disease. Aliment Pharmacol Ther 2014;39:1126–35. doi:10.1111/apt.12733 [DOI] [PubMed] [Google Scholar]
- 23.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med 2003;348:601–8. doi:10.1056/NEJMoa020888 [DOI] [PubMed] [Google Scholar]
- 24.Baert F, Vande Casteele N, Tops S, et al. Prior response to infliximab and early serum drug concentrations predict effects of adalimumab in ulcerative colitis. Aliment Pharmacol Ther 2014;40:1324–32. doi:10.1111/apt.12968 [DOI] [PubMed] [Google Scholar]
- 25.Baert F, Kondragunta V, Lockton S, et al. Antibodies to adalimumab are associated with future inflammation in Crohn's patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut 2016;65: 1126–31.. doi:10.1136/gutjnl-2014-307882 [DOI] [PubMed] [Google Scholar]
- 26.Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn's disease. Gastroenterology 2009;137:1628–40. doi:10.1053/j.gastro.2009.07.062 [DOI] [PubMed] [Google Scholar]
- 27.Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014;12:80–4.e2. doi:10.1016/j.cgh.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 28.Frederiksen MT, Ainsworth MA, Brynskov J, et al. Antibodies against infliximab are associated with de novo development of antibodies to adalimumab and therapeutic failure in infliximab-to-adalimumab switchers with IBD. Inflamm Bowel Dis 2014;20:1714–21. doi:10.1097/MIB.0000000000000138 [DOI] [PubMed] [Google Scholar]
- 29.Mazor Y, Almog R, Kopylov U, et al. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn's disease. Aliment Pharmacol Ther 2014;40:620–8. doi:10.1111/apt.12869 [DOI] [PubMed] [Google Scholar]
- 30.Colombel JF, Sandborn WJ, Allez M, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn's disease. Clin Gastroenterol Hepatol 2014;12:423–31.e1. doi:10.1016/j.cgh.2013.10.025 [DOI] [PubMed] [Google Scholar]
- 31.Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol Published Online First: 8 Dec 2015. doi:10.1016/j.cgh.2015.11.014 doi:10.1016/j.cgh.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 32.Brandse JF, Mathôt RA, van der Kleij D, et al. Pharmacokinetic features and presence of anti-drug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol 2016;14:251–8.e2. doi:10.1016/j.cgh.2015.10.029 [DOI] [PubMed] [Google Scholar]
- 33.Paul S, Del Tedesco E, Marotte H, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis 2013;19:2568–76. doi:10.1097/MIB.0b013e3182a77b41 [DOI] [PubMed] [Google Scholar]
- 34.Yarur AJ, Jain A, Sussman DA, et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut 2016;65:249–55. doi:10.1136/gutjnl-2014-308099 [DOI] [PubMed] [Google Scholar]
- 35.Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn's disease. Gut 2015;64:1539–45. doi:10.1136/gutjnl-2014-307883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imaeda H, Takahashi K, Fujimoto T, et al. Clinical utility of newly developed immunoassays for serum concentrations of adalimumab and anti-adalimumab antibodies in patients with Crohn's disease. J Gastroenterol 2014;49:100–9. doi:10.1007/s00535-013-0803-4 [DOI] [PubMed] [Google Scholar]
- 37.Drobne D, Bossuyt P, Breynaert C, et al. Withdrawal of immunomodulators after co-treatment does not reduce trough level of infliximab in patients with Crohn's disease. Clin Gastroenterol Hepatol 2015;13:514–21.e4. doi:10.1016/j.cgh.2014.07.027 [DOI] [PubMed] [Google Scholar]
- 38.Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn's disease. Gastroenterology 2014;146:681–8.e1. doi:10.1053/j.gastro.2013.11.024 [DOI] [PubMed] [Google Scholar]
- 39.Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn's disease. Clin Gastroenterol Hepatol 2015;13:1042– 50.e2. doi:10.1016/j.cgh.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 40.Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.e3. doi:10.1053/j.gastro.2015.02.031 [DOI] [PubMed] [Google Scholar]
- 41.Pariente B, Laharie D. Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2014;40:338–53. doi:10.1111/apt.12838 [DOI] [PubMed] [Google Scholar]
- 42.Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn's disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27. doi:10.1136/gutjnl-2013-305279 [DOI] [PubMed] [Google Scholar]
- 43.Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualized therapy is a long-term cost-effective method compared to dose intensification in Crohn's disease patients failing infliximab. Dig Dis Sci 2015;60:2762–70. doi:10.1007/s10620-015-3581-4 [DOI] [PubMed] [Google Scholar]
- 44.Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology 2015;149:350–5.e2. doi:10.1053/j.gastro.2015.04.016 [DOI] [PubMed] [Google Scholar]
- 45.Fasanmade AA, Adedokun OJ, Ford J, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol 2009;65:1211–28. doi:10.1007/s00228-009-0718-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buurman DJ, Maurer JM, Keizer RJ, et al. Population pharmacokinetics of infliximab in patients with inflammatory bowel disease: potential implications for dosing in clinical practice. Aliment Pharmacol Ther 2015;42:529–39. doi:10.1111/apt.13299 [DOI] [PubMed] [Google Scholar]
- 47.Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis 2014;20:2247–59. doi:10.1097/MIB.0000000000000212 [DOI] [PubMed] [Google Scholar]
- 48.Billiet T, Vande Casteele N, Van Stappen T, et al. Immunogenicity to infliximab is associated with HLA-DRB1. Gut 2015;64:1344–5. doi:10.1136/gutjnl-2015-309698 [DOI] [PubMed] [Google Scholar]
- 49.Ordás I, Mould DR, Feagan BG, et al. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–46. doi:10.1038/clpt.2011.328 [DOI] [PubMed] [Google Scholar]
- 50.Ungar B, Haj-Natour O, Kopylov U, et al. Ashkenazi Jewish origin protects against formation of antibodies to infliximab and therapy failure. Medicine (Baltimore) 2015;94:e673 doi:10.1097/MD.0000000000000673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pallagi-Kunstár E, Farkas K, Szepes Z, et al. Utility of serum TNF-α, infliximab trough level, and antibody titers in inflammatory bowel disease. World J Gastroenterol 2014;20:5031–5. doi:10.3748/wjg.v20.i17.5031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut 2014;63:1258–64. doi:10.1136/gutjnl-2013-305259 [DOI] [PubMed] [Google Scholar]
- 53.Farrell RJ, Alsahli M, Jeen YT, et al. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn's disease: a randomized controlled trial. Gastroenterology 2003;124:917–24. doi:10.1053/gast.2003.50145 [DOI] [PubMed] [Google Scholar]
- 54.Afif W, Loftus EV Jr, Faubion WA et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9. doi:10.1038/ajg.2010.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol 2004;2:542–53. [DOI] [PubMed] [Google Scholar]
- 56.Zitomersky NL, Atkinson BJ, Fournier K, et al. Antibodies to infliximab are associated with lower infliximab levels and increased likelihood of surgery in pediatric IBD. Inflamm Bowel Dis 2015;21:307–14. doi:10.1097/MIB.0000000000000284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West RL, Zelinkova Z, Wolbink GJ, et al. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn's disease. Aliment Pharmacol Ther 2008;28:1122–6. doi:10.1111/j.1365-2036.2008.03828.x [DOI] [PubMed] [Google Scholar]
- 58.Ben-Horin S, Yavzori M, Katz L, et al. The immunogenic part of infliximab is the F(ab’)2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut 2011;60:41–8. doi:10.1136/gut.2009.201533 [DOI] [PubMed] [Google Scholar]
- 59.Ainsworth MA, Bendtzen K, Brynskov J. Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn's disease. Am J Gastroenterol 2008;103:944–8. doi:10.1111/j.1572-0241.2007.01638.x [DOI] [PubMed] [Google Scholar]
- 60.Candon S, Mosca A, Ruemmele F, et al. Clinical and biological consequences of immunization to infliximab in pediatric Crohn's disease. Clin Immunol 2006;118:11–19. doi:10.1016/j.clim.2005.07.010 [DOI] [PubMed] [Google Scholar]
- 61.Steenholdt C, Frederiksen MT, Bendtzen K, et al. Time course and clinical implications of development of antibodies against adalimumab in patients with inflammatory bowel disease. J Clin Gastroenterol Published Online First: 9 Jul 2015 doi:10.1097/MCG.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 62.Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol 2013;108:40–7; quiz 48 doi:10.1038/ajg.2012.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baert F, Drobne D, Gils A, et al. Early trough levels and antibodies to infliximab predict safety and success of reinitiation of infliximab therapy. Clin Gastroenterol Hepatol 2014;12:1474–81.e2; quiz e91 doi:10.1016/j.cgh.2014.01.033 [DOI] [PubMed] [Google Scholar]
- 64.O'Meara S, Nanda KS, Moss AC. Antibodies to infliximab and risk of infusion reactions in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014;20:1–6. [DOI] [PubMed] [Google Scholar]
- 65.Kopylov U, Mazor Y, Yavzori M, et al. Clinical utility of antihuman lambda chain-based enzyme-linked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies. Inflamm Bowel Dis 2012;18:1628–33. doi:10.1002/ibd.21919 [DOI] [PubMed] [Google Scholar]
- 66.Roblin X, Rinaudo M, Del Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol 2014;109:1250–6. doi:10.1038/ajg.2014.146 [DOI] [PubMed] [Google Scholar]
- 67.Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015;13:522–30.e2. doi:10.1016/j.cgh.2014.07.029 [DOI] [PubMed] [Google Scholar]
- 68.Steenholdt C, Bendtzen K, Brynskov J, et al. Changes in serum trough levels of infliximab during treatment intensification but not in anti-infliximab antibody detection are associated with clinical outcomes after therapeutic failure in Crohn's disease. J Crohns Colitis 2015;9:238–45. doi:10.1093/ecco-jcc/jjv004 [DOI] [PubMed] [Google Scholar]
- 69.Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn's disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol 2013;11:654–66. doi:10.1016/j.cgh.2012.12.035 [DOI] [PubMed] [Google Scholar]
- 70.Paul S, Roblin X, Peyrin-Biroulet L. Letter: infliximab de-escalation based on trough levels in patients with inflammatory bowel diseases. Aliment Pharmacol Ther 2015;42:939–40. doi:10.1111/apt.13335 [DOI] [PubMed] [Google Scholar]
- 71.Van Assche G, Magdelaine-Beuzelin C, D'Haens G, et al. Withdrawal of immunosuppression in Crohn's disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology 2008;134:1861–8. doi:10.1053/j.gastro.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 72.Papamichael K, Vermeire S. Withdrawal of anti-tumour necrosis factor α therapy in inflammatory bowel disease. World J Gastroenterol 2015;21:4773–8. doi:10.3748/wjg.v21.i16.4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:330–5.e1. doi:10.1016/j.cgh.2014.07.041 [DOI] [PubMed] [Google Scholar]
- 74.Vande Casteele N, Buurman DJ, Sturkenboom MGG, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther 2012;36:765–71. doi:10.1111/apt.12030 [DOI] [PubMed] [Google Scholar]
- 75.Bendtzen K, Geborek P, Svenson M, et al. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum 2006;54:3782–9. doi:10.1002/art.22214 [DOI] [PubMed] [Google Scholar]
- 76.Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J Immunol Methods 2012;382: 177–88. doi:10.1016/j.jim.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 77.Steenholdt C, Ainsworth MA, Tovey M, et al. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohn's disease. Ther Drug Monit 2013;35:530–8. doi:10.1097/FTD.0b013e31828d23c3 [DOI] [PubMed] [Google Scholar]
- 78.Steenholdt C, Bendtzen K, Brynskov J, et al. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn's disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol 2014;109:1055–64. doi:10.1038/ajg.2014.106 [DOI] [PubMed] [Google Scholar]
- 79.Bodini G, Giannini EG, Furnari M, et al. Comparison of two different techniques to assess adalimumab trough levels in patients with Crohn's disease. J Gastrointestin Liver Dis 2015;24:451–6. [DOI] [PubMed] [Google Scholar]
- 80.Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn's disease—algorithm for practical management. Aliment Pharmacol Ther 2016;43:30–51. doi:10.1111/apt.13445 [DOI] [PubMed] [Google Scholar]
- 81.Papamichael K, Van Stappen T, Jairath V, et al. Review article: pharmacological aspects of anti-TNF biosimilars in inflammatory bowel diseases. Aliment Pharmacol Ther 2015;42:1158–69. doi:10.1111/apt.13402 [DOI] [PubMed] [Google Scholar]
- 82.Kuin S, Stolte SB, van den Brink GR, et al. Short article: remicade infusions at home: an alternative setting of infliximab therapy for patients with Crohn's disease. Eur J Gastroenterol Hepatol 2016;28:222–5. doi:10.1097/MEG.0000000000000530 [DOI] [PubMed] [Google Scholar]
- 83.Corstjens PL, Fidder HH, Wiesmeijer KC, et al. A rapid assay for on-site monitoring of infliximab trough levels: a feasibility study. Anal Bioanal Chem 2013;405:7367–75. doi:10.1007/s00216-013-7154-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunogenicity of maintenance anti-TNF therapy in IBD RCTs.
flgastro-2016-100685supp_table.pdf (194.2KB, pdf)