Abstract
Objective
Faecal calprotectin (FC) is a non-invasive marker used to differentiate irritable bowel syndrome from inflammatory bowel disease (IBD). However, false positives are common. We sought to determine the diagnostic yield of investigation in patients presenting with new lower gastrointestinal (GI) symptoms and a mildly elevated FC (100–200 µg/g).
Design
Retrospective study of electronic patient records.
Patients
Patients aged 16–50 years with new lower GI symptoms and an FC 100–200 µg/g were identified from our biochemistry laboratory database between September 2009 and 2011. Patients were excluded if they had a previous FC >200 µg/g, were taking non-steroidal anti-inflammatory drugs (NSAIDs), had IBD, positive stool cultures or ‘alarm’ symptoms.
Setting
Secondary care gastroenterology clinics.
Results
161 patients (103 female patients) were identified. Mean age was 37.3 years with a mean FC of 147 µg/g. 398 endoscopic, radiological and histological investigations were undertaken in 141 patients (an average of 2.8 investigations per patient). 131 colonoscopies were performed with abnormalities in only 24 (18.3%). In patients with a macroscopically normal upper GI endoscopy and colonoscopy, the diagnostic yield of any further investigation was only 7.3%. The negative predictive value (NPV) of an FC 100–200 µg/g was 86.7% for any pathology and 97.5% for significant luminal pathology (IBD, advanced adenoma or colorectal carcinoma). After a mean follow-up of 172.4 weeks, IBD was the final diagnosis in only 4 (2.5%) of patients.
Conclusions
In adult patients under 50 years old presenting with new lower GI symptoms, the NPV of an FC between 100 and 200 µg/g in excluding significant organic GI disease is high.
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder estimated to affect 10%–25% of the UK population.1 Approximately 50% of patients with IBS consult their general practitioner and 20% will be referred to secondary care, constituting 20%–50% of the gastroenterology outpatient workload.1 Typical symptoms include lower abdominal pain, bloating and altered bowel habit with diarrhoea, constipation or alternating between both. Similar symptoms may be present in inflammatory bowel disease (IBD). However unlike IBS, which can often be managed symptomatically in primary care, patients with IBD require long-term specialist treatment and follow-up to prevent complications such as the need for surgery or the development of colorectal cancer.
Faecal calprotectin (FC) is a calcium-binding protein which constitutes 60% of neutrophil cytosol. Lower concentrations are present in other leucocytes. FC is increasingly used as a non-invasive marker to differentiate IBS from IBD and can avoid the need for specialist referral or invasive investigation. Recent National Institute of Health and Care Excellence (NICE) guidelines recommend using FC testing to differentiate between organic or functional disease in patients with new lower GI symptoms where cancer is not suspected.2
However, as FC is a non-specific but extremely sensitive marker of luminal inflammation, false positives are common. We, and others, have previously demonstrated a low yield of diagnostic colonoscopy in patients with borderline elevations of FC (50–100 µg/g).3 Higher FC levels (100–200 µg/g) often prompt more extensive investigation.
Aim
Our aim was to determine the diagnostic yield of endoscopic, histological and radiological investigation in patients aged <50 presenting with new lower (non-‘alarm’) GI symptoms and a mildly elevated FC (100–200 µg/g).
Methods
All patients with an FC 100–200 µg/g were identified from our biochemistry laboratory database between September 2009 and September 2011. Patients aged 16–50 years attending gastroenterology outpatient clinics with new lower GI symptoms (abdominal pain and/or altered bowel habit) were identified. Patients were excluded from further analysis if they had a previous FC >200 µg/g, were taking non-steroidal anti-inflammatory drugs (NSAIDs), had known IBD, positive stool cultures, alarm symptoms or anaemia. Weight loss and bleeding per rectum represented ‘alarm symptoms’. Further investigation was at the discretion of the responsible clinician and based upon both clinical presentation and FC results. Details of investigations, diagnosis and clinical outcomes were determined electronically from the NHS Greater Glasgow and Clyde Clinical Portal. All FC assays were undertaken according to the manufacturer's instructions (Bühlmann calprotectin ELISA kit) using Roche faecal extraction device as previously described.4
Results
In all, 161 patients (103 female patients) were identified who met the inclusion criteria. The mean age was 37.3 years with a mean FC of 147 µg/g. The primary presentation was diarrhoea in 98 (60.9%) and abdominal pain in 63 (39.1%). Secondary symptoms were abdominal pain (28.6%), diarrhoea (18.6%) and constipation (1.9%). Baseline demographics and presenting symptoms are shown in table 1.
Table 1.
Patient demographics
| Mean age | 37.3 years | |
|---|---|---|
| Sex | Male | 58 (36%) |
| Female | 103 (64%) | |
| Primary symptom | Abdominal pain | 63 (39.1%) |
| Diarrhoea | 98 (60.9%) | |
| Secondary symptom | Abdominal pain | 46 (28.6%) |
| Diarrhoea | 30 (18.6%) | |
| Constipation | 3 (1.9%) | |
| Mean faecal calprotectin | 147 µg/g | |
A total of 398 endoscopic, radiological and histological investigations were undertaken in 141 patients with an average of 2.8 investigations per patient. The number of investigations performed and the diagnostic yield of each test is shown in table 2.
Table 2.
Investigations performed and diagnostic yield
| Investigation | Number | Yield (%) |
|---|---|---|
| All | 398 | 13.3 |
| Colonoscopy | 131 | 18.3 |
| Excluding colonoscopy | 267 | 10.9 |
| TI/colonic biopsies | 119 | 7.6 |
| Barium meal and follow-through | 16 | 6.3 |
| CT | 19 | 5.3 |
| MRI small bowel | 19 | 16.7 |
| Capsule endoscopy | 12 | 8.3 |
| Upper gastrointestinal endoscopy | 47 | 27.7 |
| Distal duodenal biopsies | 36 | 2.8 |
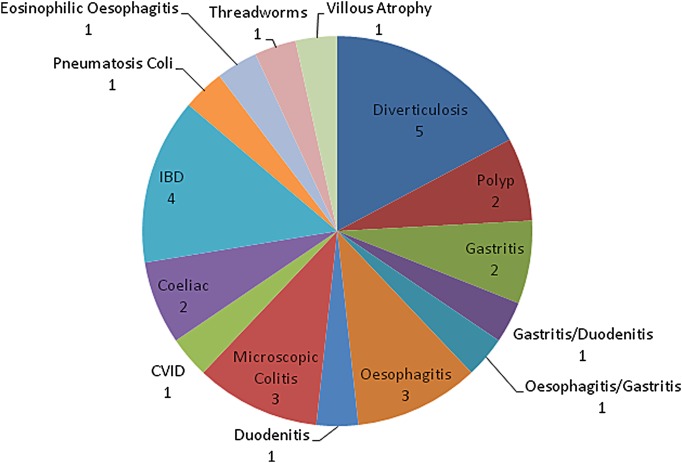
A total of 131 colonoscopies were performed with abnormalities detected in only 24 (18.3%). In patients with a macroscopically normal upper GI endoscopy and colonoscopy, the diagnostic yield of any further investigation was only 7.3%. The negative predictive value (NPV) of an FC 100–200 µg/g in this cohort was 86.7% for any pathology and 97.5% for significant luminal pathology (IBD, advanced adenoma or colorectal carcinoma). The mean duration of follow-up was 172.4 weeks with IBD the final diagnosis in only 4 (2.5%) patients while 49.7% were diagnosed as having IBS. All diagnoses made can be seen in figure 1. Following investigation, the majority of patients (74%) were deemed on clinical grounds not to require long-term follow-up. No patients with negative initial investigations were subsequently diagnosed with IBD during the follow-up period.
Figure 1.
Pie-chart of diagnoses.
Discussion
FC is now well established as a useful non-invasive marker of GI tract inflammation. A large meta-analysis of studies supports the use of FC as a screening tool for organic disease and in determining the need for further invasive investigation.5 UK national guidelines have subsequently recommended the use of FC in differentiating IBS from IBD provided cancer is not suspected.2
The commonest cut-off value advised by many of the manufacturers of FC assays is 50 µg/g. However, the sensitivity and specificity of FC vary significantly across studies.2 Similarly, the NPV and positive predictive value (PPV) are influenced by the population prevalence of IBD which differs among primary, secondary and tertiary care centres. Many of the early studies used to evaluate FC and to determine the optimal cut-off values for use in clinical practice are small and originate from specialist centres. Indeed, the largest of these studies only included a total of 240 subjects.6 The study cohort was split evenly between four groups with 60 control patients and equal numbers of patients with IBD, IBS and colorectal cancer. This study by Li et al6 was included in a meta-analysis that was performed as part of the NICE guidelines development.2 This calculated a sensitivity of 93% and 94% for an FC cut-off of 50 µg/g. However, the majority of these studies also included elderly patients and those with 'alarm symptoms’ in whom cancer should be considered and further investigation should be mandatory.
Other investigators have previously shown a low yield from investigating patients with a ‘borderline’ FC result of 50–100 µg/g. Zayyat et al7 reviewed all patients with an FC between 50 and 100 µg/g from their large dataset. Overall, 433 patients met the inclusion criteria and underwent further investigation. Only 10 cases of IBD were identified. In younger adult patients, without alarm symptoms, the yield of investigation may be even lower. Moroni et al,3 from our own group, examined patients aged 16–50 years old who presented with lower GI symptoms and with an FC of 50–100 µg/g. Patients with alarm features (PR bleeding, weight loss and anaemia) were excluded. The yield of subsequent colonoscopy in these patients was low (12.3%) and the NPV was high. The NPV for any pathology was 87.7% and for significant luminal pathology, defined as colorectal cancer, advanced adenoma or IBD, was 100%. Importantly, this group of patients is analogous to that which the NICE guidelines advise screening with FC.
In the current study, we have examined this group in even greater detail and, prompted by our own clinical experience of this patient cohort, extended the upper limit of FC in our analyses. Our findings suggest that the yield of further endoscopic, radiological and histological investigation in younger adult patients with mildly elevated FC and without alarm features is low and the NPV for significant luminal pathology remains high.
A retrospective review of electronic patient records such as this has obvious limitations compared with a prospective study. The limited use of wireless capsule endoscopy (WCE) to further investigate our cohort is a further potential criticism. Although the use of WCE as a diagnostic tool is increasing, it is often only available in tertiary centres. Studies are time-consuming and capsule retention can occur if a patient has stricturing disease. It would not be feasible or appropriate to undertake WCE in all patients presenting with new lower GI symptoms and a mildly elevated FC. Our approach to investigation is pragmatic and likely reflects the approach of most UK gastroenterologists. As a result, our findings are highly relevant to clinical practice.
A meta-analysis by Triester et al8 suggested WCE was superior in the diagnosis of small bowel CD to Barium radiology (63% vs 23% yield), CT enterography/enteroclysis (69% vs 30% yield) and ileocolonoscopy (61% vs 46% yield). Studies comparing magnetic resonance enteroclysis (MRE) have shown conflicting results. A small study (n=36) by Golder et al9 suggested WCE was superior to MRE in diagnosing small bowel CD but a subsequent study by Tillack and colleagues demonstrates a good correlation between both modalities.10 More recently, in a study by Wiarda et al,11 MRE had a higher sensitivity and PPV than WCE in the diagnosis of small bowel CD. Koulaouzidis et al12 looked at FC levels as a selection tool for further investigation of suspected IBD in patients with prior negative bi-directional endoscopy. In 35 patients with an FC >100 µg/g, 42.9% had findings on WCE consistent with small bowel CD. However, a definite diagnosis of CD was only made in 10 (28.5%) with a median FC in this group of 368 µg/g. Higher levels were more likely to predict a diagnosis of CD with 50% of patients who had an FC of >200 µg/g being diagnosed with CD following WCE. However, in this study, patients were investigated with WCE due to ongoing clinical suspicion of CD which, in some cases, included equivocal small bowel imaging. In other cases, patients proceeded to WCE without any small bowel radiology.
Despite the above limitations, our study raises a number of questions regarding our current practice and how we best use FC as a diagnostic tool. These include, what is the most appropriate cut-off value to use and how do we structure further investigation and follow-up? Previous studies suggest that the trend in serial FC values can be a useful indicator of the likelihood of significant pathology. Demir et al13 examined patients referred from primary to secondary care with GI symptoms in whom an FC had been checked. A total of 2663 patients were included. The authors looked in more detail at patients with a ‘minimally elevated’ FC (50–150 µg/g) and those with higher levels (150–3000 µg/g) who underwent repeat FC testing after an interval of 6–8 weeks. In the higher FC cohort, there were 13 new cases of IBD with a mean increase in FC from 933 to 1666 µg/g. In 66 patients with a ‘minimally elevated’ FC, none developed IBD during the 2 years of follow-up and the mean FC fell from 88 to 65 µg/g. Similarly in the study by Zayyat et al,7 in 90% (9/10) of the patients who were ultimately diagnosed with IBD a repeat FC had increased.
Furthermore, a small study by Mohammed and Smale14 demonstrated that after initial negative radiological or endoscopic GI investigations, longer term follow-up of patients with elevated FC <225 µg/g failed to identify significant pathology. In all, 67 patients were followed for 3 years with no patients found to have IBD during subsequent review. Recent work by D'Haens et al15 in IBD patients supports the observation that FC levels of this magnitude are not associated with significant mucosal inflammation. In their study which examined 126 patients with IBD, an FC <250 µg/g was associated with mucosal healing, predicting endoscopic remission (Crohn's Disease endoscopic index of severity ≤3) with 94.1% sensitivity.
Despite having a high NPV for significant luminal pathology, a number of different pathologies were identified during endoscopic investigation of these symptomatic patients. In some cases, this may have influenced management, while in others the reassurance provided by negative investigations may have been important. However, it is worth noting that the yield of investigation after a macroscopically normal upper GI endoscopy and colonoscopy falls dramatically. Given these results and in light of previous studies, we propose an alternative diagnostic approach of repeating the FC after 6–8 weeks in patients with values of 100–200 µg/g. If the FC has fallen <100 µg/g then no further investigation would be pursued; if the FC remains 100–200 µg/g or has increased, then further investigation may be considered. However, in patients with an FC between 100 and 200 µg/g who have a macroscopically normal upper GI endoscopy and colonoscopy, further investigation would seem unnecessary. Further work is needed to evaluate this strategy in a prospective study.
Conclusions
In adult patients under 50 years old presenting with new non-alarm lower GI symptoms, the NPV of an FC between 100 and 200 µg/g in excluding significant organic GI disease is high. Patients are often extensively investigated yet the overall diagnostic yield is low and the majority of these patients have functional disease. We suggest that the manufacturer's FC cut-off of 50 µg/g of stool is too low for utilisation in clinical practice and often results in unnecessary, invasive investigations. Repeat FC testing and clinical assessment may represent a more pragmatic approach in this patient cohort.
Significance of this study.
What is already known on this subject?
Faecal calprotectin (FC) is increasingly used by clinicians to differentiate Inflammatory Bowel Disease (IBD) from Irritable Bowel Syndrome (IBS) but false positives are common.
What this study adds?
In young patients without ‘alarm features’, the diagnostic yield of investigating a mildly elevated FC (100–200 μg/g) is low with a high negative predictive value for significant organic disease.
How it might impact on clinical practice in the foreseeable future?
Current, manufacturer advised, reference ranges for FC assays may be too low for utilisation in clinical practice. By increasing the threshold for further investigation and/or using serial measurements, unnecessary investigations may be avoided.
Acknowledgments
This paper was previously published in abstract form (Gut 2013;62:Suppl1 A79) prior to presentation as a poster at BSG annual meeting in 2013 (PTU-081).
Footnotes
Contributors: JPS was involved in study design, data collection, analysis and manuscript writing. FT and KR were involved in data collection. KS provided intellectual contribution. DRG was involved in study design and manuscript writing.
Funding: NHS Greater Glasgow and Clyde.
Competing interests: None.
Ethics approval: The study was performed as a retrospective audit of electronic patient records and was approved by the NHS Greater Glasgow and Clyde Clinical Effectiveness Department.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Williams JG, Roberts SE, Ali MF, et al. Gastroenterology services in the UK. The burden of disease, and the organisation and delivery of services for gastrointestinal and liver disorders: a review of the evidence. Gut 2007;56(Suppl 1):1–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence. Faecal calprotectin diagnostic tests for inflammatory diseases of the bowel (DG11). London: National Institute for Health and Care Excellence, 2013. [Google Scholar]
- 3.Moroni F, Winter JW, Morris AJ, et al. What is the clinical relevance of a mildly elevated faecal calprotectin detected in new referrals to the gastroenterology clinic? Gut 2012;61(Suppl 2):A78–9. [Google Scholar]
- 4.Naismith GD, Smith LA, Barry SJ, et al. A prospective single-centre evaluation of the intra-individual variability of faecal calprotectin in quiescent Crohn's disease. Aliment Pharmacol Ther 2013;37:613–21. [DOI] [PubMed] [Google Scholar]
- 5.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li XG, Lu YM, Gu F, et al. [Fecal calprotectin in differential diagnosis of irritable bowel syndrome]. Beijing Da Xue Xue Bao 2006;38:310–13. [PubMed] [Google Scholar]
- 7.Zayyat R, Appleby RN, Logan RPH. Can we improve the negative predictive value of faecal calprotectin for the diagnosis of IBS in primary care? Gut 2011;60(Suppl 1):A49–50. [Google Scholar]
- 8.Triester SL, Leighton JA, Leontiadis GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's disease. Am J Gastroenterol 2006;101:954–64. [DOI] [PubMed] [Google Scholar]
- 9.Golder SK, Schreyer AG, Endlicher E, et al. Comparison of capsule endoscopy and magnetic resonance (MR) enteroclysis in suspected small bowel disease. Int J Colorectal Dis 2006;21:97–104. [DOI] [PubMed] [Google Scholar]
- 10.Tillack C, Seiderer J, Brand S, et al. Correlation of magnetic resonance enteroclysis (MRE) and wireless capsule endoscopy (CE) in the diagnosis of small bowel lesions in Crohn's disease. Inflamm Bowel Dis 2008;14:1219–28. [DOI] [PubMed] [Google Scholar]
- 11.Wiarda BM, Mensink PB, Heine DG, et al. Small bowel Crohn's disease: MR enteroclysis and capsule endoscopy compared to balloon-assisted enteroscopy. Abdom Imaging 2012;37:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koulaouzidis A, Douglas S, Rogers MA, et al. Fecal calprotectin: a selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scand J Gastroenterol 2011;46:561–6. [DOI] [PubMed] [Google Scholar]
- 13.Demir OM, Ahmed Z, Logan RPH. 17 Optimising the use of faecal calprotectin for early diagnosis of IBD in primary care. J Crohn's Colitis 2013;7:S8–9. [Google Scholar]
- 14.Mohammed N, Smale S. Positive calprotectin but negative investigations—what next? Gut 2012;61(Suppl 2):A236. [Google Scholar]
- 15.D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2218–24. [DOI] [PubMed] [Google Scholar]
- 16.Seenan JP, Thomson K, Rankin, et al. Are we exposing patients with a mildly elevated faecal calprotectin to unnecessary investigations? Gut 2013;62:(Suppl1):A79. [DOI] [PMC free article] [PubMed] [Google Scholar]