Abstract
Endoscopic surveillance remains the core management of non-dysplastic Barrett's oesophagus, although questions regarding its efficacy in reducing mortality from oesophageal adenocarcinoma have yet to be definitively answered, and randomised trial data are awaited. One of the main goals of current research is to achieve risk stratification, identifying those at high risk of progression. The recent British Society of Gastroenterology (BSG) guidelines on surveillance have taken a step in this direction with interval stratification on clinicopathological grounds. The majority of Barrett's oesophagus remains undiagnosed, and this has led to investigation of methods of screening for Barrett's oesophagus, ideally non-endoscopic methods capable of reliably identifying dysplasia.
Chemoprevention to prevent progression is currently under investigation, and may become a key component of future treatment.
The availability of effective endotherapy means that accurate identification of dysplasia is more important than ever. There is now evidence to support intervention with radiofrequency ablation (RFA) for low-grade dysplasia (LGD), but recent data have emphasised the need for consensus pathology for LGD. Ablative treatment has become well established for high-grade dysplasia, and should be employed for flat lesions where there is no visible abnormality. Of the ablative modalities, RFA has the strongest evidence base. Endoscopic resection should be performed for all visible lesions, and is now the treatment of choice for T1a tumours.
Targeting those with high-risk disease will, hopefully, lead to efficacious and cost-effective surveillance, and the trend towards earlier intervention to halt progression gives cause for optimism that this will ultimately result in fewer deaths from oesophageal adenocarcinoma.
Keywords: BARRETT'S OESOPHAGUS, BARRETT'S METAPLASIA, ENDOSCOPY, SCREENING, CHEMOPREVENTION
Introduction
Barrett's oesophagus has been recognised as a precursor lesion for oesophageal adenocarcinoma (OAC) for over 50 years,1 2 and the metaplasia-dysplasia-adenocarcinoma sequence is now well characterised.3 Endoscopic surveillance is now widely practised, and endoscopic therapy has become a central part of the management of dysplasia in Barrett's oesophagus. Nonetheless, there are many areas which remain the subject of debate in the management of Barrett's oesophagus. Current controversies include the efficacy and cost-effectiveness of surveillance programmes, how to risk-stratify to identify those most likely to progress, the role of chemoprevention, whether ablative therapy is indicated for those with low-grade dysplasia (LGD), and optimal management for dysplastic Barrett's oesophagus given an ever-growing array of endotherapeutic options. This review will focus on current management strategies for Barrett's oesophagus and Barrett's oesophagaus-associated neoplasia.
Management of non-dysplastic Barrett's oesophagus
Surveillance
The increased risk of OAC for patients with Barrett's oesophagus is well recognised, and this has led to the introduction of endoscopic surveillance programmes in many countries to achieve early detection of dysplastic or malignant change, and to enable curative intervention. Historically, early detection of cancer before lymphatic spread remains the primary aim of surveillance, but with increasing endoscopic options for early disease, the accurate identification of dysplasia is now critical.
Choosing an appropriate interval for surveillance endoscopy must balance this goal against the costs and acceptability of frequent endoscopy, along with the low annual cancer risk for patients with Barrett's oesophagus, which is below 0.5% for those in surveillance, and evidence from population studies suggest it may be closer to 0.12%–0.16%, overall.4–6
Evidence that endoscopic surveillance for Barrett's oesophagus reduces mortality from OAC continues to be debated. There is evidence for improved outcomes, or earlier stage at diagnosis with surveillance,7–14 however, randomised trial data are lacking. Recent population-wide studies in Northern Ireland15 and The Netherlands16 have shown improved outcomes from OAC, with participation in surveillance programmes, with this result persisting after adjustment for lead-time and length-time bias. However, other retrospective studies have failed to demonstrate any benefit from surveillance programmes.17 18
Surveillance programmes are expensive, and with uncertain efficacy, the cost-effectiveness of such programmes has been questioned.19–21 A large, randomised, controlled trial (RCT) is underway in the UK (Barrett's Oesophagus Surveillance Study) to assess the efficacy and cost-effectiveness of surveillance.
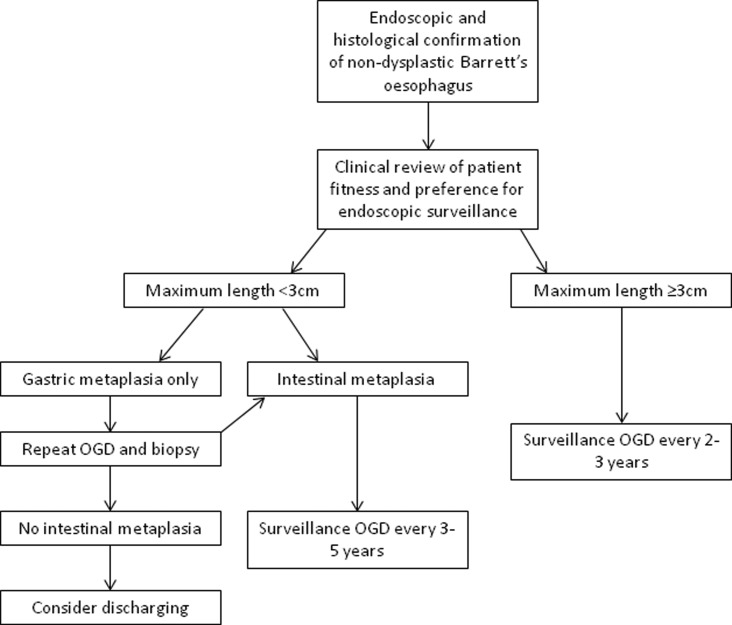
Given these concerns, risk stratification is a key goal of current research to identify those at highest risk of progression, rather than a ‘one size fits all’ policy that includes those at very low risk of malignancy. The recently updated British Society of Gastroenterology (BSG) guidelines have made several changes in line with this approach. For those with Barrett's oesophagus segment <3 cm, surveillance is now only advocated for those with intestinal metaplasia, and the recommended interval has been extended to 3–5 years.22 Surveillance every 2–3 years is reserved for those with Barrett's oesophagus segments with a length of 3 cm or greater. The current guidelines on endoscopic surveillance are summarised in figure 1.
Figure 1.
Guidelines for endoscopic surveillance of Barrett's oesophagus. Adapted from Fitzgerald et al,22 with permission. Note: the interval for repeat oesophagogastroduodenoscopy and biopsy after a finding of gastric metaplasia only, depends on the confidence of the endoscopic and histological findings and the number of biopsies taken. OGD, oesophagogastroduodenoscopy.
Further risk stratification through biomarkers has been a key aim of much recent research. The genetic changes that occur in progression from Barrett's oesophagus to invasive adenocarcinoma have been shown to be protean and complex.23–25 A large, genome-wide study of patients with disease across the spectrum from Barrett's oesophagus, dysplasia and adenocarcinoma, identified p53 and SMAD4 as the strongest markers of high-risk disease.23 As a biomarker, p53 has shown promise, and BSG guidelines advise consideration of its use as an immunostain to identify dysplasia.22 A preliminary study identified 86% of patients with dysplasia using a non-endoscopic cell collection device.23 The same genome-wide study showed that SMAD4 was highly specific, occurring only in adenocarcinoma, but since it was found in only 13% of cases it would be a low-sensitivity biomarker.23
Screening
Even highly effective surveillance can only have a limited outcome on overall survival from OAC, as only a small proportion of patients who develop adenocarcinoma have a previous diagnosis of Barrett's oesophagus. In a large, population-based study in Northern Ireland, only 7.3% of cases with OAC occurred in patients known to have Barrett's oesophagus.26 Although this may be influenced by successful surveillance programmes, this results largely from the high proportion of patients with undiagnosed Barrett's oesophagus: previous studies have estimated that up to 2% of the population have Barrett's oesophagus, with 80% undiagnosed.27–29
This has led some to consider screening for Barrett's oesophagus: several groups have examined the possibility of screening patients with chronic symptoms of gastro-oesophageal reflux disease (GORD). The prevalence of Barrett's oesophagus in patients with chronic GORD is 5%–15%.30 A meta-analysis by Taylor and Rubenstein31 found that while there was a strongly increased risk of long-segment Barrett's oesophagus with GORD symptoms (OR, 4.92, 95% CI 2.01 to 12.0), there was no association between short-segment Barrett's oesophagus and GORD (OR 1.15, 95% CI 0.76 to 1.73).
However, these symptoms are common among the general population, with around 20% having symptoms up to once a week,30 and around 6% of the population over the age of 45 years experiencing chronic symptoms.32 To screen all these individuals with endoscopy would require huge resources: estimates from the USA suggest that 6.6 million individuals would require endoscopy, with 1 OAC detected for every 1320 procedures.19 20 It must also be noted that 40% of patients with OAC (and 71% of patients with junctional adenocarcinoma of the cardia) do not report any GORD symptoms.33
Consequently, endoscopic screening in an unselected population with GORD is not currently recommended either in the USA or the UK.22 34 35 However, in the presence of multiple risk factors (chronic GORD plus 3 of the following: male, aged over 50 years, Caucasian, obese), the BSG guidelines advise consideration of screening, with a lower threshold for those with a first-degree relative with Barrett's oesophagus, or OAC.22
An alternative approach to screening is to use less invasive techniques to reduce the cost and/or morbidity. Studies assessing capsule endoscopy to identify Barrett's oesophagus have shown a relatively low sensitivity at 60%–78%.36–38 Others have used ultrathin transnasal endoscopes in patients with GORD or known Barrett's oesophagus, and demonstrated results very similar to conventional endoscopy.39 40
A further possibility currently being trialled is a swallowed cytology collection device (Cytosponge).41 The device is contained within a small capsule which is attached to a length of string: patients swallow the capsule, the gelatine capsule then dissolves in the acidic gastric secretions to reveal the collection device (analogous to a cytology brush), which is then withdrawn through the oesophagus using the string, collecting cells as it passes.
Accurate biomarkers of Barrett's oesophagus are required due to the mixed cell population collected by the device, with a previous study suggesting the most promising molecular marker using immunohistochemistry is Trefoil Factor 3 (TFF3).42 Furthermore, the acceptability of the device has been demonstrated in a primary care setting.41
The Barrett's oEsophagus Screening Trial (BEST2, ISRCTN 12730505)43 is a case-controlled study which aims to identify the sensitivity and specificity of the Cytosponge, and whether biomarkers can be used to risk-stratify patients when compared with the grade of dysplasia found at endoscopy and biopsy. Other outcomes include the safety profile of the device, along with the feasibility of high-throughput processing if the device were to be used for population-based screening.
To date, none of these technologies have entered routine clinical practice, but further trial results are awaited, and the prospect of screening could radically alter surveillance for Barrett's oesophagus and, potentially, lead on to a significant reduction in deaths from OAC.
Chemoprevention
There is increasing evidence for the use of certain drugs as chemoprevention for patients with Barrett's oesophagus. A number of cohort studies have shown a significantly reduced risk of progression to high-grade dysplasia (HGD) or OAC for patients taking proton pump inhibitors (PPI) versus no therapy, or on histamine-2 receptor antagonists (H2RA).44 45 However, data from RCTs are still awaited, and the BSG guidelines advise that ‘there is not yet sufficient evidence to advocate acid suppression drugs as chemopreventive agents’.22
Evidence for a protective effect from non-steroidal anti-inflammatory drugs (NSAID) comes from large meta-analyses of patients taking aspirin as primary or secondary prevention for cardiovascular disease.46–48 In the largest of these, incorporating data from 23 535 patients, those followed-up for 10–20 years after starting aspirin, and having taken aspirin daily for 5 years or more, had a significant reduction in risk of OAC, HR 0.36 (95% CI 0.18 to 0.71).46
Once again, there are no data from randomised trials, and given the risks of NSAIDs, such as gastrointestinal bleeding and cerebral haemorrhage, this will be crucial in informing management decisions for patients with Barrett's oesophagus.49
Aspirin and Esomeprazole for Chemoprevention in Barrett's metaplasia is a large, multicentre RCT (NCT00357682) which aims to address the lack of randomised data, and evaluate the risks and benefits of both PPIs and NSAIDs as chemoprevention in Barrett's oesophagus.50 The trial has four arms, with patients randomised to low-dose or high-dose PPI (esomeprazole)± aspirin.
There is some evidence from observational studies for a protective effect from statins against oesophageal cancer. A recent meta-analysis reviewed the effects of statins in a patient with Barrett's oesophagus,51 including 11 observational studies with a total of 1999 patients, and found an OR for progressing to OAC was 0.57 (95%CI 0.43 to 0.75) for those on a statin.
While the absence of randomised data limits current recommendations for chemoprevention, this paradigm may become a key component in the management of Barrett's oesophagus in future.
Management of Barrett's oesophagus-associated neoplasia
Endoscopic treatments aim to remove or destroy areas of neoplasia in the oesophagus, either through resection or ablation, and promote regrowth of the normal squamous lining. The goal of ablative techniques is to achieve complete eradication of dysplasia, along with complete eradication of intestinal metaplasia.52 These minimally invasive treatments can offer treatment without recourse to radical surgery and, thus, can be considered in patients with earlier-stage disease, and those for whom major surgery would be a very high risk.
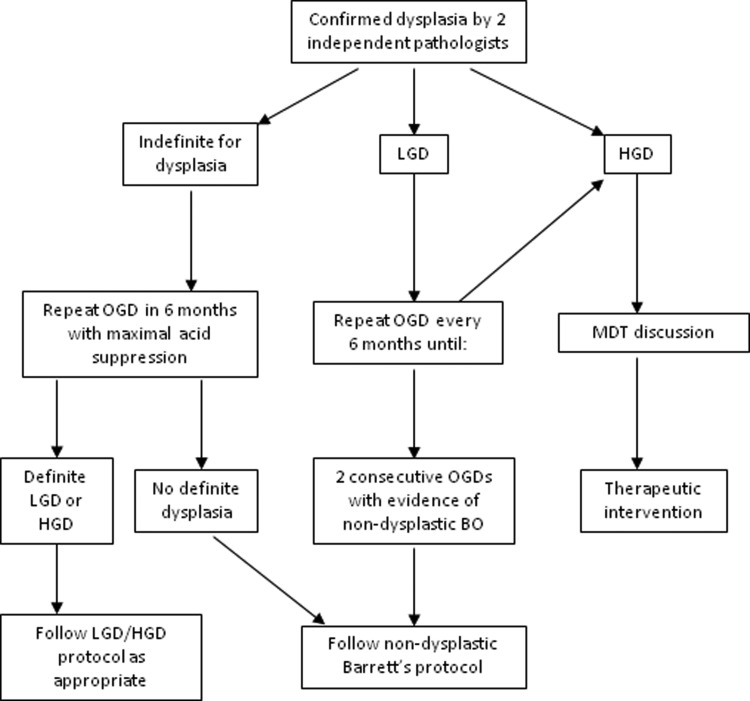
The BSG guidelines advise the following algorithm (see figure 2) for management of dysplastic Barrett's oesophagus, and this is discussed further below.
Figure 2.
Algorithm for management of dysplastic Barrett's oesophagus. Adapted from Fitzgerald et al,22 with permission. LGD, low-grade dysplasia; HGD, high-grade dysplasia; OGD, oesophagogastroduodenoscopy; MDT, multidisciplinary team.
Low-grade dysplasia
In the 2014 BSG guidelines, as shown in figure 3, patients found to have LGD are advised to have 6-monthly oesophagogastroduodenoscopy (OGD) surveillance until biopsy confirms regression to non-dysplastic Barrett's oesophagus on successive OGDs, or progression to HGD occurs.22 However, a recent RCT from European centres reported a highly significant reduction in progression for patients with LGD treated with radiofrequency ablation (RFA).53 The rate of progression to HGD or cancer over a 3-year follow-up was 1.5% in the ablation group versus 26.5% in the untreated group (p<0.001), with progression to cancer 1.5% vs 8.8%, respectively (p=0.03).
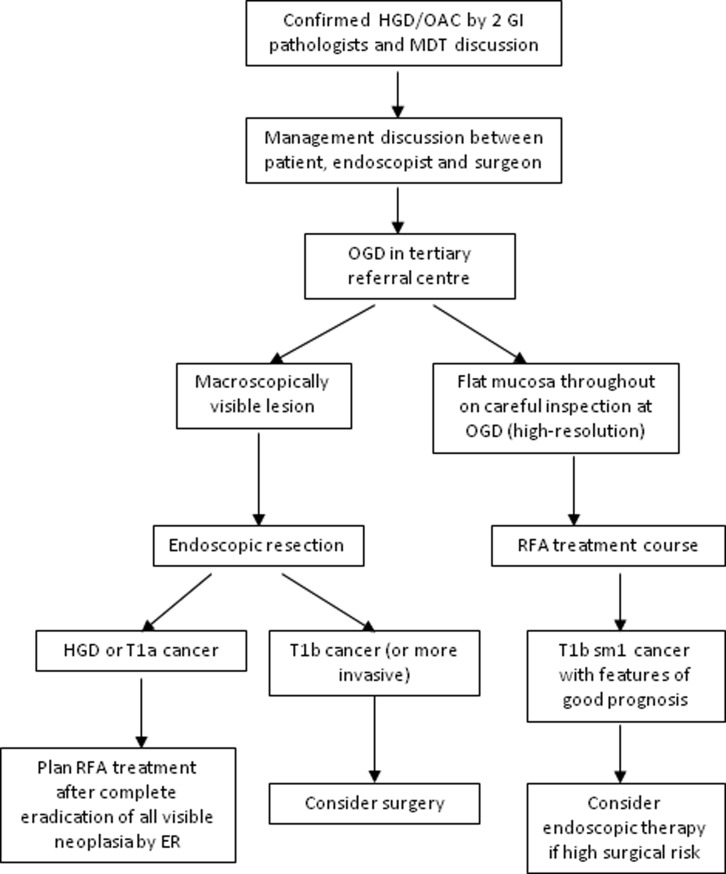
Figure 3.
Algorithm for managing HGD and early oesophageal adenocarcinoma. Adapted from Fitzgerald et al,22 with permission. HGD, high-grade dysplasia; OAC, early oesophageal adenocarcinoma; MDT, multidisciplinary team; OGD, oesophagogastroduodenoscopy; RFA, radiofrequency ablation.
It must be noted that the rate of progression in the control arm in this trial (26.5% over 3 years) was much higher than might be expected from the natural history of LGD reported in other studies, with a recent meta-analysis calculating a risk of progression to HGD or cancer of around 1% per year.54 The use of an expert pathology panel for consensus diagnosis, with a very robust classification as LGD, is likely to have influenced the composition of the group seen in the study by Phoa et al53 Of 511 patients with an initial diagnosis of LGD screened for entry to the trial, only 247 were confirmed to have LGD after review by the expert panel, and 140 went on to be randomised.53
In light of these findings, it seems likely that historical overdiagnosis of LGD has led to an underestimation of the true risk of progression with LGD.54 This suggests the need for consensus reporting of all Barrett's dysplasia specimens, and the question of whether to offer intervention in the absence of consensus is difficult.
The recently published National Institute for Health and Care Excellence guidelines now support the use of RFA for the ablation of LGD.55
High-grade dysplasia
The diagnosis of HGD has serious implications for patients. They have a high risk of progression to adenocarcinoma, and also a significant risk that a small focus of cancer may already be present, but not yet detected.56 For this reason, historically, the treatment of HGD was radical oesophagectomy for those considered fit for surgery. The advent of endotherapy led to great debate over whether these treatments could offer oncologically sound treatment. Although there have not been any randomised trials directly comparing surgery with endotherapies, the growing body of evidence for endotherapies over the past 20 years or so has resolved this debate, and endoscopic resection (ER) is now considered the treatment of choice for patients with macroscopically visible HGD or T1a adenocarcinoma.22 The management of HGD and early oesophageal cancer is shown in the algorithm in figure 3.
For visible lesions, ER is preferred over ablative therapy alone because it provides the most accurate staging information. Depth of invasion can be accurately assessed: data from stepwise ER of entire Barrett's oesophagus segments have confirmed that the most advanced disease is located in visible lesions.57 A meta-analysis of studies in which patients have undergone oesophagectomy for HGD found the risk of invasive OAC to be 11% in those with visible lesions, compared to 3% in those with no visible lesion.56
There are now numerous ablative techniques for HGD: each of these techniques has its strengths and weaknesses, and some key features are presented in table 1 below. RCT data are available for some of these modalities, and includes direct comparison of certain techniques. Currently, there is a large-scale trial (Barrett's Radiofrequency Intervention for Dysplasia by Endoscopy, NCT017337) underway aiming to compare outcomes between RFA and argon plasma coagulation.58 This pilot study has just been closed to recruitment, and initial data will be available soon. On the basis of currently available RCT data, and taking account of the side effect profiles of each treatment, RFA is recommended as the first-line therapy for HGD outside the context of RCTs.22
Table 1.
Comparison of ablative techniques for HGD
| Technique | Eradication of dysplasia (%) | Advantages | Disadvantages |
|---|---|---|---|
| Step-wise radical ER | 97–100 | Accurate diagnosis Low costs |
High risk of stenosis Only feasible <5 cm Barret's oesophagus |
| APC | 67–86 | Widely available Low costs |
Buried glands Feasible for short segments only |
| PDT | 40–77 | RCT data Treatment of nodular dysplasia |
High risk of stricture Buried glands Photosensitivity |
| RFA | 80–98 | RCT data High response rate Low complication rate |
High costs Minimal long-term follow-up data |
| Cryotherapy | 68–88 | Good safety profile | No RCT data No long-term follow-up data |
Adapted from Fitzgerald et al,22 with permission.
APC, argon plasma coagulation; ER, endoscopic resection; HGD, high-grade dysplasia; PDT, photodynamic therapy; RCT, randomised controlled trial; RFA, radiofrequency ablation.
The first major RCT of RFA randomised 127 patients on a 2:1 allocation to RFA or a sham procedure.59 Complete eradication of HGD was seen in 81.0% of treated patients versus 19.0% of controls (p<0.001). In a later report on extended follow-up of this group, 96% of those achieving eradication of HGD remained free of HGD at 3-year follow-up.60 Risk of any disease progression was reduced in the ablation group (3.6% vs 16.3%, p=0.03), as was the risk of cancer (1.2% vs 9.3%, p=0.045).
A meta-analysis of RFA for Barrett's oesophagus calculated pooled estimates for complete eradication of dysplasia of 91% (87%–95%, 95% CI), and complete eradication of intestinal metaplasia 78% (70%–86%, 95% CI).61 The commonest complication was stricture, which occurred in 5% of patients treated with RFA.
Intramucosal adenocarcinoma
The low risk of nodal metastasis with T1a cancer (0%–10%)62–69 has resulted in very good tumour-free and overall survival with ER for T1a cancers in high-volume centres. One recent large series reported tumour-free survival of 93.8% at mean follow-up of 56.6 months.70 ER for T1b tumours is associated with poorer outcomes, with 5-year tumour-free and overall survival at 60% and 58%, respectively, likely due to increased depth and higher risk of nodal involvement (up to 46%).62–69 For upper third tumours of the submucosa (T1bsm1) the risk of lymph node metastasis is relatively low (around 10%), and some series report good outcomes treating T1bsm1 tumours endoscopically.63 68 70 Decision making may be further influenced by pathological indicators of good prognosis, such as clear resection margins, and absence of vascular and lymphatic invasion.71 The use of ER for T1bsm1 cancer remains debated, however, and the current BSG guidance advises surgery for patients who are fit enough, but that ER should be offered with curative intent in patients who are high-risk surgical candidates (see figure 3).22
While ER is effective at removing Barrett's oesophagus-associated intramucosal lesions, these patients have a high rate of metachronous lesions after ER alone, affecting around 15%–20%.70 72 The risk of developing further lesions can be reduced significantly with ablative therapy to the remaining Barrett's oesophagus segment.57 72 Current guidance advises removal of all visible lesions with ER, followed by ablative therapy (currently, RFA is the preferred method) to all residual areas of Barrett's oesophagus.22
Conclusions
The recent update to BSG guidelines represents a move towards a more stratified approach to surveillance of Barrett's oesophagus. This trend is likely to continue with ongoing efforts to identify those at high risk of progression through appropriate genetic or cellular biomarkers.
Advances in endoscopic therapy have revolutionised the treatment of dysplastic Barrett's oesophagus, and this indication now extends to those with confirmed LGD, although the challenges of achieving consensus histology remain a pressing issue.
Ongoing trials of chemoprevention and screening may lead to new approaches to Barrett's oesophagus. The possibility of an efficacious, non-endoscopic test offers hope of a cost-effective means of achieving this, and large-scale randomised trial data are eagerly awaited to evaluate agents, such as aspirin and PPIs, as chemoprevention in Barrett's oesophagus.
Thus, it seems likely that the future will bring a continued increase in intervention for those with early disease through chemoprevention and endotherapy, more targeted surveillance, and a reduction in patients requiring radical surgery or presenting with disseminated disease.
Footnotes
Contributors: All the authors contributed to preparation of the manuscript.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dawson JL. Adenocarcinoma of the middle oesophagus arising in an oesophagus lined by gastric (parietal) epithelium. Br J Surg 1964;51:940–2. http://www.ncbi.nlm.nih.gov/pubmed/14226058 (accessed 17 Dec 2014). doi:10.1002/bjs.1800511218 [DOI] [PubMed] [Google Scholar]
- 2.Bani-Hani KE, Bani-Hani BK. Columnar-lined esophagus: time to drop the eponym of “Barrett”: Historical review. J Gastroenterol Hepatol 2008;23:707–15. doi:10.1111/j.1440-1746.2008.05386.x [DOI] [PubMed] [Google Scholar]
- 3.Jankowski JA, Wright NA, Meltzer SJ, et al. Molecular evolution of the metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Pathol 1999;154:965–73. doi:10.1016/S0002-9440(10)65346-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst 2011;103:1049–57. doi:10.1093/jnci/djr203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hvid-Jensen F, Pedersen L. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011;365:1375–83. http://www.nejm.org/doi/full/10.1056/Nejmoa1103042 (accessed 21 May 2014). doi:10.1056/NEJMoa1103042 [DOI] [PubMed] [Google Scholar]
- 6.Desai T, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut 2012;61:970–6. http://gut.bmj.com/content/61/7/970.short (accessed 21 May 2014). doi:10.1136/gutjnl-2011-300730 [DOI] [PubMed] [Google Scholar]
- 7.Streitz JM, Andrews CW, Ellis FH. Endoscopic surveillance of Barrett's esophagus. Does it help? J Thorac Cardiovasc Surg 1993;105:383–7; discussion 387–8. http://www.ncbi.nlm.nih.gov/pubmed/8445916 (accessed 21 May 2014). [PubMed] [Google Scholar]
- 8.Peters JH, Clark GW, Ireland AP, et al. Outcome of adenocarcinoma arising in Barrett's esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg 1994;108:813–21; discussion 821–2. http://www.ncbi.nlm.nih.gov/pubmed/7967662 (accessed 21 May 2014). [PubMed] [Google Scholar]
- 9.Van Sandick JW, van Lanschot JJB, Kuiken BW, et al. Impact of endoscopic biopsy surveillance of Barrett's oesophagus on pathological stage and clinical outcome of Barrett's carcinoma. Gut 1998;43:216–22. doi:10.1136/gut.43.2.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett's adenocarcinomas: a population-based study. Gastroenterology 2002;122:633–40. http://www.ncbi.nlm.nih.gov/pubmed/11874995 (accessed 21 May 2014). doi:10.1053/gast.2002.31879 [DOI] [PubMed] [Google Scholar]
- 11.Cooper GS, Yuan Z, Chak A, et al. Association of prediagnosis endoscopy with stage and survival in adenocarcinoma of the esophagus and gastric cardia. Cancer 2002;95:32–8. doi:10.1002/cncr.10646 [DOI] [PubMed] [Google Scholar]
- 12.Fountoulakis A, Zafirellis KD, Dolan K, et al. Effect of surveillance of Barrett's oesophagus on the clinical outcome of oesophageal cancer. Br J Surg 2004;91:997–1003. doi:10.1002/bjs.4591 [DOI] [PubMed] [Google Scholar]
- 13.Rubenstein JH, Sonnenberg A, Davis J, et al. Effect of a prior endoscopy on outcomes of esophageal adenocarcinoma among United States veterans. Gastrointest Endosc 2008;68:849–55. doi:10.1016/j.gie.2008.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper GS, Kou TD, Chak A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: a population-based study with temporal trends. Am J Gastroenterol 2009;104:1356–62. doi:10.1038/ajg.2009.159 [DOI] [PubMed] [Google Scholar]
- 15.Bhat SK, McManus DT, Coleman HG, et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett's oesophagus: a population-based study. Gut 2015;64:20–5. doi:10.1136/gutjnl-2013-305506 [DOI] [PubMed] [Google Scholar]
- 16.Verbeek RE, Leenders M, Ten Kate FJW, et al. Surveillance of Barrett's Esophagus and Mortality from Esophageal Adenocarcinoma: A Population-Based Cohort Study. Am J Gastroenterol 2014;109:1215–22. doi:10.1038/ajg.2014.156 [DOI] [PubMed] [Google Scholar]
- 17.Corley DA, Mehtani K, Quesenberry C, et al. Impact of endoscopic surveillance on mortality from Barrett's esophagus-associated esophageal adenocarcinomas. Gastroenterology 2013;145:312–9.e1. doi:10.1053/j.gastro.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macdonald CE, Wicks AC, Playford RJ. Final results from 10year cohort of patients undergoing surveillance for Barrett's oesophagus: observational study. BMJ 2000;321:1252–5. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=27527&tool=pmcentrez&rendertype=abstract (accessed 3 Jun 2014). doi:10.1136/bmj.321.7271.1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahrilas PJ. The problems with surveillance of Barrett's esophagus. N Engl J Med 2011;365:1437–8. doi:10.1056/NEJMe1108435 [DOI] [PubMed] [Google Scholar]
- 20.Vaezi MF, Kahrilas PJ. Barrett's esophagus surveillance: time to rethink if one size fits all? Gastroenterology 2013;145:503–5. doi:10.1053/j.gastro.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 21.De Jonge PJF, van Blankenstein M, Grady WM, et al. Barrett's oesophagus: epidemiology, cancer risk and implications for management. Gut 2014;63:191–202. doi:10.1136/gutjnl-2013-305490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014;63:7–42. doi:10.1136/gutjnl-2013-305372 [DOI] [PubMed] [Google Scholar]
- 23.Weaver JMJ, Ross-Innes CS, Shannon N, et al. Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nat Genet 2014;46:837–43. doi:10.1038/ng.3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaidi AH, Gopalakrishnan V, Kasi PM, et al. Evaluation of a 4-protein serum biomarker panel-biglycan, annexin-A6, myeloperoxidase, and protein S100-A9 (B-AMP)-for the detection of esophageal adenocarcinoma. Cancer 2014;120:3902–13. doi:doi:10.1002/cncr.28963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah AK, Saunders NA, Barbour AP, et al. Early diagnostic biomarkers for esophageal adenocarcinoma--the current state of play. Cancer Epidemiol Biomarkers Prev 2013;22:1185–209. doi:10.1158/1055-9965.EPI-12-1415 [DOI] [PubMed] [Google Scholar]
- 26.Bhat S. Barrett's oesophagus: risk of malignant progression and cost effectiveness of endoscopic surveillance. 2012. PhD thesis, Queen's University Belfast. [Google Scholar]
- 27.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology 2005;129:1825–31. doi:10.1053/j.gastro.2005.08.053 [DOI] [PubMed] [Google Scholar]
- 28.Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano-Monghidoro study. Gut 2008;57:1354–9. doi:10.1136/gut.2007.145177 [DOI] [PubMed] [Google Scholar]
- 29.Zeki S, Fitzgerald RC. Targeting care in Barrett's oesophagus. Clin Med 2014;14(Suppl 6):s78–83. doi:10.7861/clinmedicine.14-6-s78 [DOI] [PubMed] [Google Scholar]
- 30.Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA 2002;287:1972–81. http://www.ncbi.nlm.nih.gov/pubmed/11960540 (accessed 23 May 2014). doi:10.1001/jama.287.15.1972 [DOI] [PubMed] [Google Scholar]
- 31.Taylor JB, Rubenstein JH. Meta-analyses of the effect of symptoms of gastroesophageal reflux on the risk of Barrett's esophagus. Am J Gastroenterol 2010;105:1729, 1730–7; quiz 1738 doi:10.1038/ajg.2010.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camilleri M, Dubois D, Coulie B, et al. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clin Gastroenterol Hepatol 2005;3:543–52. http://www.ncbi.nlm.nih.gov/pubmed/15952096 (accessed 20 May 2014). doi:10.1016/S1542-3565(05)00153-9 [DOI] [PubMed] [Google Scholar]
- 33.Lagergren J, Bergström R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31. doi:10.1056/NEJM199903183401101 [DOI] [PubMed] [Google Scholar]
- 34.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol 2008;103:788–97. doi:10.1111/j.1572-0241.2008.01835.x [DOI] [PubMed] [Google Scholar]
- 35.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 2011;140:1084–91. doi:10.1053/j.gastro.2011.01.031 [DOI] [PubMed] [Google Scholar]
- 36.Lin OS, Schembre DB, Mergener K, et al. Blinded comparison of esophageal capsule endoscopy versus conventional endoscopy for a diagnosis of Barrett's esophagus in patients with chronic gastroesophageal reflux. Gastrointest Endosc 2007;65:577–83. doi:10.1016/j.gie.2006.06.035 [DOI] [PubMed] [Google Scholar]
- 37.Galmiche JP, Sacher-Huvelin S, Coron E, et al. Screening for esophagitis and Barrett's esophagus with wireless esophageal capsule endoscopy: a multicenter prospective trial in patients with reflux symptoms. Am J Gastroenterol 2008;103:538–45. doi:10.1111/j.1572-0241.2007.01731.x [DOI] [PubMed] [Google Scholar]
- 38.Ramirez FC, Akins R, Shaukat M. Screening of Barrett's esophagus with string-capsule endoscopy: a prospective blinded study of 100 consecutive patients using histology as the criterion standard. Gastrointest Endosc 2008;68:25–31. doi:10.1016/j.gie.2007.10.040 [DOI] [PubMed] [Google Scholar]
- 39.Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett's esophagus: a randomized and blinded comparison. Am J Gastroenterol 2006;101:2693–703. doi:10.1111/j.1572-0241.2006.00890.x [DOI] [PubMed] [Google Scholar]
- 40.Shariff MK, Bird-Lieberman EL, O'Donovan M, et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett's esophagus. Gastrointest Endosc 2012;75:954–61. doi:10.1016/j.gie.2012.01.029 [DOI] [PubMed] [Google Scholar]
- 41.Kadri SR, Lao-Sirieix P, O'Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study. BMJ 2010;341:c4372 doi:10.1136/bmj.c4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lao-Sirieix P, Boussioutas A, Kadri SR, et al. Non-endoscopic screening biomarkers for Barrett's oesophagus: from microarray analysis to the clinic. Gut 2009;58:1451–9. doi:10.1136/gut.2009.180281 [DOI] [PubMed] [Google Scholar]
- 43.BEST2 trial. http://www.controlled-trials.com/ISRCTN12730505 (accessed 22 May 2014).
- 44.El-Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett's esophagus. Am J Gastroenterol 2004;99:1877–83. doi:10.1111/j.1572-0241.2004.30228.x [DOI] [PubMed] [Google Scholar]
- 45.Nguyen DM, El-Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304. doi:10.1016/j.cgh.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothwell PM, Fowkes FGR, Belch JFF, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 2011;377:31–41. doi:10.1016/S0140-6736(10)62110-1 [DOI] [PubMed] [Google Scholar]
- 47.Corley DA, Kerlikowske K, Verma R, et al. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47–56. doi:10.1053/gast.2003.50008 [DOI] [PubMed] [Google Scholar]
- 48.Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012;142:442–52.e5; quiz e22–3 doi:10.1053/j.gastro.2011.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 2009;10:501–7. doi:10.1016/S1470-2045(09)70035-X [DOI] [PubMed] [Google Scholar]
- 50.ClinicalTrials.gov. A Phase III, Randomized, Study of Aspirin and Esomeprazole Chemoprevention in Barrett's Metaplasia (AspECT). http://www.clinicaltrials.gov/show/NCT00357682
- 51.Beales ILP, Hensley A, Loke Y. Reduced esophageal cancer incidence in statin users, particularly with cyclo-oxygenase inhibition. World J Gastrointest Pharmacol Ther 2013;4:69–79. doi:doi:10.4292/wjgpt.v4.i3.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leggett CL, Gorospe EC, Wang KK. Endoscopic therapy for Barrett's esophagus and early esophageal adenocarcinoma. Gastroenterol Clin North Am 2013;42:175–85. doi:10.1016/j.gtc.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phoa KN, van Vilsteren FGI, Weusten BLAM, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209–17. doi:10.1001/jama.2014.2511 [DOI] [PubMed] [Google Scholar]
- 54.Almond LM, Hodson J, Barr H. Meta-analysis of endoscopic therapy for low-grade dysplasia in Barrett's oesophagus. Br J Surg 2014;101:1187–95. doi:10.1002/bjs.9573 [DOI] [PubMed] [Google Scholar]
- 55.National Institute for Health and Care Excellence. Endoscopic radiofrequency ablation for Barrett's oesophagus with low-grade dysplasia or no dysplasia 2014. www.guidance.nice.org.uk/ipg496
- 56.Konda VJA, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high-grade dysplasia in Barrett's esophagus overestimated? Clin Gastroenterol Hepatol 2008;6:159–64. doi:10.1016/j.cgh.2007.09.013 [DOI] [PubMed] [Google Scholar]
- 57.Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection for eradication of Barrett's oesophagus with early neoplasia in a cohort of 169 patients. Gut 2010;59:1169–77. doi:10.1136/gut.2010.210229 [DOI] [PubMed] [Google Scholar]
- 58.Barrett's Radiofrequency Intervention for Dysplasia by Endoscopy. Clin. Trials.gov 2012. http://clinicaltrials.gov/show/NCT01733719?displayxml=true (accessed 19 Dec 2014). [Google Scholar]
- 59.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277–88. doi:10.1056/NEJMoa0808145 [DOI] [PubMed] [Google Scholar]
- 60.Shaheen N, Wolfsen HC, Wang KK, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology 2011;141:460–8. doi:10.1053/j.gastro.2011.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett's Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1245–55. doi:10.1016/j.cgh.2013.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004;60:703–10. http://www.ncbi.nlm.nih.gov/pubmed/15557945 (accessed 13 Jun 2014). doi:10.1016/S0016-5107(04)02017-6 [DOI] [PubMed] [Google Scholar]
- 63.Westerterp M, Koppert LB, Buskens CJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch 2005;446:497–504. doi:10.1007/s00428-005-1243-1 [DOI] [PubMed] [Google Scholar]
- 64.Stein HJ, Feith M, Bruecher BLDM, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 2005;242:566–73; discussion 573–5 doi:10.1097/01.sla.0000184211.75970.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu L, Hofstetter WL, Rashid A, et al. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol 2005;29:1079–85. http://www.ncbi.nlm.nih.gov/pubmed/16006804 (accessed 13 Jun 2014). [PubMed] [Google Scholar]
- 66.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology 2009;137:815–23. doi:10.1053/j.gastro.2009.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sepesi B, Watson TJ, Zhou D, et al. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg 2010;210:418–27. doi:10.1016/j.jamcollsurg.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 68.Alvarez Herrero L, Pouw RE, van Vilsteren FG, et al. Risk of lymph node metastasis associated with deeper invasion by early adenocarcinoma of the esophagus and cardia: study based on endoscopic resection specimens. Endoscopy 2010;42:1030–6. doi:10.1055/s-0030-1255858 [DOI] [PubMed] [Google Scholar]
- 69.Barbour AP, Jones M, Brown I, et al. Risk stratification for early esophageal adenocarcinoma: analysis of lymphatic spread and prognostic factors. Ann Surg Oncol 2010;17:2494–502. doi:10.1245/s10434-010-1025-0 [DOI] [PubMed] [Google Scholar]
- 70.Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652–60.e1. doi:10.1053/j.gastro.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 71.Estrella JS, Hofstetter WL, Correa AM, et al. Duplicated muscularis mucosae invasion has similar risk of lymph node metastasis and recurrence-free survival as intramucosal esophageal adenocarcinoma. Am J Surg Pathol 2011;35:1045–53. doi:10.1097/PAS.0b013e318219ccef [DOI] [PubMed] [Google Scholar]
- 72.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200–6. doi:10.1136/gut.2007.142539 [DOI] [PubMed] [Google Scholar]