Abstract
Introduction
Southampton General Hospital provides inflammatory bowel disease (IBD) services for a population of 650 000. Biological agents have impacted hugely on IBD but are costly drugs requiring careful supervision. These challenges led us to develop a specialist nurse-led biologics service to improve patient care.
Method
A 2010 case note audit highlighted areas for improvement in monitoring biologics and follow-up. A business case was developed to establish an IBD nurse to ensure identification and appropriate screening, education and review of biologics patients. A gain share was agreed with the local Care Commissioning Group (CCG) and £60 000 invested. Outcomes were reaudited in 2014.
Results
Biologic use has grown rapidly from 90 patients in 2011 to 330 in 2014. All records are now kept in a centralised database. Infection screening improved from 79% to 100%. In 2014, 96% of patients had follow-up ≤4 months post-induction to assess response, but two patients were seen at 7 months. 80% were followed up again at 9–12 months (100% at 9–14 months), all with treatment decisions. The initial investment was recouped via commissioners funding 368 additional outpatient appointments and 35 colonoscopies. Savings represented 15% total yearly biologic costs.
Conclusions
The introduction of the IBD biologics nurse-led service resulted in significant gains in care quality and costs. The need for improved follow-up of patients on biologics reflects increased pressures on clinic resources across the country. With continued biologics expansion, the introduction of a biologics nurse has provided invaluable support to patients and the IBD team at Southampton General Hospital.
Keywords: CROHN'S DISEASE, INFLAMMATORY BOWEL DISEASE, ULCERATIVE COLITIS, ANTIBODY TARGETED THERAPY, HEALTH ECONOMICS
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory relapsing/remitting disease affecting an estimated 620 000 people in the UK.1 IBD can impact upon growth and development, fertility and pregnancy, psychological health, personal relationships and employment. It is important to have a defined IBD team to deliver optimal patient outcomes and improve quality of life as defined by the UK IBD standards1 and endorsed by National Institute for Health and Care Excellence (NICE).2
Biological agents have had a huge impact on the management of patients who had IBD with moderate–severe disease. Infliximab (Remicade) is a chimeric anti-tumour necrosis factor (TNFα) monoclonal antibody typically administered every 8 weeks as a 1–2 hours infusion in a hospital day unit. Adalimumab (Humira) is a recombinant human immunoglobulin anti-TNFα monoclonal antibody usually self-administered subcutaneously every fortnight. Infliximab is licensed for the treatment of fistulising Crohn's, and both agents have a UK licence for moderate to severe Crohn's not responding to conventional treatment. NICE guidance3 supports infliximab as rescue therapy in acute severe ulcerative colitis (UC) when steroids have failed, and more recently has approved infliximab, adalimumab, golimumab and vedolizumab for moderate to severe UC.4 5 Biologic use continues to expand, with a number of infliximab biosimilars coming to market and numerous agents with new modes of action in clinical trials. Biologics are costly, ranging from £10 000 to £20 000 per patient annum. Treatment should be reviewed after 1 year and continuation only if clear evidence of active disease.3 The STORI study6 helps to stratify relapse risk in patients and some centres use this data to inform treatment cessation. Continuation of treatment should be based upon symptoms, biological markers, imaging and endoscopy where appropriate and should take into account patient wishes as well as the proximity of major life events. Patients who continue treatment should have regular blood monitoring and review of their disease and treatment by a specialist at least annually. Deciding when to cease treatment can be as important as initiating treatment due to the high associated costs of biologics and the importance of reducing the risk of potentially serious side effects.
The safe, cost-effective and efficient provision of biological treatment requires careful coordination and supervision. Prior to commencing biologics, patients should be screened for opportunistic infections including HIV, hepatitis B and C, varicella and tuberculosis (TB).7 In addition, patients' nutritional status should be assessed and optimised, and a careful history taken to assess the risk of TB exposure and of recurrent infection, which may suggest pre-existing immunodeficiency. Patients must be adequately counselled on treatment risks and benefits, and should feel supported in making this decision by an experienced specialist.
Once treatment has commenced, there is a need to identify primary non-responders so alternative treatments can be considered. With the increasing numbers of patients being treated with these drugs as well as the relentless increase in demands on gastroenterology services, it has become challenging to provide the level of supervision required to ensure optimal patient outcomes. In this context, we set out to develop a specialist nurse-led IBD biologics service. We present a description of the processes involved in setting up this service and subsequent evaluation of the clinical and financial impacts, for which ethical review has not been required.8
IBD services in Southampton
Southampton General Hospital is a busy teaching hospital providing specialist IBD services for a population of 650 000. In 2010, approximately 90 patients with IBD were treated with biological agents. Biological treatments were tracked using paper pharmacy records or prescriptions for the day-case unit. Hospital coders struggled to differentiate between indications for biological treatment and there was no clear financial accounting pathway for at-home treatments. Records for patients receiving adalimumab were held by gastroenterology secretaries. Patients were followed up in the IBD/general gastroenterology clinics and if they did not attend or had appointments cancelled, there was no robust system to ensure timely review. The absence of a clear pathway made it very challenging to provide the appropriate pre-treatment screening, monitoring and support required by these complex patients. Through local and national audit, we identified a number of deficiencies in our own service for patients on biological treatments including screening for opportunistic infections, timely follow-up to assess response and longer-term drug monitoring.
Preintervention audit of services
In 2010, a retrospective case note audit of the processes surrounding the supervision of biological therapies was conducted in the available notes of 85 patients. This highlighted areas for concern in the documentation of treatment, review of response to therapy, documentation of decisions regarding maintenance therapy and monitoring of therapy, which echoed the findings of the Royal College of Physicians national IBD biologics audit.9
Documentation
In 2010, only 17% of patients had a clear management plan documented. About 79% of patients had documentation of the exclusion of absolute contraindications to therapy such as opportunistic infection and cancer. The results of the pre-treatment chest X-ray were documented in the notes of only 56% of patients.
Treatment decisions and follow-up
NICE recommend that patients should be followed up after initial induction (before third infusion for infliximab and after up to 3 months for adalimumab) to identify primary non-responders, and approximately 10 months after induction to assess appropriateness of treatment continuation.3 This is important to ensure that treatment failures can be recognised promptly and an appropriate management plan initiated. Ideally this should be undertaken by a consultant with a subspecialty interest in IBD. In 2010, 80% of patients were reviewed ≤12 weeks post-anti-tumour necrosis factor (TNF) induction to assess primary response. A positive decision to continue biological treatment was documented in 83% of those continuing. A third of patients in the audit sample were reviewed in an outpatient clinic on average less than once every 6 months, with 20% seen less than once a year. This represented inadequate medical supervision of patients. Treatment continuation decisions were documented in 73%.
Implementation of a biologics nurse specialist gain share
In response to the preliminary local audit findings and the 2010 publication of NICE TA187,3 a business case was developed to establish the role of the biologics clinical nurse specialist (CNS) to ensure safe, cost-effective use of these high-cost drugs. The trust invested approximately £60 000 to deliver the improvements to the service (specialist nurse, equipment, clinics) and a scheme was agreed with local care commissioners as a gain share of savings commencing in the 2013/2014 financial year. A gain share is a collaborative arrangement between commissioners and providers in working together to create incentives that achieve better outcomes for patients and more efficient use of medicines which are not reimbursed via the National Tariff.10 National Health Service (NHS) England recommend a number of key principles to facilitate these improvements which include the prescription of more cost-effective alternatives, moving to more effective operating strategies and reducing wastage,10 all of which may be addressed by the implementation of a biologics nurse specialist.
The role of the biologics nurse specialist
The biologics nurse specialist fulfils the role of an advanced IBD nurse set out by the N-European Crohn's and Colitis Organisation (ECCO) international consensus11 with a particular focus on the care of patients receiving biological therapies. The nurse works closely with several other specialist IBD nurses and although there is some overlap with traditional IBD nursing roles, the volume of biologics patients means responsibilities are dedicated largely to patients receiving these complex treatments. Our CNS is an experienced senior sister with a background of inpatient gastroenterology nursing, as well as managing the endoscopy department and the infusion unit within the trust where infliximab is administered. The band 7 post was commenced in January 2012 at 37.5 whole time equivalent hours per week. A list of competences appropriate to the post was developed and a training programme put in place before the nurse started IBD biologics clinics after 6 months. During this time, all the patients who had IBD treated with biologics within our trust were identified from pharmacy and clinical records and from the infusion unit.
The role of the IBD biologics CNS includes:
Maintaining a database of patients treated with biologics.
Coordinating referrals for patients requiring biologics following decision to treat.
Increasing patient safety by ensuring screening for opportunistic infection and other risk factors including infection or cancer histories.
Patient/carer counselling and education.
Liaison with infusion day unit.
Injection technique and support.
Liaison with Healthcare at Home for provision and delivery of supplies.
Blood monitoring.
Coordinating regular clinic follow-up, in particular postinduction therapy review to assess response, and yearly review to plan future therapy.
Inpatient anti-TNF support.
Audit and clinical governance (leading data entry for the national biological therapies audit).
Research study recruitment.
Fortnightly biologics multi-disciplinary team (MDT) review.
Postintervention audit of services
Biologic use
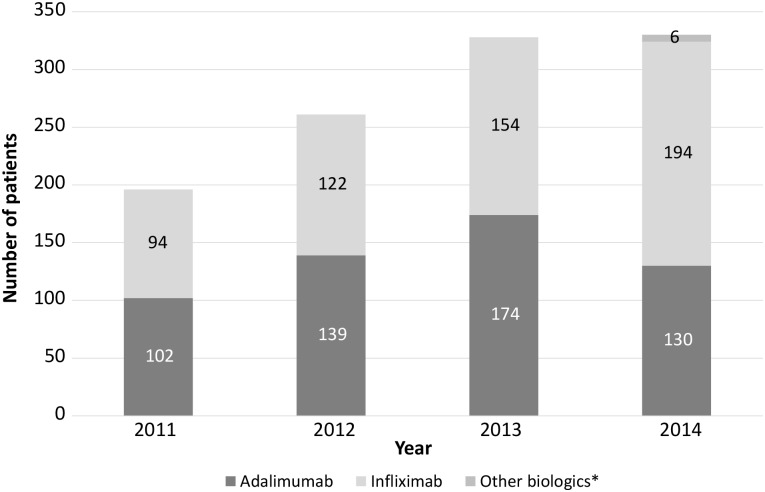
At the end of 2014, yearly biologic pharmacy data since 2011 were reviewed. A further audit of 50 patient notes commencing biological treatment between January and August 2013 was also conducted to assess the impact of the nurse-led service in a number of key areas. This time frame was selected to allow for assessment of adequacy of yearly follow-up of biological treatment. Biologic use has increased rapidly in recent years both in Southampton and across the country. In 2011, just prior to the introduction of the IBD biologics CNS, 196 patients received biologics, climbing steadily each year to 330 in 2014 (figure 1).
Figure 1.
Yearly IBD biologic use in Southampton General Hospital. IBD, inflammatory bowel disease.
Documentation
All records of biologic prescription are now kept in a centralised database. One hundred per cent of patients in the 2014 audit had documented evidence of a chest X-ray (CXR) prior to commencing biological treatment, a significant improvement upon previous findings and also comparing favourably with the 2013 national audit data rate of 93%.9 Opportunistic infection screening was again completed fully in 100% of patients compared with the national audit figures (TB 32%, hepatitis B 79%, hepatitis C 78%, varicella 61% and HIV 41%).9 All had record of absolute exclusion criteria having been reviewed.
Treatment decisions and follow-up
Only 74% of patients (37/50) had follow-up within 3 months of commencing biological therapy (vs 80% preintervention), although 96% (48/50) were seen by a physician <4 months postinduction. Two patients were not seen until 7 months postinduction. We would expect that follow-up of patients would improve postimplementation of the nurse specialist, however, this decline reflects a paucity of available outpatient slots and increasing stresses on many IBD services as IBD incidence and biologic use increase. Achievement of 12 months treatment review remained static at 80% both pre-intervention and post-intervention in those patients continuing biological therapy beyond induction (32/40). However, 100% were followed up by 14 months postinduction. One hundred per cent of patients had a documented decision regarding ongoing treatment (table 1).
Table 1.
Documentation, screening and treatment review post-CNS implementation
| Intervention | 2010—prebiologics CNS (%) | 2014—postbiologics CNS (%) |
|---|---|---|
| Prescription record | 17 | 100 |
| Documented chest X-ray | 56 | 100 |
| Documented exclusion of opportunistic infection* | 79 | 100 |
| 3 months review | 80 | 74 (96% ≤4 months) |
| 12 months review | 80 | 80 (100% ≤14 months) |
*Hepatitis B and C, HIV, TB, varicella.
CNS, clinical nurse specialist; TB, tuberculosis.
The national biologics audit revealed that across the UK as many as 29% of patients did not even have at least one follow-up encounter recorded during the course of their biological treatment.9 Nearly all clinic records reviewed in our audit sample specified timelier planned follow-up than achieved. To partly address this, Southampton General Hospital offers a telephone advice line which helps to improve appropriateness of unscheduled outpatient appointments, and has developed a highly successful yearly ‘virtual’ postal follow-up clinic for stable patients, freeing up an estimated 400 clinic slots per year (table 1).12
Discontinuation of biological treatment
The implementation of the biologics nurse specialist has led to improved monitoring and appropriateness of biological therapy. Between 2011 and 2014, a total of 165 biological treatments were discontinued for various reasons (table 2). Almost a third (n=51) of these patients had biologics discontinued due to: disease remission (n=29, mean 34.5 months treatment, range 2–85 months), patient choice (n=7, mean 17.8 months, range 3–30 months) or non-adherence (n=15, mean 13.5 months, range 2–33 months). These are all factors which may not have previously been recognised without the improved treatment supervision provided by a specialist nurse.
Table 2.
Indications for discontinuation of biological treatment
| Indication | 2012 | 2013 | 2014 | Total |
|---|---|---|---|---|
| Primary loss of response | 13 | 6 | 5 | 24 |
| Secondary loss of response | 11 | 10 | 4 | 25 |
| Side effects | 16 | 14 | 6 | 36 |
| Clinical remission | 4 | 10 | 15 | 29 |
| Bridging/induction therapy | 3 | 7 | 1 | 11 |
| Non-adherence | 3 | 4 | 8 | 15 |
| Patient choice | 5* | 1 | 1 | 7 |
| Deceased | 1† | 2† | 2† | 5 |
| Moved out of area | 1 | 1 | 6 | 8 |
| Infection | 1‡ | 0 | 3 | 4 |
| Malignancy | 0 | 0 | 1§ | 1 |
| Total patients | 58 | 55 | 52 | 165 |
*Pregnancy and life events.
†Unrelated to anti-tumour necrosis factor.
‡Tuberculosis; patient choice to discontinue.
§Diffuse large B cell lymphoma, remission on cessation of infliximab and azathioprine therapy.
Outcomes of gain share
The implementation of the biologics CNS has proved financially successful within the first year of the initiative, with significant drug-cost savings. The initial £60 000 investment by the trust was recouped within a year through activity income in the form of an additional 368 outpatient appointments and 35 colonoscopies conducted as a result of improvements to the biologics service. Based upon average costs of £11 000 per patient year of biological drug use, the first financial year (2013–2014; table 3) included estimated savings of £198 000 as a result of biologic review/discontinuation and £89 000 due to an increase in the proportion of patients on adalimumab versus infliximab. Ideal placement of the biologics nurse to facilitate patient identification and recruitment into biologics studies throughout the year led to £124 000 in drug-cost savings, with Southampton ranking highly for recruitment in several large IBD studies. When shared with local CCGs as part of the gain-share agreement, the resulting total savings from biologics CNS activities represented an estimated 15% of total yearly biologic drug costs for the trust. In response to increased demand for biologics, a specialist biologics pharmacist role was developed to work closely with the nurse specialist to facilitate efficient biologic provision.
Table 3.
Estimated cost savings for first financial year—2013–2014
| Area of change | Estimated cost saving (£) |
|---|---|
| Additional clinic capacity, IT and specialist nurse | −60 000 |
| Biologic cessation: disease remission | 110 000 |
| Primary non-responders* | 44 000 |
| Non-adherence with treatment/monitoring | 44 000 |
| Increased use of adalimumab vs infliximab | 89 000 |
| Transferred to research study | 124 000 |
| Activity income: colonoscopies | 18 445 |
| Follow-up appointments | 41 216 |
| Total savings | 410 691 |
*Estimated one-third of yearly biologics treatment received.
IT, information technology.
The future of the biologics service
Electronic management systems
Between 2011 and 2014, biologic use in Southampton expanded rapidly by 68% from 196 to 330 patients. To cope with the increased demands of this complex population of patients, we have developed two major information technology (IT) initiatives. An electronic biologics management system as part of an extensive update the pre-existing EMIS gastroenterology database will allow point of care data capture to improve monitoring. This will feed data into the national IBD Registry13 and the UK IBD Biologics audit9 as well as providing an excellent resource for biologics monitoring and research. The IBD Portal, a web-based self-management system built upon the Microsoft HealthVault platform, has also been developed to empower patients to gain greater control over their IBD. The portal incorporates educational material, clinic scheduling, test results, monitoring diaries, interactive treatment algorithms and anticipated faecal calprotectin monitoring, all supported by secure email communication with the IBD team. It has been piloted successfully in 50 patients and is intended for use in all patients with IBD, including more complex patients receiving biological therapy.
Biosimilars
Recent developments in anti-TNF biosimilar medications are anticipated to improve patient access to advanced IBD treatments through reduced drug acquisition costs. Southampton General Hospital currently employs two specialist IBD nurses in addition to the biologics CNS to manage a busy IBD service. In order to conduct a managed switching programme from Remicade to biosimilar infliximab, funding has been obtained from a further gain-share agreement with local commissioning groups for an additional biologics CNS to oversee biosimilar use and support inpatient biologics services. Locally available biosimilars are estimated to be 20%–50% cheaper than Remicade, suggesting potential procurement savings of between £300K and £800K annually based on projected 2014/2015 costs.
Conclusions
The introduction of the specialist IBD biologics nurse-led service has resulted in substantial improvements in quality of care and cost savings, and fulfils the requirements of the IBD Standards1 by maintaining a patient-centred service, educating and supporting patients and families and using data, IT and audit to support patient care and inform clinical commissioning groups. One clear area for improvement is the need for provision of adequate follow-up for patients on biologics, and this reflects increased pressures on gastroenterology clinic resources across the country as illustrated by the national biologics audit. It is hoped that increased nursing support and empowerment of patients through self-management strategies can help to alleviate some of these pressures. With the continued expansion of biologics, the introduction of a specialist biologics nurse has proved an invaluable service that allows us to continue to meet and improve upon high standards of IBD care in Southampton. We would strongly recommend the implementation of similar initiatives in other specialist IBD centres to improve IBD standards across the UK.
Key messages.
What is already known on this topic?
Specialist nurses are key to providing a comprehensive inflammatory bowel disease (IBD) service. The 2013 international Nurse-ECCO consensus describes the important role of advanced IBD nurses in the screening, education and assessment of response to biological treatments.
What this study adds?
This paper describes the implementation and outcomes of an advanced specialist biologics nursing role and the negotiation of a financial gain-share agreement with local care commissioners.
How might it impact on clinical practice in the foreseeable future?
Other healthcare providers wishing to realise similar improvements for their inflammatory bowel disease service can be guided by our experiences.
Footnotes
Contributors: NST conducted the analysis and drafted the manuscript, with revision by JRFC for intellectual content. MB advised on the role of the specialist nurse and provided biologics data. KP, SK and JW provided financial/commissioning advice, and CU provided pharmacy biologics data and advice. All authors contributed to the writing and approved the final manuscript.
Competing interests: JRFC has received lecture fees/honorarium from Napp pharmaceuticals, MSD, Abbvie, Biogen, Hospira, Celltrion and Janssen.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Anonymised audit data can be made available on application to the authors. Detailed financial data has not been made publicly available at the discretion of the trust finance department.
References
- 1.The IBD Standards Group, 2009. http://www.bsg.org.uk/attachments/160_IBDstandards.pdf (accessed Nov 2015).
- 2.NICE Quality Standard [QS81]—Inflammatory Bowel Disease. http://www.nice.org.uk/guidance/qs81/chapter/quality-statement-2-multidisciplinary-team-support (accessed Nov 2015). [Google Scholar]
- 3.NICE technology appraisal guidance 187. Infliximab (review) and adalimumab for the treatment of Crohn's disease (including a review of technology appraisal guidance 40). http://www.nice.org.uk/guidance/TA187. (accessed Nov 2015).
- 4.NICE technology appraisal guidance 329. Infliximab, adalimumab and golimumab for the treatment of moderately to severely active ulcerative colitis after failure of conventional therapy. https://www.nice.org.uk/guidance/ta329 (accessed Nov 2015).
- 5.NICE technology appraisal guidance 342. Vedolizumab for treating moderately to severely active ulcerative colitis. https://www.nice.org.uk/guidance/ta342 (accessed Nov 2015).
- 6.Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission amongst patients with Crohn's disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 2012;142:63–70. doi:10.1053/j.gastro.2011.09.034 [DOI] [PubMed] [Google Scholar]
- 7.Mowat C, Cole A. Windsor I, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011;60:571–607. doi:10.1136/gut.2010.224154 [DOI] [PubMed] [Google Scholar]
- 8.http://www.hra.nhs.uk/documents/2013/09/defining-research.pdf (accessed Apr 2016).
- 9.Executive summary of the national clinical audit of biological therapies UK Inflammatory Bowel Disease (IBD) audit 2013. https://www.rcplondon.ac.uk/sites/default/files/executive_summary_of_the_national_clinical_audit_of_biological_therapies_-_adult_report._29_august_2013_0.pdf (accessed Nov 2015).
- 10.Principles for sharing the benefits associated with more efficient use of medicines not reimbursed through national prices NHS England 2014. http://www.england.nhs.uk/wp-content/uploads/2014/01/princ-shar-benefits.pdf (accessed Nov 2015).
- 11.O'Connor M, Bager P, Duncan J, et al. N-ECCO Consensus statements on the European nursing roles in caring for patients with Crohn's disease or ulcerative colitis. J Crohns Colitis 2013;7:744–64. doi:10.1016/j.crohns.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 12.Hunter J, Claridge A, James S, et al. Improving outpatient services: the Southampton IBD virtual clinic. Frontline Gastroenterol 2012;3:76–80. doi:10.1136/flgastro-2012-100123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The IBD Registry. http://ibdregistry.org.uk/ (accessed Nov 2015).