 Chirality variation in amphiphile architecture resulted in a significant difference in detergent efficacy for membrane protein stabilisation.
Chirality variation in amphiphile architecture resulted in a significant difference in detergent efficacy for membrane protein stabilisation.
Abstract
Amphiphile selection is a crucial step in membrane protein structural and functional study. As conventional detergents have limited scope and utility, novel agents with enhanced efficacy need to be developed. Although a large number of novel agents have been reported, so far there has been no systematically designed comparative study of the protein stabilization efficacy of stereo-isomeric amphiphiles. Here we designed and prepared a novel class of stereo-isomeric amphiphiles, designated butane-1,2,3,4-tetraol-based maltosides (BTMs). These stereoisomers showed markedly different behaviour for most of the targeted membrane proteins depending on the chirality of the linker region. These findings indicate an important role for detergent stereochemistry in membrane protein stabilization. In addition, we generally observed enhanced detergent efficacy with increasing alkyl chain length, reinforcing the importance of the balance between hydrophobicity and hydrophilicity in detergent design. The stereo-isomeric difference in detergent efficacy observed provides an important design principle for the development of novel amphiphiles for membrane protein manipulation.
Introduction
Membrane proteins are crucial cellular components, responsible for a range of key biological functions including inter- and intra-cellular material transfer and signal transduction. More than 50% of all clinical drug molecules target membrane proteins and thus their structure and function are main foci of the pharmaceutical industry.1 Our understanding of membrane protein structure and function, however, is hampered by difficulties associated with handling these bio-macromolecules.2 Most membrane proteins are not soluble in aqueous solutions because they have large hydrophobic surfaces when properly folded; therefore, detergents are required to extract membrane proteins from the membranes and to maintain them in their native state in non-native environments. Conventional detergents such as n-dodecyl-β-d-maltoside (DDM), lauryldimethylamine-N-oxide (LDAO) and n-octyl-β-d-glucoside (OG) are widely used for membrane protein study, but many membrane proteins tend to denature/aggregate even when solubilized with these agents,3,4 making it difficult to conduct functional studies, spectroscopic analysis or crystallization trials.
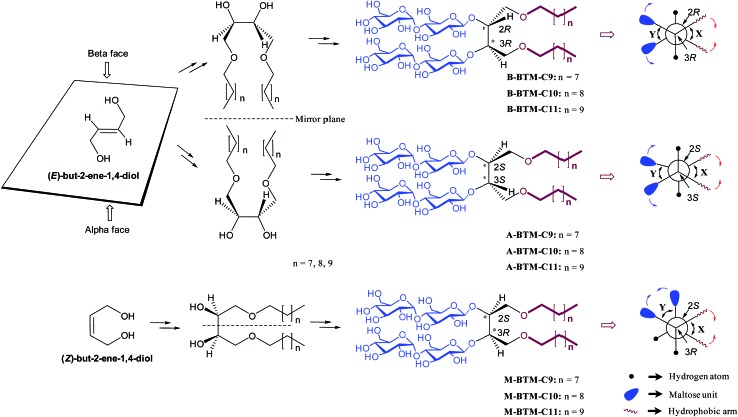
Because of the suboptimal behavior of conventional detergents, there is a persistent demand for new amphiphilic “assistants” with enhanced membrane protein solubilization and stabilization characteristics. Recent representatives include β-peptides (BPs),5 facial amphiphiles (FAs),6 the neopentyl-glycol (NG) amphiphiles7 (glucose neopentyl-glycols (GNGs), maltose neopentyl-glycols (MNGs), and neopentyl glycol triglucosides (NDTs)) and penta-saccharide amphiphiles (PSEs).8 Note that the NG class has contributed to crystal structure determination of ∼25 membrane proteins including the β2 adrenergic, acetylcholine and opioid receptors in the last few years.9 Despite considerable progress in the development of novel amphiphiles, more are still required, because many membrane proteins, particularly eukaryotic ones, are currently refractory to structural determination. Furthermore, no systematic study has been reported for the stereo-chemical effect of detergent on membrane protein stability. In this study, we designed and prepared three alkyl chain variants for three stereo-isomers (α, β and meso) of butane-1,2,3,4-tetraol-based maltosides (BTMs), designated A-, B- and M-BTMs, respectively (Fig. 1). In spite of the common head and tail groups, these stereoisomers showed distinct behaviors with target membrane proteins depending on their stereochemistry. In addition, we found that a C11 alkyl chain detergent (M-BTM-C11) was significantly superior to DDM for all the four membrane proteins targeted in this study.
Fig. 1. Chemical structures of newly prepared BTMs (middle-right) and their Newman projections (extreme right). B- and A-BTMs were derived from E-but-2-ene-1,4-diol while M-BTMs were derived from Z-but-2-ene-1,4-diols (extreme left). The Sharpless asymmetric dihydroxylation was used for facial diol generation (middle left). R or S designation was used to specify the stereochemistry of the two chiral carbons (C2 and C3) (middle right). The Newman projections for individual isomers include the two dihedral angles between the two hydrophobic arms and between the two hydrophilic groups (designated X and Y, respectively) along with their relative motions induced in an aqueous medium. The reduction in the X dihedral angle (indicated by red arrows) will increase the dihedral angle Y (indicated by purple arrows) for the A-/B-isomers, but will decrease the Y dihedral angle for the M-isomer.
Results and discussion
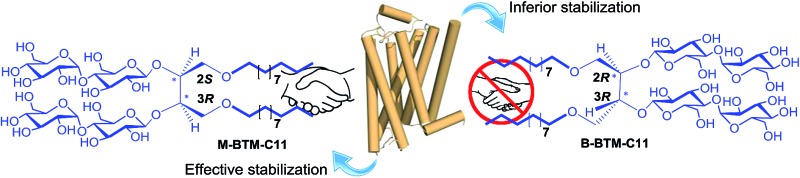
Detergent structures and physical characterizations
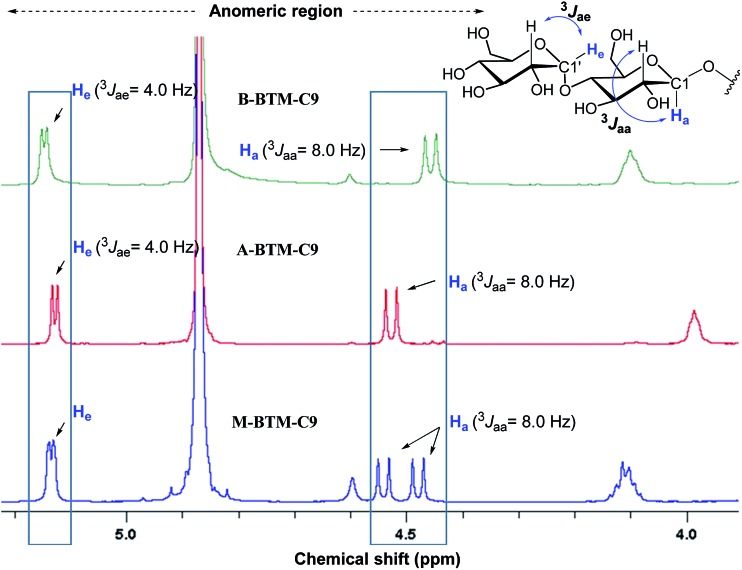
The architectures of the BTMs vary in terms of the stereochemistry in the linker region (Fig. 1). The design of the novel agents features two alkyl chains and two dimaltoside headgroups, connected by a butane-1,2,3,4-tetraol (BT) linker. The presence of two chiral centers in the BT linker (C2 and C3) allowed us to synthesize three BTM stereoisomers (A-BTMs, B-BTMs and M-BTMs). The BT linker of the A-isomers contains chiral centers with a 2S and 3S configuration while that of the B-isomer contains a 2R and 3R configuration. Thus, the non-carbohydrate regions of these agents (A-/B-BTMs) are mirror images and are thus enantiomers of each other. In contrast, the BT linker with a 2R and 3S (or 2S and 3R) configuration was used for preparation of the M-isomers. This results in a diastereomeric relationship between the non-carbohydrate regions of the M-isomer and A-/B-isomers. Note that the non-sugar units of the M-isomers are meso compounds due to the presence of a symmetry plane (Fig. 1). As each non-carbohydrate region of the A-/B-/M-BTMs was connected with two maltoside headgroups via a stereospecific β-glycosidic linkage, however, all these agents are diastereomers to each other, with different relative directions of the tail and head groups. Additionally, the carbon chain length varied from C9 to C11 in each set of BTMs and was used for detergent designation. The A- and B-isomers were prepared through introduction of two hydroxyl groups into the α and β faces of (E)-but-2-ene-1,4-diol derivatives, respectively (Fig. 1). High stereo-specificity for this reaction was achieved by the well-known Sharpless asymmetric dihydroxylation.10 In contrast, the M-isomers were derived from (Z)-but-2-ene-1,4-diol through syn-dihydroxylation using OsO4 (see ESI† for details). The M-BTMs possess synthetic advantages over the other isomers as they could be prepared in four high-yielding synthetic steps, making them highly accessible. In contrast, large scale manufacture of the other two isomers (A-/B-isomers) requires more effort and cost mainly as a result of the introduction of chirality at the C2/C3 carbons; the expensive AD-mix-α/β reagents need to be used for the Sharpless asymmetric dihydroxylation process that takes five days to complete (see ESI† for details). A synthetic protocol comprising four steps gave overall yields of 50–60%. The high diastereomeric purity of the different isomers was confirmed by their individual 1H NMR spectra (Fig. 2 and S1†). Protons of the A- and B-isomers of BTM-C9 attached to the anomeric carbon (C1), designated H a, gave rise to 1H NMR peaks at 4.53 and 4.46 ppm as doublets while those of the M-isomer gave two separated peaks centered at 4.48 and 4.54 ppm as doublets. In addition, the coupling constants (3 J) for the anomeric protons (H a) of all the isomers were 8.0 Hz, typical of a β-anomer, demonstrating successful formation of β-linkage in the glycosylation step. Note that α-glycosidic bond formation will produce a peak downfield shifted to around 5.14 ppm with a much smaller coupling constant (3 J = 4.0 Hz) for the anomeric proton, as observed for another proton (H e) attached to the anomeric carbon (C1′) of all the BTM isomers (Fig. 2).
Fig. 2. Anomeric region of the 1H NMR spectra for the three BTM-C9 isomers showing their high diastereomeric purity (see Fig. S1† for the full range of 1H NMR spectra). Each isomer gave unique spectral features in the anomeric region, indicative of the clear differentiation of the individual isomers by their 1H NMR spectra. Vicinal coupling constants (3 J aa & 3 J ae) are indicated above individual peaks to allow differentiation of the α- and β-anomeric protons (H e and H a, respectively, in blue). The chemical structure of the maltoside head group is shown at the top right corner to illustrate the anomeric protons of interest.
All new agents were water-soluble up to 20 wt% except the BTM-C11s, for which a brief sonication was required to generate clear aqueous solutions of ∼10%. Since we anticipated that any BTM-C12 would exhibit even lower solubility we did not prepare these versions of the amphiphiles. Critical micelle concentrations (CMCs) were estimated by monitoring encapsulation of the fluorescent probe (diphenylhexatriene (DPH))11 with increasing detergent concentration and the hydrodynamic radii (R h) of the micelles determined via dynamic light scattering (DLS). Table 1 shows the summarized data for the BTMs along with DDM. The CMC values of all BTMs (from 0.006 to 0.023 mM) turned out to be much smaller than that of DDM (0.17 mM), indicating a stronger tendency to form self-assemblies than DDM. The CMC values of the new agents decreased with increasing alkyl chain length from C9 to C11 irrespective of chirality variation in the linker region. This is likely due to the increased hydrophobicity resulting from the alkyl-chain extension. Notably, the M-isomers gave the lowest CMC values of the three BTM stereoisomers, followed by the A-isomers. For instance, the CMC value of M-BTM-C9 is ∼0.017 mM compared to ∼0.021 and ∼0.023 mM for A- and B-BTM-C9, respectively. As these three stereoisomers have the same tail and head groups (i.e. the same hydrophobicity and hydrophilicity), this result implies a different tendency to self-aggregate depending on the stereochemistry.12 The M-isomers have the strongest self-aggregation tendency, followed by the A- and B-isomers. The sizes of micelles formed by the BTMs tend to increase with increasing alkyl chain length. For instance, detergent micelle size increased from 3.2 to 3.5 to 4.7 nm with alkyl chain lengths of C9, C10, and C11, respectively, for the M-isomers. Note that small differences in micelle size were observed between the equivalent A- and B-isomers, which could be attributed to the enantiomeric relationship between their hydrophobic groups (Fig. 1). In contrast, the M-isomers tend to form substantially larger micelles than the other stereoisomers.
Table 1. Molecular weights (MWs) and critical micelle concentrations (CMCs) of BTMs and a conventional detergent (DDM) and hydrodynamic radii (R h; n = 4) of their micelles.
| Detergent | MW a | CMC (mM) | CMC (wt%) | R h b (nm) |
| B-BTM-C9 | 1023.2 | ∼0.023 | ∼0.0023 | 2.9 ± 0.04 |
| A-BTM-C9 | 1023.2 | ∼0.021 | ∼0.0022 | 2.9 ± 0.04 |
| M-BTM-C9 | 1023.2 | ∼0.017 | ∼0.0017 | 3.2 ± 0.05 |
| B-BTM-C10 | 1051.2 | ∼0.013 | ∼0.0014 | 3.1 ± 0.07 |
| A-BTM-C10 | 1051.2 | ∼0.011 | ∼0.0012 | 3.2 ± 0.07 |
| M-BTM-C10 | 1051.2 | ∼0.008 | ∼0.0009 | 3.5 ± 0.07 |
| B-BTM-C11 | 1079.3 | ∼0.008 | ∼0.0009 | 3.5 ± 0.08 |
| A-BTM-C11 | 1079.3 | ∼0.007 | ∼0.0008 | 3.5 ± 0.03 |
| M-BTM-C11 | 1079.3 | ∼0.006 | ∼0.0006 | 4.7 ± 0.27 |
| DDM | 510.1 | ∼0.17 | ∼0.0087 | 3.4 ± 0.03 |
aMolecular weight of detergents.
bHydrodynamic radius of detergents measured at 1.0 wt% using dynamic light scattering.
The distinct characteristics of the M-isomers compared to the A- and B-isomers (small CMC values and large micelle formation) could be due to the isomeric variation in molecular conformation adopted in an aqueous medium. In a gaseous state, each molecule would adopt an approximately staggered conformation, forming dihedral angles between the alkyl chains and between the maltoside headgroups (designated X and Y, respectively, Fig. 1) of ∼60°. Because of the hydrophobic effect, however, an aqueous environment would force the two alkyl chains of each isomer to come together, resulting in a decrease in the dihedral angle (X) between the two alkyl chains and accordingly an increase in torsional/steric strain in the molecule. Thus, the two alkyl chains of the detergent molecules dissolved in an aqueous solution will adopt a compromise position between these two opposite forces, resulting in a dihedral angle (X) less than 60°. Interestingly, depending on the stereochemistry of the isomers (A-/B-/M-isomers), the reduced dihedral angle (X) gives rise to different outcomes in the relative orientation of the two maltoside headgroups (Fig. 1). Specifically, the reduction in the X dihedral angle will decrease the dihedral angle (Y) between the two headgroups in the M-isomers, but will increase Y for the A-/B-isomers. As a result, the M-isomers will adopt a conformation with relatively small dihedral angles of both X and Y, giving small hydrophobic as well as hydrophilic volumes relative to the other isomers. This unique feature of the M-isomers appeared to generate more effective micellar packing than the A- and B-isomers with relatively large hydrophilic volumes, consistent with the relatively small CMC values observed for the M-isomers. In addition, the M-isomers are likely to have geometry closer to a cylindrical shape than the A- and B-isomers for the same reason, and this is probably responsible for the relatively large micelles formed by these isomers. When an energy-minimized conformation of each isomer was calculated using density functional theory (DFT) at the level of B3LYP/6-31G*, the Y dihedral angle was smallest for the M-isomer (Fig. S2†); in this calculation, both dihedral angles of X and Y were smaller than 60° for the M-isomer while the X dihedral angles were smaller than 60°, but the Y dihedral angles were larger than 60° for the A- and B-isomers. These findings are supportive of our hypothesis on the relative motions of the alkyl chain and the hydrophilic groups in an aqueous solution. Finally, when we investigated the size distribution for micelles formed by the BTMs via DLS, all isomers showed only one population of micelles, as does DDM (Fig. S3†).
Detergent evaluation with membrane proteins
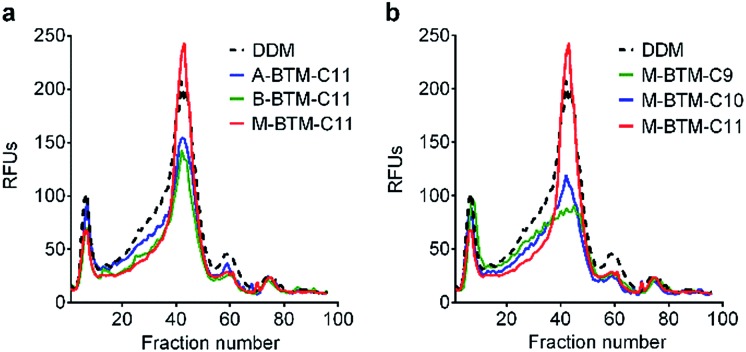
To assess the potential utility of new amphiphiles as tools for membrane protein study, multiple protein systems must be examined. We used DDM as a benchmark for conventional detergent performance in each case, because this agent is probably the most commonly employed detergent in current membrane protein research.13 As a start, the new amphiphiles were evaluated for their ability to maintain the native state of a protein transporter, UapA: a uric acid–xanthine/H+ symporter from Aspergillus nidulans.14 After protein extraction from the membranes using 1.0 wt% DDM, the thermostability of UapA was assessed through fluorescence size exclusion chromatography (FSEC)15 following heat treatment at 40 °C for 10 min. As can be seen in Fig. 3a, DDM-solubilized UapA yielded a monodispersed peak of relatively high intensity (∼40 fraction number), indicating that this agent maintains the protein in a stable state. When A-BTM-C11 and B-BTM-C11 were used for this experiment, however, these agents resulted in a substantial reduction in size of the monodisperse protein peak compared to DDM. Thus, a significant portion of the transporter solubilized in these agents had aggregated/denatured. In contrast, M-BTM-C11-stabilized UapA showed a marked increase in the recovery of the monodispersed peak compared to DDM and the other isomers. In addition, the monodisperse peak was noticeably narrower, indicating that the M-isomer is superior to the other isomers and even better than DDM at preserving protein integrity (Fig. 3a). The C9 and C10 versions of the M-isomer were markedly worse than DDM (Fig. 3b), but these isomers were still consistently better than all the A-/B-isomers regardless of chain length (Fig. S4†). Collectively, the M-isomers were superior to the A- and B-isomers at maintaining the UapA in a stable state in solution, with M-BTM-C11 additionally better than DDM.
Fig. 3. Thermo-stability of UapA solubilized in (a) BTM-C11 isomers (A-BTM-C11, B-BTM-C11 and M-BTM-C11) and (b) M-isomers with different alkyl chain length (M-BTM-C9, M-BTM-C10, and M-BTM-C11). A conventional detergent (DDM) was used as a positive control. Protein stability was accessed via fluorescence size exclusion chromatography (FSEC) after heating the samples for 10 min at 40 °C. The data is representative of two independent experiments.
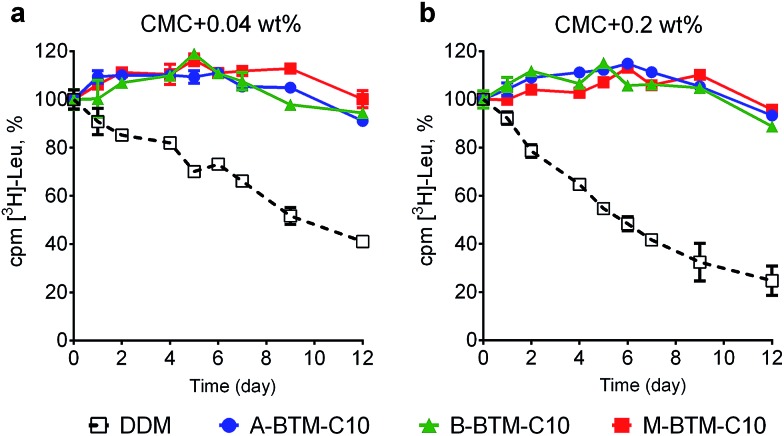
These BTM agents were also evaluated with the bacterial leucine transporter (LeuT) from Aquifex aeolicus.16,17 The transporter was initially extracted with 1.0 wt% DDM and purified in 0.05 wt% of the same detergent. DDM-purified LeuT was diluted into buffer solutions containing individual BTMs or DDM to reach a final detergent concentration of CMC + 0.04 wt%. We monitored protein activity at regular intervals by measuring radiolabeled substrate ([3H]-Leu) binding via a scintillation proximity assay (SPA)18 during a 12 day incubation at room temperature. As can be seen in Fig. 4a, all BTM-C10 isomers were markedly superior to DDM. When the detergent concentration was increased to CMC + 0.2 wt%, differences in detergent efficacy between BTM-C11s and DDM were more evident (Fig. 4b). Remarkably, all BTM-C10s fully retained the transporter activity during 12 day incubation even at the higher detergent concentration. This is in stark contrast with the more progressive degradation observed for the DDM-solubilized transporter with increasing detergent concentration. However, no clear difference between the isomers was observed in maintaining transporter activity. When the alkyl chain length was increased to C11 or decreased to C9, all the BTMs (BTM-C11s and BTM-C9s) were inferior to the C10 versions of the BTMs, indicating that the BTM architecture with the C10 alkyl chain length is optimal for LeuT stability. However, all BTM isomers with C9 or C11 alkyl chains were found to be still better than DDM, particularly at the higher detergent concentration of CMC + 0.2 wt% (Fig. S5 and S6†). This result suggests that the overall BTM architecture is favorable for LeuT stability. It is noteworthy that, similar to the C10 isomers, all BTM-C9 or BTM-C11 stereoisomers displayed very similar behavior with respective to LeuT activity. This indicates that, in contrast to UapA, for LeuT there is little effect of the stereo-chemical differences of the BTM isomers on LeuT stability.
Fig. 4. Long-term stability of LeuT solubilized in BTM-C10 isomers or DDM at the two detergent concentrations: (a) CMC + 0.04 wt% and (b) CMC + 0.2 wt%. The ligand binding activity of the transporter was measured using a radio-labeled substrate ([3H]-Leu) at regular intervals during a 12 day incubation at room temperature. LeuT activity was measured via scintillation proximity assay (SPA). Error bars, SEM, n = 3.
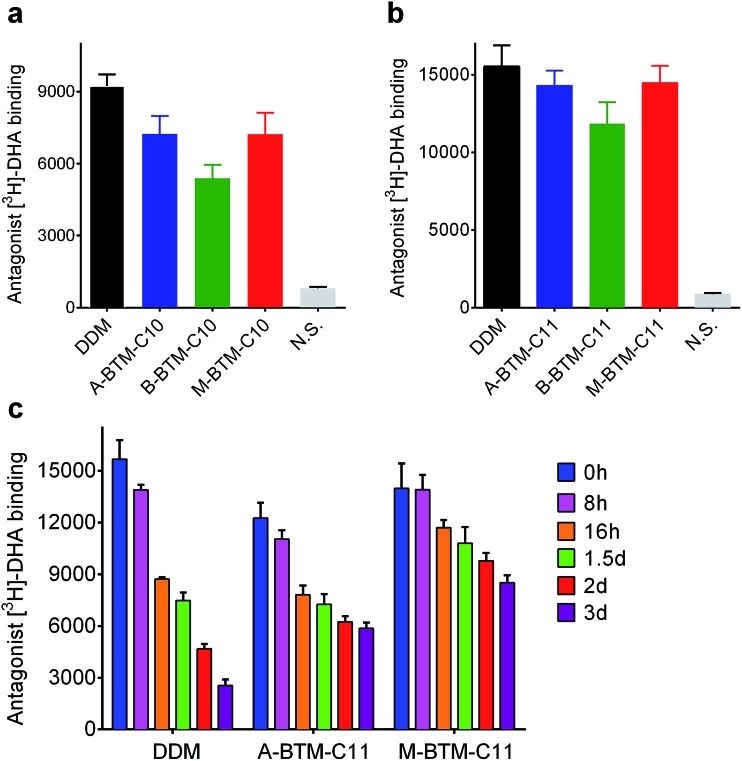
We next turned to a G protein-coupled receptor (GPCR),19 the human β2 adrenergic receptor (β2AR), to further evaluate the novel agents. For this experiment, the receptor was extracted from the membranes using DDM and purified in the same detergent. The DDM-purified receptor was diluted in buffer solutions containing either the individual BTMs without cholesteryl hemisuccinate (CHS) or DDM with CHS. The final detergent concentration was CMC + 0.2 wt%. Ligand binding activity of the receptor was assessed using the antagonist ([3H]-dihydroalprenolol (DHA)).20–22 All C10 versions of the BTMs were inferior to DDM at maintaining the ligand binding activity of the receptor (Fig. 5a). In this evaluation, the A- and M-isomers showed a similar efficacy, and these isomers were both better than the B-isomer. A similar trend was observed in efficacy order for the C11 versions of the BTMs; the A- and M-isomers were comparable to each other, and better than the B-isomer (Fig. 5b). Note that overall detergent efficacy was significantly enhanced by increasing the alkyl chain length from C10 to C11, reaching a level of receptor activity comparable to that with DDM. The slight decrease in receptor activity observed here for either A-BTM-C11 or M-BTM-C11 relative to DDM is likely attributed to the absence of CHS, known to considerably enhance the stability of GPCRs.23 In order to further differentiate detergent efficacy, the ligand binding activity of the receptor solubilized in A-BTM-C11, M-BTM-C11 and DDM was monitored at regular intervals over a three-day incubation at room temperature. The DDM-solubilized receptor showed high initial activity, but rapidly lost its activity, giving only ∼10% residual activity at the end of the three-day incubation (Fig. 5c). In contrast, the M-BTM-C11-solubilized receptor showed initial activity nearly comparable to that of the DDM-solubilized receptor, and maintained ∼70% of the initial activity even at the end of the three-day incubation (Fig. 5c). Note that M-BTM-C11 was significantly more effective than DDM at stabilizing the receptor even in the absence of CHS. A-BTM-C11-solubilized receptor showed an initial activity a little lower than that with DDM and gave a gradual loss in receptor activity over time. Thus, overall detergent efficacy order for receptor stability is M-BTMs > A-BTMs > B-BTMs, which is more or less consistent with the results obtained for UapA.
Fig. 5. Ligand binding activity of β2AR solubilized in (a) BTM-C10 isomers and (b) BTM-C11 isomers. DDM was used as a positive control. DDM-purified receptor was diluted into buffer solutions containing individual BTMs or DDM/CHS to reach the final detergent concentration of CMC + 0.2 wt%. The ligand binding activity of the transporter was measured using radio-labelled ligands ([3H]-dihydroalprenolol (DHA)). (c) Long-term stability of β2AR solubilized in BTM-C11 isomers or DDM/CHS. The activity of the transporter was measured at regular intervals during a 3 day incubation at room temperature. N. S. indicates non-specific binding. Error bars, SEM, n = 3.
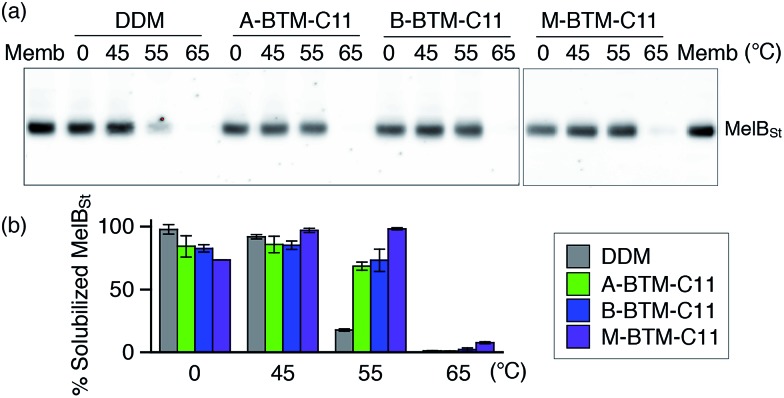
The intriguing results of BTM-C11s with UapA, LeuT and β2AR encouraged us to evaluate these agents with Salmonella typhimurium melibiose permease (MelBSt).24 For protein extraction, E. coli membranes containing MelBSt were treated with 1.5 wt% of A-, B- or M-BTM-C11s for 90 min at 0 °C. As seen in Fig. 6a and b, DDM yielded almost quantitative solubilization of MelBSt under these conditions. The three BTM isomers yielded similar amounts of soluble protein to each other, but were slightly less efficient than DDM at solubilizing the protein from the membranes at this low temperature. In order to investigate detergent efficacy for protein stability at elevated temperature, MelBSt solubilization experiments were carried out at 45 °C. All the BTM isomers efficiently extracted MelBSt and retained MelBSt solubility to a similar level to DDM (Fig. 6b). Note that the M-isomer appeared to be best among the stereo-isomers at this temperature. A more significant difference between the M-isomer and A-/B-isomers was observed when the incubation temperature was further increased to 55 °C. At this high temperature, M-BTM-C11-solubilized MelBSt retained its full solubility while the A- and B-isomer showed a marked decrease in the amount of soluble protein (Fig. 6b). DDM was much less effective in this regard under the same conditions. The superiority of the M-isomer to DDM and the other two isomers was further confirmed by MelBSt solubilization at 65 °C; only the M-isomer produced a detectable amount of soluble MelBSt.
Fig. 6. Thermostability of MelBSt solubilized in three BTM-C11 stereoisomers (A-BTM-C11, B-BTM-C11 and M-BTM-C11) or DDM. MelBSt was extracted from E. coli membranes using 1.5 wt% individual detergents for 90 min at 0, 45, 55 or 65 °C. Following ultracentrifugation to remove insoluble protein and cellular debris, the soluble MelBSt was separated by SDS-PAGE and visualized by Western blot (a). The amount of soluble MelBSt was expressed as percentage of total MelBSt in the untreated membrane (Memb) and presented as a histogram (b). Error bars, SEM, n = 2.
Here we made efforts not only to develop a novel class of amphiphiles for membrane protein study, but also to explore the effect of detergent stereo-isomerism on stabilization of a number of membrane proteins. The new agents (BTMs), particularly BTM-C11s, displayed significantly enhanced behavior toward the solubilization and stabilization of multiple membrane proteins compared to the most representative conventional detergent (DDM). Furthermore, significant differences were observed in detergent efficacy for both UapA and β2AR stability as well as MelBSt solubility, depending on the detergent stereochemistry (efficacy differences between the stereoisomers was minimal for LeuT stability). Of the isomers, the M-BTMs displayed generally the most favorable behavior, followed by the A-BTMs and B-BTMs. The efficacy differences between the A- and B-isomers were relatively small for most target membrane proteins (e.g., UapA and MelBSt) while the differences were rather substantial for β2AR. However, the M-isomers are significantly better than the other isomers at stabilizing these membrane proteins despite the fact that these agents have the same head and tail groups. The effects of the stereo-chemical differences of these BTM isomers could be interpreted by considering two kinds of molecular interaction involved in membrane protein stability: protein–detergent and detergent–detergent interactions. In view of protein–detergent interactions, a target protein could more favorably interact with one stereoisomer than the others because of the presence of inherent chirality in the protein architecture. In this type of interaction, detergent hydrophobic groups interact directly with the hydrophobic surfaces of membrane proteins and thus chirality in this group is important in determining stereo-chemical outcomes. Note that the non-carbohydrate regions of the A- and B-isomers including the hydrophobic alkyl chains are mirror images of each other (enantiomers) but are non-mirror image stereoisomers (diastereomers) with the non-carbohydrate regions of the M-isomers (Fig. 1). Thus, it is likely that the M-isomer gives distinct micellar properties from the other isomers as indicated by the CMCs and micelle sizes (Table 1). Resultantly, the M-isomers are likely to have detergent efficacies distinct from the other isomers, as was observed for UapA, β2AR and MelBSt. Although the protein–detergent interaction plays a role in discriminating stereo-isomeric detergent efficacy, this could be a minor contribution to the overall efficacy difference between the BTM isomers observed in this study. This is due to the fact that the chiral centers (C2 and C3) of the BTM isomers are quite distant from the alkyl tips which closely interact with the protein surfaces. In addition, because of the irregular shape of the protein surfaces, any favorable detergent interactions with certain regions of a protein surface could be counterbalanced by unfavorable detergent interactions with other regions of the protein surface. Thus, a collective difference between the stereoisomers could be minimal as observed in the comparison of the A- and B-isomers with most of the target membrane proteins (UapA, LeuT and MelBSt). A more important factor for detergent efficacy differentiation seemingly originates from detergent–detergent interaction, given that detergent molecules interact with each other around membrane proteins through the micellar arrangement. Favorable detergent–detergent interaction will generate tightly packed detergent micelles, positively associated with micellar stability as well as membrane protein stability. Detergent CMC value may correlate with micellar stability since favorable detergent–detergent interaction would result in a high propensity to self-assemble. In the current study, the M-isomers gave lower CMC values than the other isomers, thus forming more stable micelles. This could contribute to the superior ability of the M-isomer to stabilize three target membrane proteins (UapA, β2AR and MelBSt) compared to the other isomers. At this point, it is unclear why we could not observe any detergent efficacy difference between the stereo-isomers for LeuT.
Over more than two decades, there has been substantial interest in developing novel detergents for membrane protein study, but, in almost every case, an achiral hydrophobic group was connected to a polar head group (e.g., glucose, maltose, or N-oxide).7,12,25 In contrast, the FAs6 and GDN26 contain multiple chiral centers within the hydrophobic moiety, but in these cases the chirality is fixed and hard to vary. Because of the facile chiral induction in the linker region, in contrast, we could conveniently prepare three BTM stereoisomers for comparative evaluation with multiple membrane proteins. This comparison enabled us to find marked differences in their effects on membrane protein stabilization, depending on the detergent chirality. The important role of detergent stereochemistry in membrane protein study has been implicated by previous studies with α-DDM and β-DDM.27 The stereochemistry of these detergents differs by only a single glycosidic bond and thus the overall architecture of these stereoisomers is very similar. In contrast, the BTM isomers bear variations in two chiral centers of the BT linker, resulting in significant changes in the relative orientation of two alkyl chains and molecular geometry. This variability is likely to account for the rather large difference in detergent efficacy observed for the BTMs. In addition, there has been little systematic analysis of the effect of detergent stereochemistry on membrane protein stability. More importantly, by interpreting the detergent stereo-chemical outcome in terms of detergent–detergent interactions in the micellar environment, the current study has provided the first insights into why a particular stereoisomer is superior to others at stabilizing the target membrane proteins.
We also varied the alkyl chain length from C9 to C11 in order to find the best detergent for each membrane protein system. In this variation, BTMs with C10 alkyl chain length were superior for LeuT while the C11 alkyl chain BTMs outperformed the shorter alkyl chain BTMs (BTM-C9s and BTM-C10s) for UapA and β2AR. Initially, we prepared and evaluated BTMs with a C9 or C10 alkyl chain, but all of these agents were inferior to DDM for β2AR and UapA stability. This result prompted us to prepare BTMs with a C11 alkyl chain. These C11 alkyl chain BTMs turned out to be superior to DDM for all four membrane proteins although these agents were slightly inferior to the C10 versions for LeuT. It is important to note that the dimensions of the detergent need to closely match the dimensions of the target membrane protein for protein stability. Thus, it is believed that there is an optimal range of detergent alkyl chain length that varies depending on the target membrane protein. The enhanced efficacy of BTM-C10s relative to BTM-C11s for LeuT suggests that the C10 alkyl chain length is a good match to the dimensions of this particular transporter. However, the computational approach showed that UapA, LeuT and MelBSt have comparable hydrophobic thickness (29–30.5 Å) whereas β2AR is slightly thicker (32 Å) (Table S1†).28 This seemingly contradictory data indicates that other protein properties are also associated with the alkyl chain length optimal for protein stability. For instance, a long alkyl chain detergent would be more effective at preventing protein aggregation than a short alkyl chain agent while the opposite would be true for minimizing protein denaturation.7d Accordingly, the protein’s tendency to aggregate/denature is likely to be another variable affecting optimal detergent alkyl chain length. The optimal alkyl chain length of a detergent for any given membrane protein is almost certainly determined by a combination of multiple factors. Detergent alkyl chain length is also related to hydrophile–lipophile balance (HLB), another important factor in determining protein stability.29 Along with synthetic availability and water-solubility, therefore, multiple detergent properties should be considered when developing novel agents for membrane protein study. As M-BTM-C11 was significantly superior to DDM at stabilizing all targeted membrane proteins introduced here, such multiple properties appeared to be incorporated into the single BTM architecture, a difficult thing to achieve by molecular design.
Conclusions
In conclusion, with variation of stereochemistry and alkyl chain length within the BTM architecture, we have identified M-BTM-C11 as displaying general utility for all four membrane proteins targeted in this study. Because of its convenient synthesis and enhanced efficacy toward multiple membrane proteins, this novel agent will find wide utility in future membrane protein research. Additionally, we have introduced an important detergent structure-property-efficacy relationship related to detergent stereochemistry, which has not been explored in detail by other researchers. Furthermore, our study indicates that an optimal detergent alkyl chain length necessary for protein stability is dependent upon multiple factors including the hydrophobic dimensions of the protein and potentially its tendency to aggregate/denature. Thus, our collective effort involving synthetic chemists, structural biologists and membrane protein scientists not only provides novel detergent tools useful for membrane protein study, but also reveals two important guidelines for future novel amphiphile design.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (grant number 2008-0061891 and 2016R1A2B2011257 to P. S. C. and M. D.). The work was also supported by Biotechnology and Biological Sciences Research Council grant BB/K017292/1 to B. B. and by the Danish Council for Independent Research Sapere Aude program 0602-02100B (to C. J. L.).
Footnotes
References
- (a) Sanders C. R., Myers J. K. Annu. Rev. Biophys. Biomol. Struct. 2004;33:25–51. doi: 10.1146/annurev.biophys.33.110502.140348. [DOI] [PubMed] [Google Scholar]; (b) Overington J. P., Al-Lazikani B., Hopkins A. L. Nat. Rev. Drug Discovery. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Lacapere J. J., Pebay-Peyroula E., Neumann J. M., Etchebest C. Trends Biochem. Sci. 2007;32:259–270. doi: 10.1016/j.tibs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- (a) Garavito R. M., Ferguson-Miller S. J. Biol. Chem. 2001;276:32403–32406. doi: 10.1074/jbc.R100031200. [DOI] [PubMed] [Google Scholar]; (b) Serrano-Vega M. J., Magnani F., Shibata Y., Tate C. G. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10744–10749. doi: 10.1073/pnas.0804396105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Newstead S., Ferrandon S., Iwata S. Protein Sci. 2008;17:466–472. doi: 10.1110/ps.073263108. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) He Y., Wang K., Yan N. Protein Cell. 2014;5:658–672. doi: 10.1007/s13238-014-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Parker J. L., Newstead S. Protein Sci. 2012;21:1358–1365. doi: 10.1002/pro.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H., Lee S. C., Moeller A., Roy R. S., Siu F. Y., Zimmermann J., Stevens R. C., Potter C. S., Carragher B., Zhang Q. Nat. Methods. 2013;10:759–761. doi: 10.1038/nmeth.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Zhang Q., Ma X., Ward A., Hong W.-X., Jaakola V. P., Stevens R. C., Finn M. G., Chang G. Angew. Chem., Int. Ed. 2007;46:7023–7025. doi: 10.1002/anie.200701556. [DOI] [PubMed] [Google Scholar]; (b) Lee S. C. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E1203–E1211. doi: 10.1073/pnas.1221442110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Chae P. S. Nat. Methods. 2010;7:1003–1008. doi: 10.1038/nmeth.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chae P. S., Rana R. R., Gotfryd K. Chem. Commun. 2013;49:2287–2289. doi: 10.1039/c2cc36844g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cho K. H., Bae H. E., Das M., Gellman S. H., Chae P. S. Chem.–Asian J. 2014;9:632–638. doi: 10.1002/asia.201301303. [DOI] [PubMed] [Google Scholar]; (d) Cho K. H., Husri M., Amin A., Gotfryd K., Lee H. J., Go J., Kim J. W., Loland C. J., Guan L., Byrne B., Chae P. S. Analyst. 2015;140:3157–3163. doi: 10.1039/c5an00240k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Sadaf A., Mortensen J. S., Capaldi S., Tikhonova E., Hariharan P., Ribeiro O., Loland C. J., Guan L., Byrnec B., Chae P. S. Chem. Sci. 2016;7:1933–1939. doi: 10.1039/c5sc02900g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsan M. J. Am. Chem. Soc. 2016;138:3789–3796. doi: 10.1021/jacs.5b13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Rasmussen S. G. F. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kruse A. C. Nature. 2012;482:552–556. doi: 10.1038/nature10867. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Manglik A., Kruse A. C., Kobilka T. S., Thian F. S., Mathiesen J. M., Sunahara R. K., Pardo L., Weis W. I., Kobilka B. K., Granier S. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ring A. M., Manglik A., Kruse A. C., Enos M. D., Weis W. I., Garcia K. C., Kobilka B. K. Nature. 2013;502:575–579. doi: 10.1038/nature12572. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Miller P. S., Aricescu A. R. Nature. 2014;512:270–275. doi: 10.1038/nature13293. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Karakas E., Furukawa H. Science. 2014;344:992–997. doi: 10.1126/science.1251915. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Suzuki H., Nishizawa T., Tani K., Yamazaki Y., Tamura A., Ishitani R., Dohmae N., Tsukita S., Nureki O., Fujiyoshi Y. Science. 2014;344:304–307. doi: 10.1126/science.1248571. [DOI] [PubMed] [Google Scholar]; (h) Frick A., Eriksson U. K., de Mattia F., Oberg F., Hedfalk K., Neutze R., Grip W. J., Deen P. M. T., Tornroth-Horsefield S. Proc. Natl. Acad. Sci. U. S. A. 2014;111:6305–6310. doi: 10.1073/pnas.1321406111. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Yin J., Mobarec J. C., Kolb P., Rosenbaum D. M. Nature. 2015;519:247–250. doi: 10.1038/nature14035. [DOI] [PubMed] [Google Scholar]; (j) Kang Y., Zhou X. E. Nature. 2015;523:561–567. doi: 10.1038/nature14656. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Perez C., Gerber S., Boilevin J., Bucher M., Darbre T., Aebi M., Reymond J.-L., Locher K. P. Nature. 2015;524:433–438. doi: 10.1038/nature14953. [DOI] [PubMed] [Google Scholar]; (l) Taniguchi R., Kato H. E., Font J., Deshpande C. N., Wada M., Ito K., Ishitani R., Jormakka M., Nureki O. Nat. Commun. 2015;13:8545. doi: 10.1038/ncomms9545. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Dong Y. Y. Science. 2015;347:1256–1259. doi: 10.1126/science.1261512. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Paulsen C. E., Armache J.-P., Gao Y., Cheng Y., Julius D. Nature. 2015;520:511–517. doi: 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless B. K., Amberg W., Bennani Y. L., Crispino G. A., Hartung J., Jeong K.-S., Kwong H.-L., Morikawa K., Wang Z.-M., Xu D., Zhang X.-L. J. Org. Chem. 1992;57:2768–2771. [Google Scholar]
- (a) Chattopadhyay A., London E. Anal. Biochem. 1984;139:408–412. doi: 10.1016/0003-2697(84)90026-5. [DOI] [PubMed] [Google Scholar]; (b) Sonoda Y., Newstead S., Hu N. J., Alguel Y., Nji E., Beis K., Yashiro S., Lee C., Leung J., Cameron A. D., Byrne B., Iwata S., Drew D. Structure. 2011;19:17–25. doi: 10.1016/j.str.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Caffrey M., Li D., Dukkipati A. Biochemistry. 2012;51:6266–6288. doi: 10.1021/bi300010w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid N. I., Conn C. E., Brooks N. J., Ahmad N., Seddon J. M., Hashim R. Langmuir. 2013;29:15794–15804. doi: 10.1021/la4040134. [DOI] [PubMed] [Google Scholar]
- (a) Prive G. G. Methods. 2007;41:388–397. doi: 10.1016/j.ymeth.2007.01.007. [DOI] [PubMed] [Google Scholar]; (b) Chae P. S., Laible P. D., Gellman S. H. Mol. BioSyst. 2010;6:89–94. doi: 10.1039/b915162c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alguel Y., Amillis S., Leung J., Lambrinidis G., Capaldi S., Scull N. J., Craven G., Iwata S., Armstrong A., Mikros E., Diallinas G., Cameron A. D., Byrne B. Nat. Commun. 2016;7:11336. doi: 10.1038/ncomms11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J., Karachaliou M., Alves C., Diallinas G., Byrne B. Protein Expression Purif. 2010;72:139–146. doi: 10.1016/j.pep.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Deckert G., Warren P. V., Gaasterland T., Young W. G., Lenox A. L., Graham D. E., Overbeek R., Snead M. A., Keller M., Aujay M., Huber R., Feldman R. A., Short J. M., Olsen G. J., Swanson R. V. Nature. 1998;392:353–358. doi: 10.1038/32831. [DOI] [PubMed] [Google Scholar]
- Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Quick M., Javitch J. A. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3603–3608. doi: 10.1073/pnas.0609573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum D. M., Cherezov V., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Yao X. J., Weis W. I., Stevens R. C., Bobilka B. K. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- Mansoor S. E., McHaourab H. S., Farrens D. L. Biochemistry. 2002;41:2475–2484. doi: 10.1021/bi011198i. [DOI] [PubMed] [Google Scholar]
- Yao X., Parnot C., Deupi X., Ratnala V. R. P., Swaminath G., Farrens D. Nat. Chem. Biol. 2006;2:417–422. doi: 10.1038/nchembio801. [DOI] [PubMed] [Google Scholar]
- Swaminath G., Steenhuis J., Kobilka B., Lee T. W. Mol. Pharmacol. 2002;61:65–72. doi: 10.1124/mol.61.1.65. [DOI] [PubMed] [Google Scholar]
- (a) Hanson M. A., Cherezov V., Griffith M. T., Roth C. B., Jaakola V. P., Chien E. Y., Velasquez J., Kuhn P., Stevens R. C. Structure. 2008;16:897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Thompson A. A., Liu J. J., Chun E., Wacker D., Wu H., Cherezov V., Stevens R. C. Methods. 2011;55:310–317. doi: 10.1016/j.ymeth.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Ethayathulla A. S., Yousef M. S., Amin A., Leblanc G., Kaback H. R., Guan L. Nat. Commun. 2014;5:3009. doi: 10.1038/ncomms4009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Amin A., Hariharan P., Chae P. S., Guan L. Biochemistry. 2015;54:5849–5855. doi: 10.1021/acs.biochem.5b00660. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Amin A., Ethayathulla A. S., Guan L. J. Bacteriol. 2014;196:3134–3139. doi: 10.1128/JB.01868-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y., Newstead S., Hu N. J., Alguel Y., Nji E., Beis K., Yashiro S., Lee C., Leung J., Cameron A. D., Byrne B., Iwata S., Drew D., Caffrey M., Li D., Dukkipati A. Structure. Biochemistry. 2011;2012;1951:17–25. 6266–6288. doi: 10.1016/j.str.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae P. S., Rasmussen S. G. F., Rana R. R., Gotfryd K., Kruse A. C., Nurva S., Gether U., Guan L., Loland C. J., Byrne B., Kobilka B. K., Gellman S. H. Chem.–Eur. J. 2012;18:9485–9490. doi: 10.1002/chem.201200069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliano C., Barera S., Chimirri F., Saracco G., Barber J. Biochim. Biophys. Acta. 2012;1817:1506–1515. doi: 10.1016/j.bbabio.2011.11.001. [DOI] [PubMed] [Google Scholar]
- http://opm.phar.umich.edu/about.php .
- Chae P. S., Kruse A. C., Gotfryd K., Rana R. R., Cho K. H., Rasmussen S. G. F., Bae H. E., Chandra R., Gether U., Guan L., Kobilka B. K., Loland C. J., Byrne B., Gellman S. H. Chem.–Eur. J. 2013;19:15645–15651. doi: 10.1002/chem.201301423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.