Abstract
A 36-year old man with known HIV infection presented to an outpatient genitourinary service with jaundice, rash and sore throat. Investigations revealed marked biochemical abnormalities, including alkaline phosphatase and alanine transaminase >10 times the upper limit of normal. Liver ultrasound was normal, but stricturing and beading of the intrahepatic biliary tree was seen on magnetic resonance cholangiopancreatography (MRCP), similar to changes associated with sclerosing cholangitis. Serological syphilis antibodies were detected with a positive immunoglobulin M (IgM) and rapid plasma reagin of 1:128, in keeping with early infection. Liver biopsy showed large bile duct obstruction with portal oedema, bilirubinostasis and neutrophil polymorph infiltration around proliferating ductules; specific stains for spirochaetes were negative. Symptoms and biochemical markers improved rapidly after treatment for secondary syphilis with oral steroids and intramuscular benzathine penicillin. A repeat MRCP 18 months post syphilis treatment showed resolution. This case illustrates syphilis presenting as acute sclerosing cholangitis.
Keywords: ACUTE HEPATITIS, HIV/AIDS, HISTOPATHOLOGY, LIVER IMAGING, MAGNETIC RESONANCE IMAGING
Background
Men who have sex with men are a known risk group for syphilis infection, accounting for 74% of 3249 cases of early syphilis in 2013 in the UK.1 2 Owing to the variable presentation of syphilis, patients may attend any medical department with their symptoms. However, outside of genitourinary (GU) settings patients and doctors may feel uncomfortable discussing sexual history, and hence syphilis serology is less likely to be considered. In this case, return to baseline liver function occurred following timely treatment of syphilis; without early testing, the outcome might have been different.
This case emphasises that the triad of rash, pharyngitis and lymphadenopathy is a strong indicator for syphilis testing in a patient with deranged liver function tests. It also adds to the limited literature on magnetic resonance cholangiopancreatography (MRCP) and histological findings in cases of syphilitic hepatitis, particularly in HIV infection.
Case presentation
A 36-year-old man presented to an outpatient GU medicine clinic with jaundice. He had been diagnosed with HIV 8 years previously and had an undetectable HIV viral load on emtricitabine, tenofovir and efavirenz. His CD4 count was stable at 246 cells/µl, and he had no history of AIDS-defining illnesses.
He gave a 4-week history of lethargy and sore throat, a 2-week history of dark urine and a 5-day history of visible jaundice noted by friends. He also complained of a rash over his trunk and back, which had prompted his presentation. Four weeks previously he had visited his general practitioner (GP) with epigastric pain following alcohol excess and was treated with omeprazole. He took no other medication, was a non-smoker and denied any recreational drug use. He had a long-term male partner. His last travel had been 4 months previously to Gran Canaria where he reported oral sex with a casual male partner.
On examination, he was afebrile. He had mild jaundice of the skin and sclera and small volume cervical lymphadenopathy. There were no features of encephalopathy or stigmata of chronic liver disease. Systems examination was unremarkable. A widespread macular erythematous rash was present over his trunk and back.
Investigations
Initial investigations were as follows: bilirubin 124 µmol/l (5–21), alanine transaminase 549 IU/l (<40), alkaline phosphatase 3290 IU/l (70–300), with normal haematology, renal function and synthetic liver function. Viral parameters showed hepatitis B surface antigen not detected, hepatitis B core antibody not detected, antibodies to hepatitis B surface antigen 97 IU/l, hepatitis A immunoglobulin M (IgM) not detected, hepatitis C RNA PCR not detected, Epstein–Barr virus IgM not detected and cytomegalovirus PCR not detected. An autoantibody screen was negative other than a weakly positive antimitochondrial antibody 1/100 dilution that reverted to negative at follow-up testing. Syphilis antibodies were detected by enzyme immune assay with a positive IgM, Treponema pallidum particle agglutination assay 1:>20 480 and rapid plasma reagin (RPR) 1:128, confirmed on a repeat sample. Syphilis serology had been negative 4 months previously.
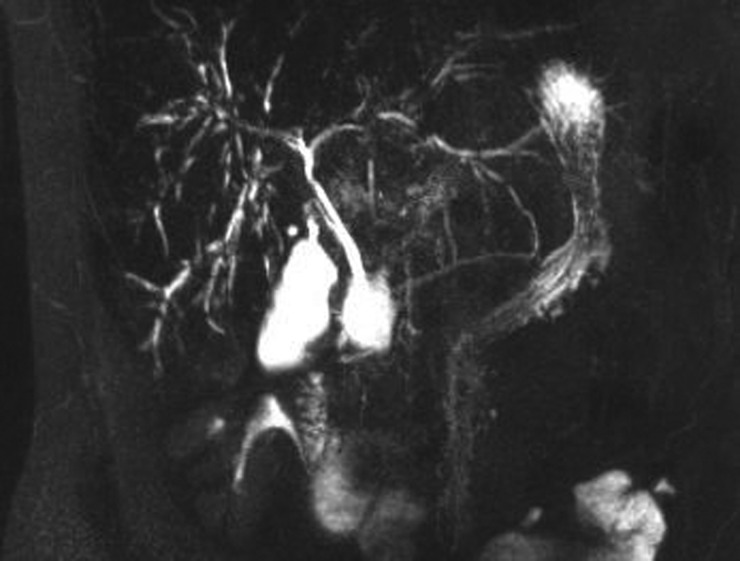
Abdominal ultrasound scan was normal, but MRCP revealed stricturing and beading of the intrahepatic biliary tree, consistent with sclerosing cholangitis (figure 1). Liver biopsy showed changes typical of acute large bile duct obstruction with neutrophil polymorph infiltration around proliferating ductules and portal oedema (figures 2 and 3). There were no signs of chronic cholestatic liver damage. No copper-associated protein or fibrosis was detected and there was no bile duct loss. Stains for spirochaetes and mycobacteria were negative. A single small non-necrotising granuloma was found.
Figure 1.
Magnetic resonance cholangiopancreatography (MRCP) image showing diffuse irregularity of the intrahepatic biliary tree, with areas of stricturing and mild dilatation. The extra hepatic biliary tree is normal.
Figure 2.
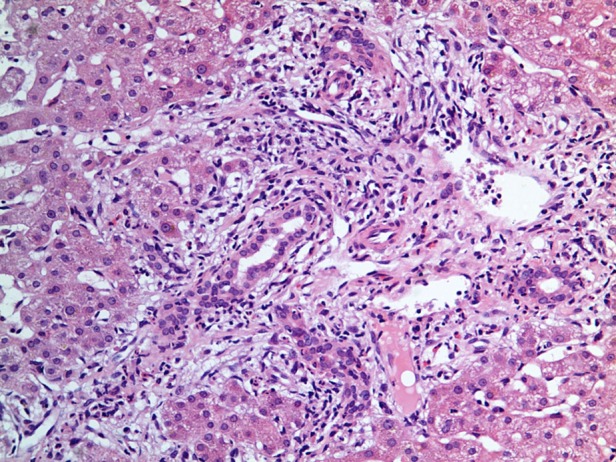
H&E stain, ×40 magnification. Bile duct obstruction with surrounding infiltration by neutrophil polymorphs.
Figure 3.
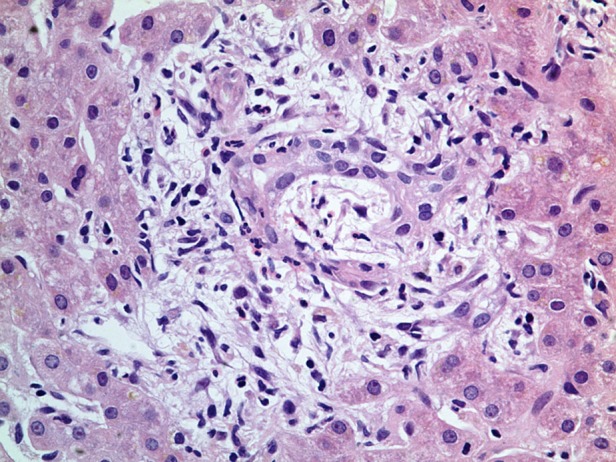
H&E stain, ×40 magnification. Oedema of the portal tract.
Differential diagnosis
The deranged liver function tests and normal ultrasound in this man with HIV infection had a wide differential diagnosis for possible cause, including viral hepatitis, opportunistic infections and drug-related hepatotoxicity. However, his stable CD4 count with an undetectable HIV viral load made AIDS cholangitis unlikely, and an initial non-invasive liver screen had not pointed to a viral or autoimmune cause. There was no recent change in antiretroviral medication to imply a drug reaction. Stricturing and beading on MRCP suggested sclerosing aetiologies including primary sclerosing cholangitis, but there are many recognised secondary causes, including infection, drug toxicity and neoplasm.3 Based on the clinical and serological picture and the additional information gained from the scans and histopathology, syphilis was believed to be the underlying infectious cause in this case. Syphilis in its secondary stage can affect any body system and cause end organ damage, and hence is well known as the ‘Great Pretender’.
Treatment
The patient was treated for early syphilis with 10 days of intramuscular procaine penicillin 600 mg once daily. He received prednisolone 20 mg three times daily for 3 days to prevent a Jarisch–Herxheimer reaction, and was then given a reducing dose of prednisolone over a month to cover for alternative sclerosing cholangitis aetiologies. He was also prescribed ursodeoxycholic acid for 1 month.
Outcome and follow-up
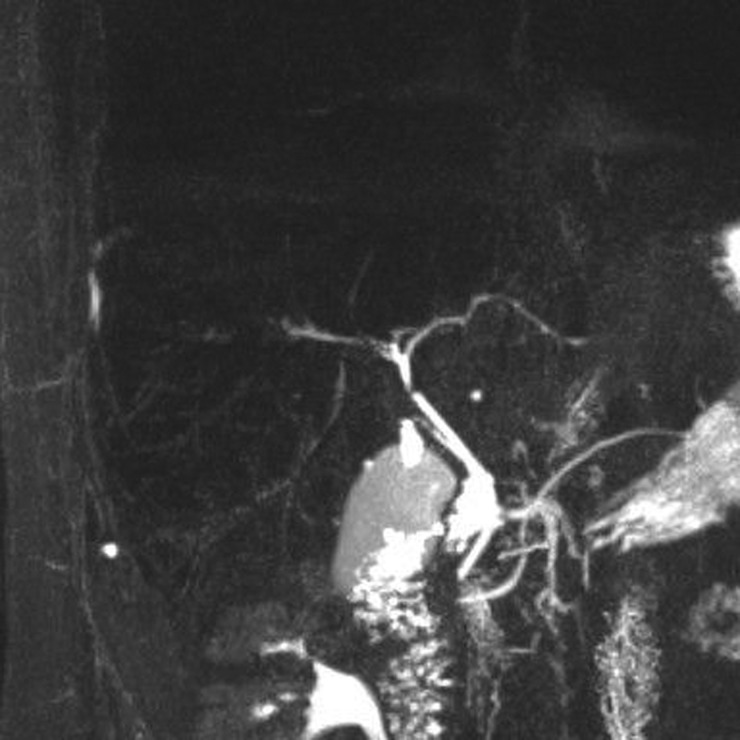
Both his symptoms and biochemical parameters improved rapidly. Within a week, his alkaline phosphatase level had reduced by half, and his liver function tests were normal within 3 months. The RPR also responded appropriately to treatment, with a titre fall to 1:4 at 3 months follow-up, and subsequently negative when tested at 1 year. A repeat MRCP at 6 months showed resolution of the bile duct dilatation and only mild irregularity of the hepatic ducts, with further improvement at 18 months (figure 4).
Figure 4.
Magnetic resonance cholangiopancreatography (MRCP) image. Following treatment, the intrahepatic ducts have returned to a normal appearance.
Discussion
Syphilitic hepatitis has been well described in early syphilis infection;4 however, hepatitis in HIV-infected patients is less well documented,5–15 and descriptions are mainly confined to specialty infection journals. In particular, only five published case reports contain histopathological information.5–9 Imaging information is also scarce, with ultrasound and computerised topographic scans often being normal,6 7 11 or showing non-specific abnormalities: mild to moderate hepatosplenomegaly,5 8 9 and one case of circumferential common bile duct wall thickening on ultrasound scan.5 This is the first case to report appearances of cholangitis on MRCP imaging with syphilis as the causative agent. Lack of previous MRCP investigations may indicate that this is an unrecognised pathological process.
In two retrospective studies involving 62 and 147 patients respectively, the incidence of hepatic involvement in HIV-infected patients with early syphilis was recorded as between 19.3% and 30.6%.14 15 Men who have sex with men are a high-risk group for both syphilis and HIV infection.1 2
The most commonly reported presenting feature of syphilitic hepatitis in HIV-infected patients is rash5 14; pharyngitis and lymphadenopathy are also well described.5 This is in contrast to the historical presentation of abdominal pain, fever, hepatomegaly and liver tenderness.7 The characteristic biochemical feature described is a disproportionate rise in alkaline phosphatase level; hyperbilirubinaemia is reported less commonly.4–6 In this case, jaundice was the presenting symptom, and there was significant hyperbilirubinaemia, but rash, pharyngitis and lymphadenopathy were also noted.
Existing histological descriptions of syphilitic hepatitis may either pre-date the penicillin era, or are in samples where there has been failure to adequately exclude other causes. Hepatocyte necrosis around a central vein, mild non-specific granulomatous reactions and pericholangiolar inflammation with lymphocyte infiltrates have been described in HIV-negative patients.4 6 9 Pathological findings are variable if HIV infection is present. The liver biopsy in this case showed changes of acute obstruction of the large bile ducts with bilirubinostasis, portal oedema and neutrophil polymorph infiltration; spirochaete stains were negative and only a single small non-necrotising granuloma was seen in a portal tract. This information adds to the already published findings of five cases of liver biopsy in HIV-infected patients with syphilitic hepatitis,5–9 where histology has ranged from periportal inflammation with neutrophil infiltration5 7 8 to piece-meal necrosis,7 and lymphomonocytic infiltrate with an isolated microgranulomatous lesion.9
Absence of spirochaetes on liver biopsy is not felt to exclude syphilitic hepatitis. A case series of early syphilis in HIV-negative patients found spirochaetes in only seven out of 15 liver biopsies for clinical syphilitic hepatitis,16 and in the five published reports with histology in HIV-positive patients only one found spirochaetes on Warthin–Starry staining.9
In this case, the patient revealed risk factors through his sexual history that required consideration of syphilis as a unifying diagnosis. Some patients may not reveal sexual risk factors even on direct questioning, especially in settings outside of GU medicine clinics. As such, serological testing for syphilis should be considered as part of an initial non-invasive screen for deranged liver function tests, especially if rash, pharyngitis or lymphadenopathy is also present.
Acknowledgments
Thanks to Dr SL Allstaff, Consultant in Genitourinary Medicine, Dr N Scott, Consultant Histopathologist, and Dr M Sheridan, Consultant Radiologist, for their contributions to this work.
Footnotes
Contributors: HEW wrote the initial draft. LCH, EFM and RLJ all contributed to amendments and the final report.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sexually transmitted infections and chlamydia screening in England, 2013. Public Health England infection report, Vol 8 (24) 17 June 2014. https://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables (accessed Jan 2015).
- 2.Table 1: STI diagnoses and rates in England by gender, 2004 to 2013. Public Health England. https://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables (accessed Jan 2015).
- 3.Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology 2006;44:1063–74. doi:10.1002/hep.21405 [DOI] [PubMed] [Google Scholar]
- 4.Veeravahu M. Diagnosis of liver involvement in early syphilis, a critical review. Arch Intern Med 1985;145:132–4. doi:10.1001/archinte.1985.00360010170028 [PubMed] [Google Scholar]
- 5.Crum-Cianflone N, Weekes J, Bavaro M. Syphilitic hepatitis among HIV infected patients. Int J STD AIDS 2009;20:278–84. doi:10.1258/ijsa.2008.008324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullick CJ, Liappis AP, Benator DA, et al. . Syphilitic hepatitis in HIV-infected patients: A report of 7 cases and review of the literature. Clin Infect Dis 2004;39:e100–105. doi:10.1086/425501 [DOI] [PubMed] [Google Scholar]
- 7.Dooley DP, Tomski S. Syphilitic pneumonitis in an HIV infected patient. Chest 1994;105:629–31. doi:10.1378/chest.105.2.629 [DOI] [PubMed] [Google Scholar]
- 8.Sabbatani S, Manfredi R, Attard L, et al. . The “Great Imitator”. Syphilis as causative agent of isolated, concurrent acute hepatitis and meningitis. Infect Dis Clin Pract 2005;13:261–4. doi:10.1097/01.idc.0000168478.73470.69 [Google Scholar]
- 9.Sabbatini S, Manfredi R, Attard L, et al. . Secondary syphilis presenting with acute severe hepatic involvement in a patient with undiagnosed HIV disease. AIDS Patient Care STDS 2005;19:545–9. doi:10.1089/apc.2005.19.545 [DOI] [PubMed] [Google Scholar]
- 10.Cooper C, MacPherson P, Angel JB. Liver toxicity resulting from syphilis and Jarisch–Herxheimer reaction in cases of coinfection with HIV and hepatitis C Virus. Clin Infect Dis 2005;40:1211–2. doi:10.1086/428848 [DOI] [PubMed] [Google Scholar]
- 11.Doyle RJ, Desai M, White J. Response to Crum-Cianflone et al. Syphilitic hepatitis among HIV infected patients. Int J STD AIDS 2009;20:739–40. doi:10.1258/ijsa.2009.009297 [DOI] [PubMed] [Google Scholar]
- 12.Saxon CJ, Helbert MR, Komolafe AJ, et al. . Rash and hepatitis within days of starting a new antiretroviral regime: Nevirapine hypersensitivity, secondary syphilis or both?. Int J STD AIDS 2014;25:228–30. doi:10.1177/0956462413497703 [DOI] [PubMed] [Google Scholar]
- 13.Pilozzi-Edmonds L, Kong LY, Szabo J, et al. . Rapid progression to gummatous syphilitic hepatitis and neurosyphilis in a patient with newly diagnosed HIV. Int J STD AIDS Published Online First: 17 Dec 2014; doi:10.1177/0956462414564401 [DOI] [PubMed] [Google Scholar]
- 14.Manavi K, Dhasmana D, Cramb R. Prevalence of hepatitis in early syphilis among an HIV cohort. Int J STD AIDS 2012;23:e4–6. doi:10.1258/ijsa.2009.009386 [DOI] [PubMed] [Google Scholar]
- 15.Palacios R, Navarro F, Narankiewicz D, et al. . Liver involvement in HIV infected patients with early syphilis. Int J STD AIDS 2013;24:31–3. doi:10.1177/0956462412472316 [DOI] [PubMed] [Google Scholar]
- 16.Feher J, Somogyi T, Timmer M, et al. . Early syphilitic hepatitis. Lancet 1975;7941:896–9. doi:10.1016/S0140-6736(75)92129-7 [DOI] [PubMed] [Google Scholar]